Prevalence, Time Trends, and Utilization Patterns of Psychotropic Polypharmacy Among Pediatric Medicaid Beneficiaries, 1999–2010
Abstract
Objectives:
This study estimated the prevalence, time trends, and state-level variation of same- and multiclass psychotropic polypharmacy among youths in Medicaid fee-for-service plans.
Methods:
Using pharmacy records from 29 Medicaid states from 1999 to 2010, the authors constructed ten two-year cohorts of beneficiaries between ages 0 and 17 years who received at least one psychotropic to treat a mental disorder. Polypharmacy was defined as any period in which dispensed days' supply of psychotropics overlapped for more than 45 days. Same- and multiclass psychotropic polypharmacy prevalence was stratified by age and state.
Results:
A total of 692,485 children were included across each two-year cohort. The prevalence of any-class and multiclass psychotropic polypharmacy grew steadily, from 21.2% and 18.8% in 1999–2000 to 27.3% and 24.4% in 2009–2010, respectively. The prevalence increased with older age, with highest estimates for late adolescents. For same-class psychotropic polypharmacy, a constant upward trend was noted over time, except for antidepressants. Polypharmacy increased over the decade for central nervous system stimulants, from .1% to .6%, and for alpha-agonists, from .1% to .4%. Heterogeneous prevalences of psychotropic polypharmacy were noted across states, ranging from 6.9% to 48.8% for any-class psychotropic polypharmacy, from .4% to 6.4% for same-class antidepressant polypharmacy, and from .1% to 4.6% for antipsychotics.
Conclusions:
The study found an overall increasing trend of psychotropic polypharmacy coupled with significant variation across the examined states. A more granular assessment that considers patient characteristics and local contextual factors is warranted.
Compared with a decade ago, use of psychotropics has steadily increased between two- to sevenfold in the United States (1–6) and also abroad (7,8). The prevalence of psychotropic polypharmacy—the concomitant use of two or more psychotropic medications—has increased as well. For example, physician office visits by adult patients with mental disorder diagnoses that involved prescription of two or more psychotropics increased from 42.6% in 1996–1997 to 59.8% in 2005–2006 (4). In addition, about 30% of these visits involved prescription of three or more psychotropic medications simultaneously. Among hospitalized psychiatric patients, psychotropic monotherapy declined by almost 30% in favor of psychotropic polypharmacy from the 1970s through the 1990s (9).
For children, information about psychotropic polypharmacy use remains fragmented. According to a previous review, only 13 pediatric psychotropic polypharmacy studies were published between 1994 and 2009 (6). The review found that approximately one-third of children who were given at least one psychotropic agent received psychotropic polypharmacy for at least 14 days. However, the methodology failed to differentiate concomitant use from augmentation and switching (6). Most previous studies have focused on same-class psychotropic polypharmacy. For example, the prevalence of concomitant use of multiple antipsychotics was estimated at 8% among children and adolescents in a state Medicaid program (10). Other studies have focused on specific mental disorders (e.g., autism) (11) or specific settings (e.g., office-based psychiatric facilities) (12).
Fragmented utilization data are complemented by insufficient data on efficacy and safety of pediatric polypharmacy. The scarce evidence originates from studies in adults, raising concerns whether extrapolations to youths are appropriate (4,13,14). For example, potentially harmful drug-drug interactions may pose an additional threat to younger patients (15).There is an urgent need to characterize the magnitude and types of psychotropic polypharmacy use in the pediatric population.
Finally, even though geographic differences in prescribing patterns were identified as an important research agenda almost 20 years ago (16), the application of geography-based analysis to quantify regional variation in psychotropic prescribing patterns remains underutilized. Therefore, we sought to quantify geographic variation in the prevalence of any-class, same-class, and multiclass polypharmacy among children and adolescents enrolled in Medicaid fee-for-service plans. We also quantified time trends in these prevalences throughout the last decade.
Methods
This study utilized a data repository of medical inpatient and outpatient encounter and pharmacy billing records from 1999 to 2010 from Medicaid programs in 29 states, representing approximately 85% of the entire population enrolled in Medicaid fee-for-service plans (17). The repository, the MedicAid EXtract (MAX) Files, is provided for research purposes by the Center for Medicare and Medicaid Services (CMS) (18).
Study Population
We built 10 cohorts (10 two-year blocks, from 1999 to 2010) of patients with a pharmacy billing record for at least one psychotropic agent and at least two years of continuous Medicaid enrollment after the first psychotropic dispensing in the above-specified plans. Patients had to be between the ages of 0 and 17 during the entire two‐year follow‐up period. The first psychotropic pharmacy claims in each study year determined the start of follow‐up (index date) and the assignment to a two-year cohort. Each beneficiary could be counted in multiple cohorts if he or she met the inclusion criteria.
