Nutrition and Exercise for Wellness and Recovery: A Randomized Controlled Trial of a Community-Based Health Intervention
Abstract
Objective:
The purpose of this study was to examine the efficacy of the Nutrition and Exercise for Wellness and Recovery (NEW-R) intervention for improving competency and behaviors related to diet, physical activity, and weight management.
Methods:
Participants with psychiatric disabilities were recruited from four community mental health agencies and a hospital-based psychiatric outpatient clinic and randomly assigned to the NEW-R intervention (N=55) or control condition (N=58). Outcome measures included the Perceived Competence Scale, Health-Promoting Lifestyle Profile (HPLP), and weight change; random-effects regression models were used. A follow-up analysis examined the interactions of group, time, and site.
Results:
Fifty of the 55 intervention participants and 57 of the 58 control participants completed the study. The two groups did not differ significantly on any measured baseline characteristic. The intervention group had statistically significant improvements, compared with the control group, in perceived competence for exercise and healthy eating, total HPLP score, and scores on two HPLP subscales (nutrition and spiritual growth). No significant difference between groups was found for weight loss. A study condition × time × site effect was observed: at the three sites where mean weight loss occurred, NEW-R participants lost significantly more weight than did control participants.
Conclusions:
NEW-R offers promise as an intervention that can initiate the change to healthy lifestyle behaviors and boost perceived competence in a healthy lifestyle. It may also be effective for weight loss when administered in supportive settings.
HIGHLIGHTS
The Nutrition and Exercise for Wellness and Recovery (NEW-R) intervention was designed as an 8-week peer-led intervention that can be easily implemented in community-based mental health settings.
Participants in the NEW-R intervention improved nutrition and spirituality behaviors and perceptions of competence regarding a healthy lifestyle, compared with the control group.
Although NEW-R participants did not lose more weight than control participants overall, they had a statistically significant mean weight loss of 5.7 pounds between baseline and 8-month follow-up at three of the five sites (responder sites), versus a weight gain of 7.7 pounds at the two other sites (nonresponder sites).
The prevalence of obesity in the United States is 42.4% (1). Adults with psychiatric disabilities are disproportionately vulnerable to obesity (2, 3) because of factors such as poverty (4), low physical activity (5, 6), high volume of food intake (7), low fruit and vegetable intake (6), and side effects of psychotropic medications (8, 9). The primary health risks of obesity include premature death, type 2 diabetes, heart disease, stroke, hypertension, hyperlipidemia (1, 10), and poor COVID-19 outcomes (11, 12). Being overweight or obese is often associated with psychological issues, including increased internal stigma, reduced self-confidence, and persistent social isolation (13). Moreover, research shows that the job discrimination and wage disparities (14, 15) experienced by obese individuals are compounded by co-occurring mental illness (16). Together, obesity and psychiatric disability inhibit meaningful, productive community participation beyond the effects of either condition alone.
The term “obesogenic environment” denotes an environment that both promotes weight gain and interferes with weight-loss efforts (17). The ecological model of obesity proposes that people in obesogenic environments struggle against a culture that promotes consumption of high-fat and sugar-laden foods and encourages sedentary behaviors. Although the average American lives in an obesogenic environment behaviorally determined by poor diet and lack of exercise, many people with psychiatric disabilities live in obesogenic environments that are both behaviorally and pharmacologically determined (18), given their high use of psychotropic medications that are associated with weight gain (19).
These concerns have led to the development of weight-management interventions for people with psychiatric disabilities. Research reviewed by Naslund et al. (20) identified 17 randomized controlled trials comparing weight-loss interventions with control conditions or usual care. The interventions were effective for modest weight reduction, with programs of longer duration achieving more consistent results. Despite these promising findings, implementation of complex, long-term interventions can be difficult for many community-based mental health programs with limited financial resources and multiple demands on staff time.
Our primary study aim was to test the effectiveness of Nutrition and Exercise for Wellness and Recovery (NEW-R), an 8-week manualized intervention using a strengths-based approach intended to circumvent the impact of obesogenic environments. We hypothesized that compared with persons in the control group, NEW-R participants would experience greater improvements in competence for healthy eating, exercise, and weight management; the ability to engage in health-promoting behaviors and practices; and weight loss.
Methods
Study Procedures
Study participants were recruited, by university investigators, from four community mental health agencies and a hospital-based psychiatric outpatient clinic in Chicago and the surrounding suburbs. Six separate waves of study recruitment, followed by delivery of NEW-R classes, began in October 2016 and ended in March 2019 (two waves occurred at one site). Inclusion criteria were assessed via self-report and program records. If criteria were met, written informed consent was obtained by using human subjects procedures approved by the institutional review board of the University of Illinois at Chicago. Randomization occurred at each site and was performed by interviewers at the conclusion of baseline interviews by using computer-generated allocation sequences that concealed study condition until assignment (21). Information regarding participant characteristics and outcomes was obtained during in-person interviews at baseline, postintervention (2 months postbaseline), and follow-up (8 months postbaseline) by researchers blind to study condition.
