Predictors of Physical Functioning Among Adults With Severe Mental Illness
Abstract
OBJECTIVES: Living conditions and treatment-related factors contribute to high rates of comorbid medical illness among adults with severe mental illness and undermine their physical well-being. This study examined physical functioning scores for this population and compared them with national norms. METHODS: A total of 309 residential care clients completed interviews. Diagnostic and sociodemographic variables were examined for significant associations with physical functioning, which was assessed with the Medical Outcomes Health Survey Short Form 36 (SF-36), and selected variables were entered into a hierarchical multiple regression analyses. Cross-sectional comparisons examined differences in scores between ten-year age groups in this sample and in the national data for men and women. RESULTS: In the final regression model, male gender, younger age, having earned income in the past six months, having less lifetime smoking, and having an admission diagnosis other than depression or schizophrenia spectrum disorders predicted better physical functioning. Functioning scores for the sample deviated from the national normed scores. Differences in functioning increased with each ten-year increment in age; study participants' functioning resembled that of cohorts ten to 20 years older. CONCLUSIONS: Determinants of physical functioning in this sample included well-known demographic predictors of health outcomes. The more striking finding was a deviation from normative data that began for study participants at the age of 25 years, indicating an early onset of limitations. The consistent trend of poorer functioning with each successive age group raises questions about the pace of physical decline among adults with mental illness that may be addressed in longitudinal studies.
Severe mental disorders take a toll on the body, but specific health risks change over time, reflecting changes in living conditions and treatment practices. For example, institutional care led to increased mortality from tuberculosis, but cigarette consumption among patients in asylums was sometimes restricted (1). Individuals in contemporary community-based care do not suffer from the infections common in institutions, but they experience health risks associated with new living conditions (2,3), and they smoke at extremely high rates (4). Inactivity and poor nutritional options contribute to a range of obesity-related problems (5). High rates of alcohol and drug abuse have long-term health implications even for those who have achieved sobriety (6). Current and former users of intravenous drugs and crack cocaine have elevated risks of HIV-AIDS and hepatitis C (7,8,9), which change risk profiles dramatically from those of older cohorts.
Psychiatric illness and treatment compound these problems. Depressive symptoms clearly increase the risk of cardiovascular disease and diabetes (10,11). Schizophrenia syndromes may predispose persons to metabolic syndrome (hypertension, hypercholesteremia, insulin resistance, and abdominal adiposity), which is more generally attributed to neuroleptic treatment (12,13,14). Selected agents within virtually all classes of psychotropic medications contribute to weight gain (15), and some second-generation neuroleptics are associated with still more obesity and metabolic dysfunction (16,17). These agents have most affected the treatment of schizophrenia, but their increasing use for mood disorders heralds effects on a broader diagnostic spectrum (18,19).
Morbidity studies document the impact of behavioral and treatment-related factors, showing consistently elevated rates of cardiovascular disease, chronic respiratory disorders, diabetes, and liver disease. Himmelhoch and colleagues (20), using data from the National Health and Nutrition Examination Study, found a 22.6 percent prevalence of chronic obstructive pulmonary disease among adults with severe mental illness compared with a reported rate of 6 percent in the general population. Using data from the National Ambulatory Medical Care Survey, Daumit and colleagues (21) found that obesity, smoking, and diabetes were reported twice as frequently among persons with severe mental illness. In a retrospective cohort study of 3,022 persons with schizophrenia, Curkendall and associates (22) described adjusted relative risks of 2.2 for cardiovascular disease and 1.8 for diabetes.
Dickey and colleagues (23) used statewide Medicaid data in Massachusetts to examine treated prevalence rates for eight common disorders among Medicaid recipients with nonaffective psychoses who were in the disabled benefit category and recipients with no mental illness who were in the Aid to Families With Dependent Children benefit category. Rates for all eight disorders—diabetes, hypertension, heart disease, asthma, gastrointestinal disorders, skin infections, malignant neoplasms, and acute respiratory disorders—were higher among those with mental illness. In a previous study we found that substance abuse increased rates of treatment for specific disorders but did not fully account for excess morbidity among 781 persons with mental illness (24).
Medical disorders reduce life expectancy among persons with mental illness. High rates of suicide and accidental deaths sometimes obscure medical mortality in this population; however, an international literature confirms the high mortality rates attributable to medical disorders, especially cardiovascular disorders, respiratory illness, and diabetes (25,26,27,28,29,30). This excess mortality is generally expressed as a ratio between rates observed among persons with severe mental illness and rates expected for persons with no mental illness who are of the same age and gender. Thus elevated ratios do not indicate different causes of death than those for the general population but rather earlier deaths from similar causes.
Although medical morbidity and mortality are well documented, less attention has focused on the functional impact of medical morbidity. Individuals with physical disabilities often have problems performing activities that are necessary for independent living, such as shopping or housekeeping. However, these abilities are not systematically assessed among adults with psychiatric diagnoses—at least until they enter services designed for elderly persons with mental illness. In view of the early mortality from chronic medical disorders among adults with severe mental illness, we suspected that they might experience early onset of physical limitations associated with chronic medical illness. To examine this phenomenon, this study looked at a sample of community-based adults with severe mental illness in order to examine demographic and clinical determinants of physical functioning and to compare them with national age and gender-matched norms.
Methods
Sample and setting
The sample for this cross-sectional study included 309 adults with severe mental illness who were recruited from July 2001 to December 2002 for a randomized trial and assessed at baseline. Persons were recruited for the study in four crisis residential programs operated by the San Francisco Progress Foundation (31). These home-like settings provide short-term treatment to persons referred from inpatient or crisis services in the city and county of San Francisco. Clients in these programs are drawn from a citywide population and approximate a treated community sample, or those receiving acute and subacute services, of adults with severe mental illness (24). Non-English speakers were excluded, as were persons with cognitive impairments that precluded participation in interviews and persons with no diagnosis except adjustment disorder. The clinical trial tested the benefits of adding an active health promotion intervention to basic primary care services for this population. The study and informed consent procedures were approved by the committee on human research of the University of California, San Francisco.