Polypharmacy Definition
The National Association of State Mental Health Program Directors has defined six types of polypharmacy (19): same class, multiclass, augmentation, adjunctive, any class, and total. We used same class, which refers to use of more than one medication from the same therapeutic class; multiclass, which refers to use of more than one medication from different classes; and any class, which includes use of more than one medication from the same class or from multiple classes.
Psychotropic Medications
For each two‐year cohort, we extracted pharmacy billing records for all psychotropic medications approved by the Food and Drug Administration (FDA) that had an indication for treatment of mental disorders. [A list of these medications in available in an online supplement to this article.] These included alpha-agonists, anticonvulsants, antidepressants, antimanic agents, first- and second-generation antipsychotics, and sedatives-hypnotics-anxiolytics.
Polypharmacy Measurement
To measure psychotropic polypharmacy, we identified the period when a patient was covered, on the basis of the pharmacy-recorded dispensed days’ supply on each claim. Patients were assumed to start taking their medication on the dispensing date. The end date was calculated on the basis of the dispensed days’ supply plus a 10-day grace period to account for late refills. This resulted in a matrix for each patient showing the calendar days during the study period covered by each of the considered psychotropic medications. Because polypharmacy was defined as an overlap of greater than 45 days in the active periods of two or more psychotropic medications with different active ingredients, we were able to differentiate polypharmacy from switching and short-term use. This more stringent consecutive overlap method allowed examination of true psychotropic polypharmacy patterns, which is consistent with previous studies of polypharmacy and is a generally accepted threshold.
Analysis
The prevalence of the various types of psychotropic polypharmacy was the proportion of eligible children who met the psychotropic polypharmacy definition within the two‐year follow‐up period. The stratified analysis by age group (age five or younger, ages six to nine, ages ten to 14 [early adolescents], and ages 15 to 17 [late adolescents]) and by state (N=29) was based on each patient’s first two-year follow-up period during the entire study period. Note that for state-level comparisons, Kentucky and Iowa were removed from the analysis because of small sample sizes (large managed care penetration resulted in only a small population in fee-for-service plans). All analyses were conducted with SAS 9.4 and ArcMap 10.3. This study was approved by the University of Florida (UF) Institutional Review Board and the UF and CMS privacy boards.
Results
Table 1 presents information on each two-year cohort. On average, 692,485 children and adolescent beneficiaries with at least one psychotropic medication were included in each cohort. Overall, gender proportions remained similar across cohorts. The proportion of African Americans decreased slightly, from about one-third in 1999–2000 to about one-quarter in 2009–2010. In contrast, representation of Hispanics increased from 15.4% in 1999–2000 to 23.5% in 2009–2010. Age distributions remained similar across the years. The proportion of beneficiaries who qualified for Medicaid because of poverty increased over time.