Inclusion and Exclusion Criteria
Participants met all six inclusion criteria: psychiatric disability status, defined in the Alcohol, Drug Abuse, and Mental Health Administration Reorganization Act of 1992 (22) as a documented DSM diagnosis (23) accompanied by severe functional impairment (24); enrolled in outpatient mental health services; overweight or obese, with a body mass index (BMI) ≥25 kg/m2; age 18 or older; ability to understand spoken English; and willingness to provide informed written consent. Exclusion criteria mirrored those used in similar studies and included residence in a nursing home, a terminal illness expected to result in death within 1 year, pregnancy, history of an eating disorder, history of heart disease, diagnosis of dementia, or cognitive impairment severe enough to prohibit informed consent.
Study Enrollment and Retention
A total of 121 individuals attended study recruitment sessions, and 113 individuals consented to participate in the study. Of these, all 113 had a baseline interview and were randomly assigned to the experimental condition (N=55) or control condition (N=58) (a CONSORT diagram is available in the online supplement to this article). Of the 113 persons interviewed, 95% (N=107: NEW-R, N=50; control, N=57) had one or more follow-up interviews, and follow-up proportions did not differ significantly by study condition.
Intervention
NEW-R was designed as an 8-week program to make implementation feasible in community mental health settings, informed by a 12-month weight-loss intervention for this population called RENEW (25, 26). The model’s foundations include self-determination theory (27), psychiatric rehabilitation theory (28), and the ecological model of obesity (17). Eight experts in weight-loss and wellness programming for people with psychiatric disabilities helped identify salient content for the shorter program. NEW-R applies psychiatric rehabilitation principles, including instruction that compensates for cognitive impairments that co-occur with some types of mental illnesses, skill building and transfer of training to new environments, provision of social and instrumental support, and goal setting. In addition, the focus on intentionality for lifestyle changes promoting weight management was designed to encourage participants to enter more intensive weight-loss programs if desired.
Each of the eight 90-minute closed NEW-R sessions starts with a weigh-in, followed by didactic presentation of session content and activities to personalize and apply the material, individualized goal setting for healthier eating or physical activity, and 20 minutes of exercise. The first session presents an overview of the intervention, the effects of obesity on general medical and mental health, and the value of making a weekly NEW-R plan. The second and third sessions provide a comprehensive overview of healthy eating. The fourth session covers the benefits of exercise and strategies for increasing physical activity. The fifth and sixth sessions focus on healthy eating in restaurants, meal preparation at home with recipes, and food shopping on a limited budget. Session 7 introduces participants to cooking tools and techniques to reduce carbohydrates and fat when cooking. During the final session, participants reflect on lifestyle changes that they have made to promote weight management and describe their plan for continued progress toward weight-loss goals. Certificates of completion are presented, and students enjoy a graduation celebration with healthy snacks. Throughout the program, there is an emphasis on personal growth and positive change.
Instructor training and supervision.
The same two instructors taught at all study sites: one was a master’s-level graduate student, and the other had completed a peer certification program. Training included a 1-hour webinar, followed by in-person practice of each of the eight sessions (including exercise segments) observed by study leadership, instruction in procedures for fidelity assessment and attendance tracking, and human subjects research education. Instructors were supervised by research staff at weekly meetings, held throughout the period of intervention delivery, during which they reviewed fidelity and attendance statistics, discussed content of the upcoming session, and resolved logistical issues.
Intervention fidelity.
Fidelity was monitored by researchers via completion of checklists with instructors within 48 hours of each session. Checklists tracked adherence to each session’s prescribed topics, time frames, and instructional modalities. Each curriculum component was scored as 1 if the prescribed element occurred and as 0 if it did not. Fidelity scores were computed as the proportion of prescribed elements present for that session. We also used direct observation of two randomly selected sessions to validate fidelity reporting.
Control Condition
Participants in the control condition received services as usual in the program from which they were recruited.
Measures
The primary outcome of perceived competence for healthy eating and exercise was assessed by the four-item Perceived Competence Scale (29), which uses 7-point Likert responses (range 4 to 28, with higher scores indicating greater perceived competence) to measure respondents’ degree of confidence in their ability to maintain a healthy diet and be physically active (baseline Cronbach’s α=0.79). A secondary outcome was frequency of engaging in health-promoting behaviors and practices, as assessed by three subscales of the Health-Promoting Lifestyle Profile (HPLP) (30). We included subscales measuring nutrition, spiritual growth, and physical activity, given their consistency with the intervention’s targeted behaviors and theoretical underpinnings. The spiritual growth subscale addresses behaviors such as personal growth, being open to new challenges, and setting new goals. Following the scale’s authors (30), we also computed a total score that summed responses to the 26 subscale items (range 26–104, with higher scores indicating a greater health-promoting lifestyle). HPLP total and subscale baseline Cronbach’s alphas ranged from 0.76 to 0.88. Another secondary outcome was weight change, measured in pounds with a medical-grade digital scale.