| Characteristic | 1999–2000 | 2001–2002 | 2002–2003 | 2003–2004 | 2004–2005 | 2005–2006 | 2006–2007 | 2007–2008 | 2008–2009 | 2009–2010 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample sizea | 637,174 | 683,280 | 769,872 | 823,047 | 966,613 | 809,466 | 639,182 | 552,980 | 557,371 | 485,874 |
| Male | 59.1 | 58.9 | 58.7 | 58.8 | 58.5 | 59.3 | 59.8 | 59.6 | 59.5 | 59.7 |
| Race-ethnicity | ||||||||||
| White | 44.5 | 45.4 | 46.8 | 48.8 | 49.6 | 48.1 | 46.6 | 45.4 | 44.6 | 44.5 |
| Black | 32.4 | 33.2 | 31.6 | 31.5 | 29.5 | 28.8 | 28.5 | 26.9 | 26.8 | 25.4 |
| American Indian/Alaska Native | .7 | .7 | .8 | .9 | .8 | .9 | 1.1 | 1.3 | 1.3 | 1.1 |
| Asian | 1.0 | .9 | .9 | .9 | .8 | .8 | .9 | 1.0 | 1.1 | 1.2 |
| Hispanic/Latino | 15.4 | 15.3 | 15.8 | 14.2 | 15.6 | 17.4 | 18.6 | 21.2 | 21.9 | 23.5 |
| Native Hawaiian/Pacific Islander | .2 | .2 | .2 | .1 | .2 | .1 | .1 | .1 | .1 | .1 |
| Hispanic/Latino and ≥1 races | 1.7 | 1.3 | 1.1 | .9 | .8 | 1.0 | 1.2 | 1.3 | 1.5 | 1.7 |
| ≥1 races | .1 | .1 | .1 | .1 | .1 | .1 | .2 | .2 | .2 | .3 |
| Unknown race | 3.1 | 3.0 | 2.7 | 2.7 | 2.7 | 2.9 | 2.8 | 2.6 | 2.6 | 2.3 |
| Age | ||||||||||
| ≥5 | 30.6 | 30.5 | 31.1 | 28.5 | 29.8 | 27.9 | 25.7 | 26.7 | 27.1 | 26.1 |
| 6–9 | 28.9 | 28.0 | 26.8 | 26.8 | 25.8 | 25.9 | 26.2 | 26.4 | 26.9 | 27.3 |
| 10–14 | 32.4 | 32.9 | 33.3 | 35.2 | 34.6 | 35.4 | 36.3 | 35.2 | 34.4 | 34.8 |
| 15–17 | 8.1 | 8.5 | 8.9 | 9.6 | 9.8 | 10.8 | 11.8 | 11.8 | 11.6 | 11.8 |
| Medicaid eligibility category | ||||||||||
| Foster care | 11.5 | 11.3 | 10.6 | 10.3 | 9.2 | 10.3 | 12.5 | 12.5 | 11.3 | 11.7 |
| Cash assistance | 46.5 | 43.0 | 41.0 | 39.2 | 38.2 | 37.1 | 36.4 | 33.9 | 33.5 | 33.1 |
| Poverty | 35.6 | 39.0 | 41.6 | 43.6 | 44.4 | 45.2 | 46.5 | 49.6 | 52.0 | 52.4 |
| Disability | 24.2 | 22.3 | 20.6 | 20.6 | 19.6 | 21.5 | 23.4 | 22.8 | 23.1 | 23.4 |
TABLE 1. Demographic characteristics (in percentages) of pediatric Medicaid beneficiaries in 29 states who had at least one psychotropic medication claim, by two-year cohort
Figure 1 shows the prevalence across time of any-class and multiclass psychotropic polypharmacy among children who received at least one psychotropic medication. Starting with prevalence estimates of 21.2% and 18.8%, respectively, for any-class and multiclass polypharmacy in 1999–2000, both psychotropic polypharmacy types exhibited a steady increase over the entire time period, of 6.1% and 5.6%, respectively, resulting in 27.3% and 24.4% in 2009–2010. [A table in the online supplement presents additional data.]
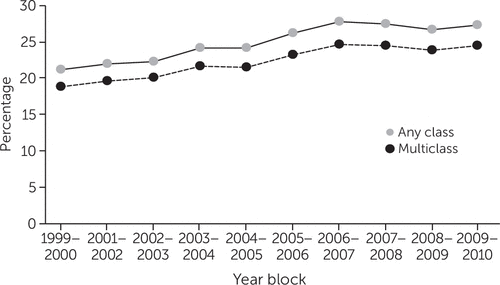
FIGURE 1. Prevalence of psychotropic polypharmacy among pediatric Medicaid beneficiaries in 29 states who had at least one psychotropic medication claim, 1999–2010a
aAny class, use of more than one medication from the same class or from multiple classes; multiclass, use of more than one medication from different classes
Figure 2 shows psychotropic polypharmacy prevalence by age group. Both any-class and multiclass polypharmacy prevalence showed similar trends for children of all age groups. For any-class polypharmacy, we noted a 18% absolute difference between prevalences for preschoolers and early adolescents (4.5% and 22.5% respectively); prevalence for late adolescents (ages 15–17) was 23.7%. Likewise, for multiclass polypharmacy, prevalence increased from 3.4% among preschoolers to 20.7% and 20.9% among early and late adolescents, representing a 17.3% and 17.5% difference, respectively.