Statistical Analysis
Success of randomization was assessed with chi-square and t tests. An intention-to-treat approach was used for the main analysis of outcomes (31), in which the effect of study condition was examined by using random-effects regression models with random intercepts and a compound symmetry covariance structure for repeated measures. The study’s randomized block design was addressed by inclusion of site as a fixed effect (32, 33). Each model included random intercept, study condition, time, and site. The longitudinal intervention effects were modeled with a study condition × time interaction term. To explore site variation found in earlier work (25, 26), we used a separate model with a three-way study condition × time × site interaction. No additional covariates were included in the models, given the statistical equivalence of study conditions in terms of baseline characteristics (described below). Data were analyzed in SAS, version 9.4, and SPSS, version 25.
Results
Intervention Fidelity and Attendance
Intervention fidelity across all classes in the six waves of intervention delivery ranged from a low of 97% to a high of 100%. Program fidelity across all program sites, course sessions, and waves of classes was 98%. On average, participants attended 6.7±2.8 of eight classes, and 84% (N=46) completed at least seven of the eight classes. There were no significant differences in class attendance by study site.
Participant Baseline Characteristics and Equivalence of Study Conditions
Table 1 presents characteristics of the study population by randomly assigned study condition. Study conditions did not differ significantly on any measured characteristic.
| Total sample (N=113) | NEW-R (N=55) | Control (N=58) | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % |
| Gender | ||||||
| Male | 37 | 33 | 15 | 27 | 22 | 38 |
| Female | 73 | 65 | 39 | 71 | 34 | 59 |
| Transgender | 3 | 3 | 1 | 2 | 2 | 3 |
| Race and ethnicityb | ||||||
| Black, African American | 86 | 76 | 42 | 76 | 44 | 76 |
| White | 24 | 21 | 14 | 26 | 10 | 17 |
| Asian | 2 | 2 | 0 | — | 2 | 3 |
| American Indian | 8 | 7 | 4 | 7 | 4 | 7 |
| Native Hawaiian, Pacific Islander | 1 | 1 | 1 | 2 | 0 | — |
| Alaska Native | 1 | 1 | 0 | — | 1 | 2 |
| Latinx | 9 | 8 | 2 | 4 | 7 | 12 |
| Age (M±SD) | 47.1±12.4 | 48.0±12.6 | 46.2±12.1 | |||
| Educationc | ||||||
| Less than high school | 28 | 25 | 17 | 31 | 11 | 19 |
| High school graduate | 31 | 28 | 15 | 27 | 16 | 28 |
| Some college | 33 | 30 | 12 | 22 | 21 | 37 |
| College graduate | 20 | 18 | 11 | 20 | 9 | 16 |
| Employed | 9 | 8 | 4 | 7 | 5 | 9 |
| Marital status | ||||||
| Never married | 82 | 73 | 41 | 75 | 41 | 71 |
| Married or cohabiting | 4 | 4 | 1 | 2 | 3 | 5 |
| Divorced | 20 | 18 | 10 | 18 | 10 | 17 |
| Separated | 3 | 3 | 1 | 2 | 2 | 3 |
| Widowed | 4 | 4 | 2 | 4 | 2 | 3 |
| Living situationd | ||||||
| Own house or apartment | 83 | 75 | 44 | 82 | 39 | 68 |
| Home of friend or relative | 23 | 21 | 8 | 15 | 15 | 26 |
| Agency housing | 4 | 4 | 1 | 2 | 3 | 5 |
| Shelter or street | 1 | 1 | 1 | 2 | 0 | — |
| Diagnosisb | ||||||
| Schizophrenia or schizoaffective disorder | 57 | 50 | 26 | 47 | 31 | 53 |
| Depressive disorder | 43 | 38 | 20 | 36 | 23 | 40 |
| Bipolar disorder | 38 | 34 | 20 | 36 | 18 | 31 |
| Anxiety disorder | 26 | 23 | 12 | 22 | 14 | 24 |
| Self-reported history of difficulties with alcohol, recreational drugs, or misusing prescribed medications | 32 | 28 | 16 | 29 | 16 | 28 |
| Comorbid medical disordere | 99 | 88 | 47 | 86 | 52 | 90 |
| Current cigarette use | 30 | 27 | 15 | 27 | 15 | 26 |
| Currently taking a medication with risk of weight gain | 20 | 18 | 8 | 15 | 12 | 21 |
| Household annual incomef | ||||||
| <$10,000 | 61 | 56 | 30 | 57 | 31 | 54 |
| $10,000–$19,999 | 29 | 26 | 15 | 28 | 14 | 25 |
| ≥$20,000 | 20 | 18 | 8 | 15 | 12 | 21 |
| Insuranceb | ||||||
| Medicaid | 90 | 80 | 41 | 75 | 49 | 85 |
| Medicare | 46 | 41 | 20 | 36 | 26 | 45 |
| Private | 6 | 5 | 4 | 7 | 2 | 3 |
| Body mass index (kg/m2) | ||||||
| Overweight (25–29) | 22 | 19 | 8 | 15 | 14 | 24 |
| Obese (30–39) | 55 | 49 | 25 | 45 | 30 | 52 |
| Severely obese (≥40) | 36 | 32 | 22 | 40 | 14 | 24 |
| Perceived Competence Scale score (M±SD)g | 18.2±5.6 | 17.8±5.6 | 18.7±5.6 | |||
| Health-Promoting Lifestyle Profile total scale (M±SD)h | 64.3±11.6 | 63.8±12.0 | 64.8±11.4 | |||
| Nutrition subscalei | 22.3±4.9 | 22.2±5.1 | 22.4±4.7 | |||
| Spiritual growth subscalei | 26.3±5.4 | 25.7±5.6 | 26.8±5.2 | |||
| Physical activity subscalej | 15.7±4.7 | 15.8±4.7 | 15.5±4.7 | |||
| Weight in pounds (M±SD) | 232.2±46.5 | 239.1±52.7 | 225.6±39.2 | |||
TABLE 1. Baseline demographic and clinical characteristics of participants in the Nutrition and Exercise for Wellness and Recovery (NEW-R) intervention and in the control conditiona
Primary and Secondary Outcomes for the Intervention Versus Control Condition
The results of random-effects regression analyses (Table 2) revealed a significant and positive intervention × time interaction in which scores on the Perceived Competence Scale for healthy diet and exercise increased from baseline to follow-up in the intervention group, compared with the control group. A significant increase over time was also noted in the intervention group, compared with the control group, for total HPLP scale score and for scores on the HPLP nutrition and spiritual growth subscales.
| Outcome | Parameter estimate | SE | p |
|---|---|---|---|
| Perceived Competence Scale (assessing exercise and healthy diet) | |||
| Intercept | 24.44 | 3.61 | <.001 |
| Intervention | −.69 | 1.09 | .527 |
| Time | −.53 | .37 | .155 |
| Intervention × time | 1.24 | .54 | .024 |
| Health-Promoting Lifestyle Profile (HPLP) total scale | |||
| Intercept | 74.22 | 15.46 | <.001 |
| Intervention | −.66 | 2.38 | .781 |
| Time | −1.15 | .87 | .185 |
| Intervention × time | 2.62 | 1.27 | .040 |
| HPLP nutrition subscale | |||
| Intercept | 22.63 | 1.01 | <.001 |
| Intervention | .06 | .90 | .950 |
| Time | −.40 | .26 | .130 |
| Intervention × time | .79 | .38 | .041 |
| HPLP spiritual growth subscale | |||
| Intercept | 26.63 | 1.19 | <.001 |
| Intervention | −.74 | .98 | .450 |
| Time | −.42 | .27 | .127 |
| Intervention × time | .95 | .40 | .019 |
| HPLP physical activity subscale | |||
| Intercept | 15.42 | .60 | <.001 |
| Intervention | .66 | .86 | .446 |
| Time | .30 | .27 | .280 |
| Intervention × time | −.05 | .40 | .907 |
| Weight in pounds | |||
| Intercept | 173.24 | 32.88 | <.001 |
| Intervention | 14.30 | 8.88 | .110 |
| Time | 1.89 | .87 | .032 |
| Intervention × time | −1.96 | 1.28 | .128 |
| Weight in pounds at three responder sites onlyb | |||
| Intercept | 250.89 | 19.58 | <.001 |
| Intervention | 9.29 | 12.51 | .461 |
| Time | 1.58 | 1.15 | .173 |
| Intervention × time | −4.33 | 1.66 | .010 |
TABLE 2. Random-effects regression models of the effect of the Nutrition and Exercise for Wellness and Recovery intervention versus the control condition over time, by outcome (N=113)a
Between baseline and final assessment, among those with available weight measurements at final assessment, 46% (N=17) of 37 intervention participants lost weight, compared with 29% (N=13) of 45 control participants, a statistically nonsignificant difference. Among those who lost weight, participants in the intervention group lost a mean±SD of 12.29±13.22 pounds and those in the control group lost 12.51±7.49 pounds, a statistically nonsignificant difference. In the random-effects regression analysis, the effect of intervention on weight loss was not significant over time.