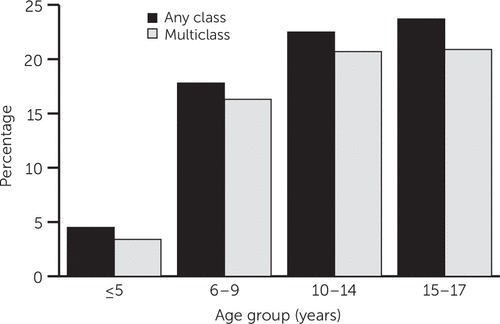
FIGURE 2. Prevalence of psychotropic polypharmacy among pediatric Medicaid beneficiaries, by age, 1999–2010a
aAny class, use of more than one medication from the same class or from multiple classes; multiclass, use of more than one medication from different classes
Figure 3 shows the secular trends of five same-class categories of psychotropic polypharmacy prevalence. Medications for attention-deficit hyperactivity disorder (ADHD) (central nervous system [CNS] stimulants and alpha-agonists) had small and nonvarying polypharmacy prevalences from 1999 to 2004 (.1%–.2%); prevalence then rose to .6% and .4%, respectively, in 2009–2010. Between 1999–2000 and 2006–2007, polypharmacy prevalence for same-class antipsychotics tripled (from .6% to 1.9%), and polypharmacy rates for same-class second-generation antipsychotics increased more than fivefold (.3% versus 1.7%). After 2007, the trend in antipsychotic polypharmacy showed a small decline. Antidepressant polypharmacy showed a less pronounced growth up to 2002–2004, when the trend took a downward trajectory to levels even lower than at the beginning of the study period. In the 1999–2000 cohort, the polypharmacy prevalence for same-class antidepressants was higher than for antipsychotics (1.8% versus .6%). However, in 2009–2010, both psychotropic classes were used at a similar proportion (1.5% versus 1.6%).
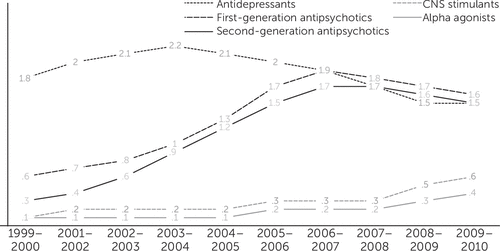
FIGURE 3. Secular trends in same-class polypharmacy prevalence among pediatric Medicaid beneficiaries, by drug class, 1999–2010a
aSame class, use of more than one medication from the same therapeutic class
Figure 4 shows any-class psychotropic polypharmacy prevalence and corresponding estimates for same-class antidepressant polypharmacy and same-class antipsychotic polypharmacy across 27 states. Nine states had any-class prevalences ranging from 6.9% (Illinois) to 12.6% (North Carolina), and prevalences in 11 states ranged between 14.4% (Missouri) and 25.2% (Indiana). The largest proportion of any-class polypharmacy was reported in Nebraska (48.8%). For same-class antidepressants, 18 states had polypharmacy prevalences below 2%, and rates in three Midwestern states (Minnesota, Kansas, and Nebraska) were higher than 4%. For antipsychotics, Nebraska had the highest polypharmacy prevalence, at 4.6%, followed by 2.6% in Kansas. The remaining states had antipsychotic polypharmacy prevalences below 2%.
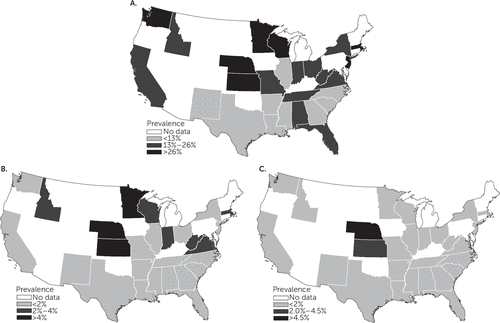
FIGURE 4. Polypharmacy prevalence among pediatric Medicaid beneficiaries who had at least one psychotropic medication claim, by state, 1999–2010a
aSource: Medicaid, 1999–2010. Panel A, any class; panel B, antidepressants; panel C, antipsychotics
Discussion
To the best of our knowledge, this is the first study to evaluate the prevalence of psychotropic polypharmacy among pediatric Medicaid enrollees over a span of 10 years. First, the prevalence of any-class and multiclass psychotropic polypharmacy among children who received at least one psychotropic medication increased steadily. Second, at the end of the study period, more than one-quarter of youths who were using at least one psychotropic received a psychotropic polypharmacy regimen. Third, psychotropic polypharmacy increased consistently with age until it plateaued at about the time children began high school (ten- to 14-year age group). Fourth, both any and, in particular, second-generation antipsychotic polypharmacy showed a sharp increase, but the rate leveled off during later study years, and antidepressant polypharmacy declined over time, reaching similar prevalences as antipsychotics at the end of the study period. Polypharmacy prevalences for same-class CNS stimulants and same-class alpha-agonists showed a constant increase beginning in 2005–2006 until the end of the study period. Finally, we noted large variation in psychotropic polypharmacy prevalence across states.