Site Differences in Weight Change
Investigation of the study condition × time × site effect found a significant three-way interaction associated with weight management. Figure 1 displays different patterns of weight change over time among NEW-R participants at the five study sites, with three “responder” sites demonstrating weight loss at postintervention, follow-up, and overall (sites 2, 3, and 5), and two “nonresponder” sites demonstrating weight gain at one or both postbaseline time points and overall (sites 1 and 4). Table 3 shows differences in weight change over time by study condition and by site over time within the intervention condition. Across sites, the intervention group lost 1.8 pounds during the 8-week course and gained 1.7 pounds in the 6 months after the intervention (follow-up at 8 months postbaseline), for an overall negligible net change of −0.1 pounds from baseline to follow-up. In contrast, the control group lost 0.7 pounds during the 8 weeks following baseline and gained 5.6 pounds during the 8-month study period, for an overall gain of 4.9 pounds. We repeated the outcome analysis by looking at only the three responder sites and found a significant decrease in weight over time in the intervention condition, compared with the control condition, as shown in Table 2
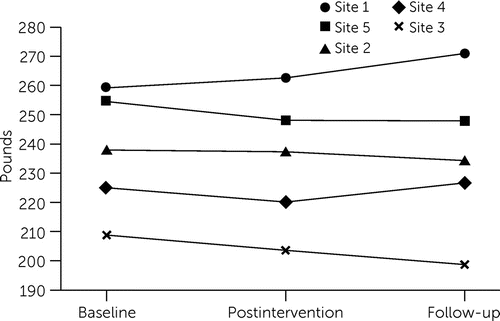
FIGURE 1. Patterns of weight change at baseline and at 8 weeks and 8 months postbaseline among participants in the Nutrition and Exercise for Wellness and Recovery intervention at the five study sites
| Condition and site | Weight at baseline | Weight at postintervention | Change from baseline to postintervention | Weight at follow-up | Change from postintervention to follow-up | Change from baseline to follow-up | |||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||||
| NEW-R | 239.1 | 52.7 | 237.3 | 56.5 | −1.8 | 239.0 | 59.1 | +1.7 | −.1 |
| Control | 225.6 | 39.2 | 224.9 | 40.0 | −.7 | 230.5 | 40.1 | +5.6 | +4.9 |
| NEW-R only | |||||||||
| Site 1 (outpatient clinic) | 259.2 | 45.1 | 262.6 | 49.0 | +3.4 | 271.0 | 48.9 | +8.4 | +11.8 |
| Site 2 (agency) | 237.9 | 67.7 | 237.3 | 73.6 | −.6 | 234.3 | 77.0 | −3.0 | −3.6 |
| Site 3 (agency) | 208.8 | 46.1 | 203.6 | 49.8 | −5.2 | 198.7 | 42.6 | −4.9 | −10.1 |
| Site 4 (agency) | 225.0 | 47.9 | 220.1 | 49.6 | −4.9 | 226.7 | 57.4 | +6.6 | +1.7 |
| Site 5 (agency) | 254.5 | 47.7 | 248.1 | 46.9 | −6.4 | 247.9 | 50.0 | −.2 | −6.6 |
TABLE 3. Changes in weight at postintervention (8 weeks postbaseline) and follow-up (8 months postbaseline) among participants in the Nutrition and Exercise for Wellness and Recovery (NEW-R) intervention and in the control condition (total N=113)a
This pattern of responder and nonresponder sites is in keeping with previous studies of weight management (26, 34). Figure 2 shows the different patterns of weight change in the intervention condition at the responder and nonresponder sites. Together, NEW-R participants at responder sites experienced a mean±SD loss of 5.7±11.0 pounds between baseline and 8-month follow-up, and those at nonresponder sites experienced a mean gain of 7.7±16.9 pounds in the same period. Statistical analysis revealed no significant differences between responder and nonresponder sites in baseline weight or in demographic or clinical characteristics that might account for the differing patterns.
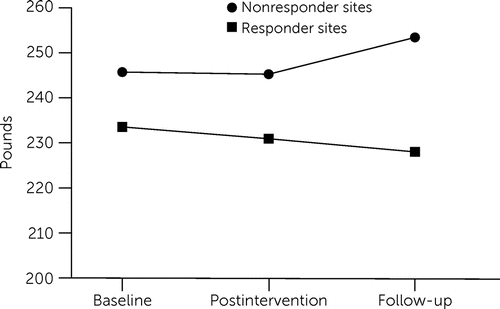
FIGURE 2. Patterns of weight change among participants in the Nutrition and Exercise for Wellness and Recovery intervention at responder and nonresponder sitesa
aThree sites (2, 3, and 5) where weight loss was demonstrated at postintervention, follow-up, and overall were labeled responder sites. Two sites (1 and 4) where weight gain was demonstrated at one or both postbaseline time points and overall were labeled nonresponder sites.
BMI
At baseline, 19% of the total group were overweight, 49% were obese, and 32% were severely obese (Table 1). An exploratory longitudinal multivariable analysis of change in BMI (not shown) that used the standard categories (normal, overweight, obese, and severely obese) found a significant study condition × time interaction in which BMI declined to a greater extent among intervention versus control participants (standardized β=−0.08, p=0.04). However, analysis of BMI as a continuous variable revealed no significant differences between study conditions.
Discussion
Our results indicate NEW-R’s effectiveness in increasing perceived competence for exercise and healthy eating. Perceived competence, also known as self-efficacy (35), includes a person’s belief in her or his ability to make the behavioral changes needed to meet life goals, including those related to diet, exercise, and weight loss (36). In addition, we found improvements in health-promoting lifestyle activities, including nutrition behaviors and health-related spirituality practices, among those receiving the intervention, indicating that participants were implementing behavioral changes. These results are consistent with NEW-R’s use of an intentional approach to slowly shift a person’s behaviors to health-promoting behaviors.
One of the NEW-R instructors was a peer, and this may have enhanced the intervention’s efficacy, given evidence that peer education and support have been shown to promote health behavior change among people with serious mental illness and various co-occurring conditions (37–40). Peer instructors who have adopted lifestyles promoting successful weight management may inspire participants to embrace healthy eating, increase physical activity, and develop other weight-management skills (41–43). The importance of generating optimism and maintaining motivation regarding weight management may be especially relevant to participants who deal with psychiatric medication–induced obesity and the compounded effects of both weight and mental illness stigma (44, 45).
Another important feature of NEW-R is its emphasis on gradual change. This emphasis is supported by evidence that gradual versus rapid weight loss has benefits for preserving resting metabolic rates (46) and preventing rapid weight regain (47). In fact, some experts advocate for a weight-neutral approach that encourages health-promoting behaviors, such as physical activity and healthy eating, in the absence of weight-loss goals (48).
Nevertheless, the nonsignificant finding regarding weight loss for NEW-R participants was disappointing. One explanation is that contamination may have occurred if NEW-R participants shared information from the intervention with control participants. This was not reported by any control participant at the time of the final assessment, and we did not recruit study participants from the same family or household (including group residences) in an effort to avoid this kind of contamination. Another possibility is that a more intensive, longer-term intervention may be necessary to achieve significant weight loss. For example, in a large randomized controlled trial of an 18-month intervention, Daumit et al. (49) found increasing amounts of weight loss over the study period, with the intervention group losing 7 more pounds than the control group. On the other hand, another long-term (12 months) study of 428 participants found that lifestyle coaching was not effective in promoting weight loss or reducing cardiovascular disease risk (50).
Nevertheless, 46% of NEW-R participants lost weight, compared with 29% of control participants. Another explanation lies in potential differences between study sites. Three of the five sites in this study were characterized as responder sites, because at these sites, NEW-R participants lost a mean total of 5.7 pounds from baseline to follow-up, and NEW-R participants lost significantly more weight than control participants. All three responder sites offered comprehensive psychosocial rehabilitation services, including health-and-wellness support, residential services, employment assistance, medication management, peer support, and case management. At the two nonresponder sites, NEW-R participants gained 7.7 pounds from baseline to follow-up, and no significant differences in weight change were noted between NEW-R participants and control participants. Services at these two sites were more limited, consisting primarily of psychiatric medication management, traditional psychotherapy, and supportive counseling. It is possible that weight-management interventions such as NEW-R are more effective when participants are receiving other services that encourage physical activity, healthy eating, and active community participation in work, school, and leisure pursuits.
Our finding of site variation in weight change is comparable to the site differences identified in research on a precursor to the NEW-R intervention (26). Another study of weight loss in this population also found site differences and attributed them to varying organizational contexts in which the intervention was implemented (34). Authors of a study of a nurse-led weight-management intervention for people with schizophrenia noted that site characteristics, such as having a gym on the premises or being located next to parks and other recreation areas, were a likely source of variation (51). If environmental factors contribute to the impact of weight-loss interventions, then it may be important to address those factors before implementing new interventions. NEW-R may work best in settings already attuned to the importance of reducing the obesogenic environment, where the intervention can “jump start” weight-management lifestyle changes for people who receive continued support from wellness programming.
A large systematic review of behavioral weight-loss programs found better weight-loss outcomes with continued availability of services after the study intervention ended (52). Additional environmental supports may come from so-called nudge strategies, in which a modification to the environment allows for choice but influences people to act in a predictable way (53). Nudge strategies for weight loss include listing calorie amounts on menus, facilitating interaction with peers who model exercise and good nutrition, making healthy foods conveniently available, and introducing environmental cues, such as signs encouraging people to use the stairs. A meta-analysis revealed significant (though small) weight loss in studies examining a variety of nudge approaches (54).