Balancing the risks and benefits of psychotropic polypharmacy among youths remains a challenging endeavor for prescribers. Even though long-term use raises far more concerning questions regarding efficacy, safety, and financial value (6), most of our knowledge about the prevalence of psychotropic polypharmacy is grounded in cross-sectional studies (20–22) or on studies using short and specific time frames, such as a single office visit (4,12). To our knowledge, only studies among adults have evaluated long-term psychotropic polypharmacy by using concurrent, overlapping definitions over time (23–25). Overall, we found lower polypharmacy prevalences for any-class psychotropics than have been found in previous cross-sectional work involving pediatric populations (10). The difference is explained in part by the use of less stringent operational definitions of psychotropic polypharmacy in other studies. For example, McIntyre and Jerrell (26) reported a pediatric psychotropic polypharmacy prevalence of 41.6%, defined as “physician who prescribed two or more psychotropic medications” in 2005. Duffy and colleagues (27) reported a 53% prevalence among youths, defined as “concurrent use of two or more psychotropic medications for the treatment of a psychiatric disorder.” Thus the proportion of youths in these previous studies who were receiving persistent, long-term psychotropic polypharmacy versus short-term, acute psychotropic polypharmacy is unknown. Also, cross-sectional definitions fail to capture a large portion of psychotropic polypharmacy users. For example, Chen and colleagues (6) estimated that cross-sectional definitions fail to identify between 18% and 44% of persons classified as long-term psychotropic polypharmacy users. Additional research is warranted to help clarify the true long-term prevalence of psychotropic polypharmacy among youths outside Medicaid by using more pertinent operational definitions.
For same-class psychotropic polypharmacy, our longitudinal trends match previous findings from the Centers for Disease Control and Prevention (CDC) for psychotropic monotherapy. The CDC found an overall upward trajectory for ADHD medications and antipsychotics and a downward trend for antidepressants (1). The latter may be attributable to the 2004 black box warning, which noted an increased risk of suicidal ideation among youths after antidepressant use (28,29). Furthermore, the warning may have encouraged clinicians to use other options, such as antipsychotics, to treat adolescents with suicidal tendencies. Indeed, a previous study found a threefold increase in the odds of receiving second-generation antipsychotics (compared with fluoxetine) among patients with concomitant depression and suicidal ideation (30). This tendency may explain the simultaneous and inversely proportional increase in our data of same-class antipsychotic polypharmacy. The finding that same-class antipsychotic polypharmacy exceeded same-class antidepressant use by the end of the study period suggests that antipsychotics are evolving as an alternative to antidepressant regimens. Also, an increased prevalence of schizophrenia and bipolar disorder diagnosis and the widespread use of antipsychotics for disruptive behavior disorders (31) may help explain the trend. However, this “medicalization” is worrisome, because pediatric guidelines do not support the generalized use of psychotropic polypharmacy (31).
Regarding same-class psychotropic polypharmacy, previous studies have reported trends similar to ours, including increased use of same-class antipsychotic polypharmacy (10,32,33), decreased use of same-class antidepressant polypharmacy (34), and a steady increase of CNS stimulant polypharmacy over time (35). Of note, our definition of CNS stimulant polypharmacy did not account for the simultaneous use of long- and short-acting stimulants (same active substance), which is considered appropriate for ADHD treatment. Thus our reported psychotropic polypharmacy prevalence estimates captured only combinations of different CNS stimulants, which are typically discouraged by clinical guidelines (for example, concomitant use of methylphenidate and amphetamine) (36). On the other hand, some combinations may be appropriate in very specific circumstances (for example clonidine and guanfacine are FDA-approved as augmentative to stimulant medication [35]). However, these short-term augmentation regimens are not supported by a particularly strong empirical base. In summary, our data suggest that use of same-class psychotropic polypharmacy among children is rising, except for same-class antidepressant polypharmacy.
The steady overall increase in psychotropic polypharmacy may be explained in part by an increase of complexity, severity, or refractoriness of illness among youths (37), but other aspects are noteworthy. First, the common perception by clinicians, patients, and parents that psychotropic monotherapy is insufficient for symptom reduction or remission has been increasing (13). Also, patients are seeking treatment options more aggressively in part because the stigma surrounding mental illness is eroding. Third, increased awareness among clinicians of diagnosing and treating mental conditions may have also contributed to the rise in psychotropic medication use (38). In addition, the industry has invested heavily in direct-to-consumer (DTC) marketing, contributing to the upward trajectory of psychotropic use. From 1996 to 2005, spending on marketing tripled for psychotropic drugs, including a 500% increase in DTC advertising (39,40). Compared with the United Kingdom, which does not allow DTC advertising, U.S. prescription rates are 25 times higher for ADHD medications for children and adolescents (41,42). Finally, the pharmaceutical industry and medical education have intensified the promotion of pharmacotherapy to the detriment of behavioral and psychosocial alternatives, leaving psychotherapy commonly reserved for resistant or refractory disorders (43–45).