Limitations of this study include the fact that all participants resided in the Chicago area, reducing the generalizability of our findings. The measures of perceived competence and lifestyle behaviors were based on self-report and not verified with more objective measures, such as observation. The lack of an active comparator condition was another limitation of the study. In addition, we did not collect data about specific services received by study participants that may have affected study outcomes, such as nutritional support, exercise classes, and other wellness programming. This information might have helped to elucidate the site differences. The exclusion of participants with a history of heart disease may have prevented these individuals from benefiting from the intervention. Finally, NEW-R’s 90-minute session length may not match community programs’ 60-minute block schedules, although length can be shortened by moving material into additional sessions.
Conclusions
Significant health and mortality disparities continue to exist for people with psychiatric disabilities, and these differences have become even more pronounced during the COVID-19 pandemic (55, 56). NEW-R was designed as a practical intervention to promote healthy lifestyle behaviors related to diet, physical activity, and weight management. NEW-R offers promise as an intervention that can initiate the change to healthy lifestyle behaviors and boost perceived competence in maintaining these behaviors. The program may also be effective for weight loss when administered in a supportive setting. Additional research on environmental prerequisites that support NEW-R’s success is warranted.
1. Adult Obesity Facts. Atlanta, Centers for Disease Control and Prevention, 2021. https://www.cdc.gov/obesity/data/adult.html. Accessed Oct 1, 2021Google Scholar
2. : Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry 2003; 64:1426–1435Crossref, Medline, Google Scholar
3. : Obesity in adults with serious and persistent mental illness: a review of postulated mechanisms and current interventions. Ann Clin Psychiatry 2011; 23:131–140Medline, Google Scholar
4. : Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry 2006; 14:212–222Crossref, Medline, Google Scholar
5. : Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis 2005; 193:641–646Crossref, Medline, Google Scholar
6. : Dietary intake, physical activity and sedentary behaviour patterns in a sample with established psychosis and associations with mental health symptomatology. Psychol Med (Epub Aug 23, 2021). doi:
7. : Dietary intake of people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry 2019; 214:251–259Crossref, Medline, Google Scholar
8. : Second-generation antipsychotics and metabolic side effects: a systematic review of population-based studies. Drug Saf 2017; 40:771–781Crossref, Medline, Google Scholar
9. : Weight gain, obesity, and psychotropic prescribing. J Obes 2011; 2011:893629Crossref, Medline, Google Scholar
10. : Medical comorbidities in 181 patients with bipolar disorder vs schizophrenia and related psychotic disorders: findings from a single-center, retrospective study from an acute inpatients psychiatric unit. Front Psychiatry 2021; 12:702789Crossref, Medline, Google Scholar
11. : Body mass index and risk for COVID-19–related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death—United States, March–December 2020. MMWR Morb Mortal Wkly Rep 2021; 70:355–361Crossref, Medline, Google Scholar
12. : COVID-19 prevalence and mortality among schizophrenia patients: a large-scale retrospective cohort study. Schizophr Bull 2021; 47:1211–1217Crossref, Medline, Google Scholar
13. : Psychosocial origins of obesity stigma: toward changing a powerful and pervasive bias. Obes Rev 2003; 4:213–227Crossref, Medline, Google Scholar
14. : The role of automatic obesity stereotypes in real hiring discrimination. J Appl Psychol 2011; 96:790–805Crossref, Medline, Google Scholar
15. : Obesity and hiring discrimination. Econ Hum Biol 2020; 37:100850Crossref, Medline, Google Scholar
16. : The impact of mental illness on employment: consumers’ perspectives. Work 2003; 20:267–276Medline, Google Scholar
17. : Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev Med 1999; 29:563–570Crossref, Medline, Google Scholar
18. : The challenge of obesity. Psychiatr Rehabil J 2013; 36:129–132Crossref, Medline, Google Scholar
19. : Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015; 14:119–136Crossref, Medline, Google Scholar
20. : Lifestyle interventions for weight loss among overweight and obese adults with serious mental illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2017; 47:83–102Crossref, Medline, Google Scholar
21. : Eliminating bias in randomized controlled trials: importance of allocation concealment and masking. Fam Med 2007; 39:132–137Medline, Google Scholar
22. ADAMHA Reorganization Act. PL 102–321, S 1306, 102d Cong. Washington, DC, Office of the Legislative Counsel for the US House of Representatives, 1992. https://www.govinfo.gov/content/pkg/STATUTE-106/pdf/STATUTE-106-Pg323.pdfGoogle Scholar
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA, American Psychiatric Association, 2013Google Scholar
24. : Estimation methodology for adults with serious mental illness (SMI). Fed Regist 1999; 64:33890–33897Google Scholar