Ideally, monotherapy (optimal choice) or switching to a same-class option (second choice) and discouraging the initiation of psychotropic polypharmacy for “psychotropicnaive” patients should be considered adequate practice (46–48). However, if monotherapy achieves only a suboptimal response and augmentation is considered as the next logical step, the addition of a new medication should first consider options that have been approved for the intended use. This approach should mitigate the risk of side effects. For example, aripiprazole helps increase treatment response rates for antidepressants and has been approved by the FDA as an add-on therapy for depression (49). However, off-label augmentation is a more common practice, even though evidence supporting it remains weak (50). Future research is urgently needed to clarify which clinical scenarios benefit the most from switching or augmenting strategies.
Regarding age differences, even though the data showed a sustained use of psychotropic polypharmacy as age progresses, this trend may be explained by the parallel changes in disease epidemiology. Most psychiatric conditions become manifest, disruptive, or clinically significant when children begin school or among prepubescent and pubescent youths. However, the prevalence of psychotropic polypharmacy among preschoolers cannot be solely explained by disease epidemiology. Use of psychotropic polypharmacy among preschoolers is a highly discouraged practice because of the unknown effects on cognitive and physical development (51).
This study found a vast degree of variability for any-class psychotropic and same-class antidepressant and antipsychotic polypharmacy across 27 states. The variability may be partly driven by the large uncertainty around use, safety, and effectiveness of psychotropic pharmacotherapy. Some factors, such as diverse degrees of access to care and availability of health care resources, are more commonly evaluated when interpreting geographic variation (52–54). However, other factors, such as state policies to monitor psychotropic polypharmacy use, patient preferences, literacy levels, and income, may also affect variation in medication use.
This study had several limitations. First, even though we included data for Medicaid beneficiaries from 29 states, representing about 85% of Medicaid beneficiaries in fee-for-service plans, our results cannot be generalized to the remaining states, nor can they be generalized to patients with private insurance. Second, even though we required a comparably large overlap to define concomitant use (>45 days), some youths may have had longer tapering periods before being switched to another medication, which would result only in short-term but not ongoing psychotropic polypharmacy. Third, because we did not account for psychotropic dosing, we could not differentiate augmentation from polypharmacy. Fourth, several clinical factors are not captured in administrative data, and some of these factors may explain scenarios in which use of psychotropic polypharmacy was appropriate; however, we did not intend to measure appropriate versus inappropriate psychotropic polypharmacy. On the basis of these results, future research is warranted to stratify policy- and clinically relevant subgroups (race-ethnicity, socioeconomic status, and mental disorder spectrum) to confirm whether our findings hold across substrata and to draw inferences to support tailored interventions.
Even though the scope and length of this work is notable, the descriptive design warrants additional analyses to confirm some of the findings. To begin with, our results should be confirmed by studies calculating the bivariate and multivariable associations between psychotropic polypharmacy and patient demographic factors, medication type, prescribing adjustments, event year, and geographic regions. For example, the effects of changing demographic patterns (higher rates of Medicaid enrollees below the poverty line and increased proportion of youths from racial and ethnic minority groups in the last year of the study) on psychotropic polypharmacy rates need to be quantified. In addition, we need to measure the length of psychotropic polypharmacy use and evaluate determinants of persistence. A cohort design with longer follow-up time would help identify the most common prescribing practices, which may become relevant for informing clinical practice. Finally, analysis of the complexity surrounding psychotropic polypharmacy use (multimorbidity, time-varying treatment patterns, and idiosyncratic preferences of physicians across regions) would benefit from a methodologic framework, such as structural equation modeling, which is capable of estimating the effect of both latent and observed variables within one and the same (causal) model.
Conclusions
Between one-fifth and one-quarter of youths who were treated with psychotropics received psychotropic polypharmacy between 1999 and 2010. The overall increase in the prevalence of any-class psychotropic polypharmacy was 6.1% across the study period. Even though antipsychotic polypharmacy evolved to the most common same-class psychotropic polypharmacy by the end of the study period, the steepest temporal growth was for CNS stimulants and alpha-agonists. Pronounced variation in psychotropic polypharmacy across states emphasizes the need for further research on determinants of psychotropic polypharmacy as well as the safety and effectiveness of such combinations to enhance evidence-based treatment.
1 Psychotropic Medication Use Among Adolescents: United States, 2005–2010. Atlanta, Centers for Disease Control and Prevention, Dec 2013. www.cdc.gov/nchs/data/databriefs/db135.htmGoogle Scholar
2 : Psychotropic medication use among Medicaid-enrolled children with autism spectrum disorders. Pediatrics 121:e441–e448, 2008Crossref, Medline, Google Scholar
3 : Psychotropic drug prescriptions by medical specialty. Psychiatric Services 60:1167, 2009Link, Google Scholar
4 : National trends in psychotropic medication polypharmacy in office-based psychiatry. Archives of General Psychiatry 67:26–36, 2010Crossref, Medline, Google Scholar
5 : Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA 279:526–531, 1998Crossref, Medline, Google Scholar
6 : The definition and prevalence of pediatric psychotropic polypharmacy. Psychiatric Services 62:1450–1455, 2011Link, Google Scholar
7 : Psychotropic drug prescription in a psychiatric university hospital in Germany. Progress in Neuro-Psychopharmacology and Biological Psychiatry 30:1109–1116, 2006Crossref, Medline, Google Scholar
8 : Research on psychotropic drug use: a review of findings and methods. Social Science and Medicine 16:1179–1196, 1982Crossref, Medline, Google Scholar
9 Rittmannsberger H: The use of drug monotherapy in psychiatric inpatient treatment. Progress in Neuropsychopharmacology and Biological Psychiatry 26:547–551, 2002Google Scholar
10 : Antipsychotic polypharmacy in the treatment of children and adolescents in the fee-for-service component of a large state Medicaid program. Clinical Therapeutics 32:949–959, 2010Crossref, Medline, Google Scholar
11 Spencer D, Marshall J, Post B, et al: Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics 132:833–840, 2013Google Scholar
12 : National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996–2007. Journal of the American Academy of Child and Adolescent Psychiatry 49:1001–1010, 2010Crossref, Medline, Google Scholar
13 Kingsbury S, Lotito M: Psychiatric polypharmacy: the good, the bad, and the ugly: page 3 of 3. Psychiatric Times, April 1, 2007. www.psychiatrictimes.com/bipolar-disorder/psychiatric-polypharmacy-good-bad-and-ugly/page/0/2Google Scholar
14 : A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation. Current Medicinal Chemistry 11:313–327, 2004Crossref, Medline, Google Scholar
15 : Pediatric psychotropic polypharmacy. Psychiatry 2:14–19, 2005Medline, Google Scholar
16 : Variability in prescription drug utilization: issues for research. Canadian Medical Association Journal 154:635–640, 1996Google Scholar
17 : Psychotropic drug utilization in children with concurrent attention-deficit/hyperactivity disorder and anxiety. Journal of Anxiety Disorders 28:530–536, 2014Crossref, Medline, Google Scholar
18 Assessing the Usability of Encounter Data for Enrollees in Comprehensive Managed Care Across MAX 2007–2009. Brief 15. Washington, Mathematica Policy Research, Dec 2012. www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/Downloads/MAX_IB_15_AssessingUsability.pdfGoogle Scholar
19 Psychiatric Polypharmacy. Alexandria, National Association of State Mental Health Program Directors, Oct 2001. www.nasmhpd.org/content/psychiatric-polypharmacyGoogle Scholar
20 : Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics 132:833–840, 2013Crossref, Medline, Google Scholar
21 : Psychotropic medication patterns among youth in foster care. Pediatrics 121:e157–e163, 2008Crossref, Medline, Google Scholar
22 : Use of multiple psychotropic medications among adolescents aging out of foster care. Psychiatric Services 59:1052–1055, 2008Link, Google Scholar
23 : Prevalence, trends, and factors associated with antipsychotic polypharmacy among Medicaid-eligible schizophrenia patients, 1998–2000. Journal of Clinical Psychiatry 65:1377–1388, 2004Crossref, Medline, Google Scholar
24 : Combination antipsychotic therapy in clinical practice. Psychiatric Services 54:55–59, 2003Link, Google Scholar
25 : Long-term antipsychotic polypharmacy in the VA health system: patient characteristics and treatment patterns. Psychiatric Services 58:489–495, 2007Link, Google Scholar
26 : Polypharmacy in children and adolescents treated for major depressive disorder: a claims database study. Journal of Clinical Psychiatry 70:240–246, 2009Crossref, Medline, Google Scholar