25. : Weight loss intervention for people with serious mental illness: a randomized controlled trial of the RENEW program. Psychiatr Serv 2011; 62:800–802Link, Google Scholar
26. : Treatment response to the RENEW weight loss intervention in schizophrenia: impact of intervention setting. Schizophr Res 2014; 159:421–425Crossref, Medline, Google Scholar
27. : Self-determination theory; in Handbook of Theories of Social Psychology. Edited by Van Lange PAM, Kruglanski AW, Higgins ET. Newbury Park, CA, SAGE, 2012Crossref, Google Scholar
28. : Psychiatric Rehabilitation Programs: Putting Theory Into Practice. Baltimore, Johns Hopkins University Press, 1989Google Scholar
29. : Internalization of biopsychosocial values by medical students: a test of self-determination theory. J Pers Soc Psychol 1996; 70:767–779Crossref, Medline, Google Scholar
30. : The Health-Promoting Lifestyle Profile: development and psychometric characteristics. Nurs Res 1987; 36:76–81Crossref, Medline, Google Scholar
31. : Understanding the intention-to-treat principle in randomized controlled trials. West J Emerg Med 2017; 18:1075–1078Crossref, Medline, Google Scholar
32. : Fixed Effects Regression Models. Thousand Oaks, CA, SAGE, 2009Crossref, Google Scholar
33. : Should blocks be fixed or random?; in
34. : Effectiveness of a psychosocial weight management program for individuals with schizophrenia. J Behav Health Serv Res 2014; 41:370–380Crossref, Medline, Google Scholar
35. : Health promotion by social cognitive means. Health Educ Behav 2004; 31:143–164Crossref, Medline, Google Scholar
36. : The effect of self-efficacy on behavior and weight in a behavioral weight loss intervention. Health Psychol 2017; 35:714–722Crossref, Google Scholar
37. : Comparative effectiveness of peer leaders and community health workers in diabetes self-management support: results of a randomized controlled trial. Diabetes Care 2014; 37:1525–1534Crossref, Medline, Google Scholar
38. : Peer-led self-management interventions and adherence to antiretroviral therapy among people living with HIV: a systematic review. AIDS Behav 2020; 24:998–1022Crossref, Medline, Google Scholar
39. : Self-management and peer support among people with arthritis on a hospital joint replacement waiting list: a randomised controlled trial. Osteoarthritis Cartilage 2009; 17:1428–1433Crossref, Medline, Google Scholar
40. : Randomized trial of a peer-led, telephone-based empowerment intervention for persons with chronic spinal cord injury improves health self-management. Arch Phys Med Rehabil 2017; 98:1067–1076.e1Crossref, Medline, Google Scholar
41. : Feasibility of behavioral weight loss treatment enhanced with peer support and mobile health technology for individuals with serious mental illness. Psychiatr Q 2016; 87:401–415Crossref, Medline, Google Scholar
42. : Peer support groups for weight loss. Curr Cardiovasc Risk Rep 2020; 14:19Crossref, Google Scholar
43. : Improving weight in people with serious mental illness: the effectiveness of computerized services with peer coaches. J Gen Intern Med 2017; 32:48–55Crossref, Medline, Google Scholar
44. : The effect of peer support on knowledge and self-efficacy in weight management: a prospective clinical trial in a mental health setting. Community Ment Health J 2021; 57:979–984Crossref, Medline, Google Scholar
45. : The double stigma of obesity and serious mental illnesses: promoting health and recovery. Stigma Health 2015; 1:86–91Crossref, Google Scholar
46. : Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry 2020; 7:64–77Crossref, Medline, Google Scholar
47. : Weight gain and associated factors in patients using newer antidepressant drugs. Gen Hosp Psychiatry 2015; 37:46–48Crossref, Medline, Google Scholar
48. : Effects of gradual weight loss v rapid weight loss on body composition and RMR: a systematic review and meta-analysis. Br J Nutr 2020; 124:1121–1132Crossref, Medline, Google Scholar
49. : A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med 2013; 368:1594–1602Crossref, Medline, Google Scholar
50. : The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry 2016; 15:155–165Crossref, Medline, Google Scholar
51. : A randomized controlled trial undertaken to test a nurse‐led weight management and exercise intervention designed for people with serious mental illness who take second generation antipsychotics. J Adv Nurs 2013; 69:1539–1548Crossref, Medline, Google Scholar
52. : Association between characteristics of behavioural weight loss programmes and weight change after programme end: systematic review and meta-analysis. BMJ 2021; 374:n1840Crossref, Medline, Google Scholar
53. : The efficacy of nudge theory strategies in influencing adult dietary behaviour: a systematic review and meta-analysis. BMC Public Health 2016; 16:676Crossref, Medline, Google Scholar
54. : The nudge strategies for weight loss in adults with obesity and overweight: a systematic review and meta-analysis. Health Policy 2021; 125:1527–1535Crossref, Medline, Google Scholar
55. : All-cause and cause-specific mortality in people with mental disorders and intellectual disabilities, before and during the COVID-19 pandemic: cohort study. Lancet Reg Health Eur 2021; 11:100228Crossref, Medline, Google Scholar
56. : Increased risk of COVID‐19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry 2021; 20:124–130Crossref, Medline, Google Scholar