27 : Concomitant pharmacotherapy among youths treated in routine psychiatric practice. Journal of Child and Adolescent Psychopharmacology 15:12–25, 2005Crossref, Medline, Google Scholar
28 : Suicidality in pediatric patients treated with antidepressant drugs. Archives of General Psychiatry 63:332–339, 2006Crossref, Medline, Google Scholar
29 : Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. American Journal of Psychiatry 164:884–891, 2007Link, Google Scholar
30 : Receipt of evidence-based pharmacotherapy and psychotherapy among children and adolescents with new diagnoses of depression. Psychiatric Services 67:316–323, 2016Link, Google Scholar
31 : The metabolic syndrome and antipsychotics in children and adolescents [in Hebrew]. Harefuah 150:791–796, 2011Medline, Google Scholar
32 : Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophrenia Research 138:18–28, 2012Crossref, Medline, Google Scholar
33 : Second-generation antipsychotic medication combinations for schizophrenia. Psychiatric Services 59:235, 2008Link, Google Scholar
34 : National patterns in antidepressant medication treatment. Archives of General Psychiatry 66:848–856, 2009Crossref, Medline, Google Scholar
35 : Pediatric psychopharmacology for treatment of ADHD, depression, and anxiety. Pediatrics 136:351–359, 2015Crossref, Medline, Google Scholar
36 : Medication use and spending trends among children with ADHD in Florida’s Medicaid program, 1996–2005. Psychiatric Services 63:115–121, 2012Link, Google Scholar
37 : The increasing use of polypharmacotherapy for refractory mood disorders: 22 years of study. Journal of Clinical Psychiatry 61:9–15, 2000Crossref, Medline, Google Scholar
38 : Mental disorders in primary care. Dialogues in Clinical Neuroscience 5:115–128, 2003Medline, Google Scholar
39 : Inappropriate prescribing. Monitor on Psychology, June 2012. www.apa.org/monitor/2012/06/prescribing.aspxGoogle Scholar
40 : A decade of direct-to-consumer advertising of prescription drugs. New England Journal of Medicine 357:673–681, 2007Crossref, Medline, Google Scholar
41 Is the drug industry developing cures or hyping up demand? New York Times. Dec 15, 2013. http://www.nytimes.com/roomfordebate/2013/12/15/is-the-drug-industry-developing-cures-or-hyping-up-demand/consumer-drug-advertising-should-be-bannedGoogle Scholar
42 Kelley T: The big bucks in keeping kids focused. Bloomberg Businessweek, Oct 14, 2013. www.bloomberg.com/news/articles/2013-10-10/shires-adhd-drugs-face-resistance-in-a-skeptical-europeGoogle Scholar
43 : A meta-analysis of psychotherapy and medication in unipolar depression and dysthymia. Journal of Affective Disorders 110:197–206, 2008Crossref, Medline, Google Scholar
44 : Receipt of psychotherapy by adolescents taking antidepressants. Psychiatric Services 59:963, 2008Link, Google Scholar
45 : Prevalence of psychotherapy surrounding initiation of psychotropic polypharmacy in the Medicaid-insured population, 1999–2010. Psychiatric Services 68:1120–1126, 2017Link, Google Scholar
46 : Third-generation antidepressants: do they offer advantages over the SSRIs? CNS Drugs 15:941–954, 2001Crossref, Medline, Google Scholar
47 : Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depression and Anxiety 6:10–18, 1997Crossref, Medline, Google Scholar
48 : Are the SSRIs and atypical antidepressants safe and effective for children and adolescents? Current Opinion in Psychiatry 18:21–25, 2005Medline, Google Scholar
49 : Augmentation treatment in major depressive disorder: focus on aripiprazole. Neuropsychiatric Disease and Treatment 4:937–948, 2008Medline, Google Scholar
50 Augmentation strategies in resistant depression: some are effective and well tolerated. Drug and Therapy Perspectives 17:6–9, 2001Crossref, Google Scholar
51 : The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews 32:1–19, 2008Crossref, Medline, Google Scholar
52 Geographic Variation in Health Care Spending. Washington, Congress of the United States, Congressional Budget Office, Feb 2008. www.cbo.gov/sites/default/files/cbofiles/ftpdocs/89xx/doc8972/02-15-geoghealth.pdfGoogle Scholar
53 : Geographic variation in the prevalence of stimulant medication use among children 5 to 14 years old: results from a commercially insured US sample. Pediatrics 111:237–243, 2003Crossref, Medline, Google Scholar
54 : Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspectives in Biology and Medicine 46:69–79, 2003Crossref, Medline, Google Scholar