Data collection
Data were obtained by a structured face-to-face interview conducted at enrollment. Demographic data were recorded, and two questions about health care were asked: Do you see a regular doctor or nurse practitioner for health care? Do you go to a regular place for health care? Data on drug and alcohol use, including smoking, were obtained by use of the relevant section of the Addiction Severity Index (32). Admitting diagnoses were extracted from clinical records.
Health status was measured by using the Medical Outcomes Health Survey Short Form 36 (SF-36). This self-report measure of physical and emotional well-being has been used widely in health outcomes studies (33). Eight scales contribute to summary measures for physical and emotional health status. The instrument has been tested extensively for reliability and validity and normed for men and women in the general population (33). The SF-36 has been employed successfully in studies of people with schizophrenia (34,35,36), bipolar disorder (37,38,39), and major depressive disorder (40,41,42). It has been used in more than 300 studies of mental disorders, although the vast majority of such studies focus on depressive symptoms within the context of a medical illness. The key variable of interest for this research, physical functioning, was measured with the physical functioning scale of the SF-36, with scores standardized from 0 to 100 (33).
A number of sources question the independence of physical and mental summary scores on this instrument, because both are affected by mood (43), and because individual scales may contribute to both summary measures (44,45), and because of the general conceptual problems inherent in separating physical and emotional well-being (44). However, the physical functioning scale consists of items eliciting concrete responses about physical limitations due to health, such as in carrying groceries, using stairs, bending, and lifting. This scale has the least association with emotional functioning of all eight SF-36 scales (33,46). For this reason we selected it as the best option for a generic measure of physical health status, independent of specific medical diagnoses.
Analysis
The sample was described by using means, medians (age, education, measures of health status, and physical functioning), and proportions (marital status, ethnicity, living situation, and income sources) for the total group by gender. Health-related characteristics were described by categories of admission diagnoses (schizophrenia and other nonaffective psychoses, bipolar disorder, depression, and others). We then examined several variables that we thought might be associated with physical functioning: gender, age, ethnicity, marital status, living situation in the past six months, recent employment (yes or no), and income (earned income, Social Security benefits, and family or other support). We also examined physical functioning in relation to lifetime substance use (number of years of use of alcohol to intoxication, opiates, stimulants, and smoking) and the number of drugs administered intravenously.
Associations between physical functioning and these variables were examined with Pearson correlations for continuous variables and with one-way analyses of variance (ANOVAs) for categorical variables. Variables that had significant bivariate associations (p<.05) with physical functioning were selected as predictors for hierarchical multiple regression analyses. Our strategy for these analyses was to enter the set of sociodemographic predictors first, then the predictors related to substance use, and finally psychiatric diagnosis (dummy coded). Because current living situation was coded in five categories, dummy variables to represent the different living situations were entered as a group after entering the dichotomous sociodemographic predictors at step 1 but before entering the substance use predictors.
To compare the scores of study participants with national norms, we computed new physical functioning scores for the participants as deviations from the relevant norm group means. Normative means were available for women and men across approximate tenyear age intervals (33). The deviation scores were tested against zero with a one-way ANOVA. We also tested for differences on these deviation scores among the age groups by using the Brown-Forsythe F*test as a guard against unequal variances among the groups and skewness in the distributions (47).
Results
The sample included 309 adults, ranging in age from 18 to 60 (mean age, 38.2). Gender was self-reported, and the sample included 210 men, 91 women, and eight trans-gender persons, all of whom were male-to-female and considered as female in the analyses. Sociodemographic characteristics by gender are presented in Table 1. Table 2 reports health-related characteristics of the sample by admission diagnosis.
Results of the hierarchical regression analyses (Table 3) showed that six sociodemographic variables (gender, age, being in a partnered relationship, living with at least one child under 18 years of age, having earned income in the past six months, and having family support, alimony, child support, or other support in the past six months) predicted 20 percent of the variance in physical functioning when entered into the model at the first step. Years of lifetime opiate use and smoking increased R2 by 4 percent at the second step. Finally, psychiatric diagnosis at admission increased R2 by 3 percent at the third step. The final model R2 was .27 (F=9.92, df=11, 290, p<.001). Five predictors contributed significantly to the regression, with p values of .05 or less; these predictors made unique contributions to R2, ranging from .6 percent to 3.5 percent. Of note, psychiatric diagnosis uniquely predicted 3.5 percent of the variance in physical functioning. Participants with diagnoses of schizophrenia and other nonaffective psychoses as well as those with depression were predicted to have lower physical functioning scores than those with bipolar disorder or "other" diagnoses.
With each ten-year increment in age, the magnitude of differences increased between the mean scores for participants and the scores for the normative groups (Figures 1 and 2). The mean deviation between the physical functioning score and the normative group mean differed from zero (F=97.03, df=1, 304, p<.001, eta squared=.24), and the test for differences among the study participants' age groups was also significant (F*=4.53, df=4, 60, p=.003, eta squared=.06). Differences from normative groups explained 24 percent of the variance in physical functioning scores; differences among age groups explained 6 percent. Separate analyses were also conducted by age group for each gender, and the results were consistent with this analysis. Therefore, only the overall analysis by age group is reported.
Several post hoc analyses were conducted to describe the findings related to age groups further. Post hoc one-sample t tests were carried out for each age group against the expected mean difference of zero to investigate the significant differences between these groups and the relevant normative groups. We controlled for type 1 error with the Bonferroni correction, using p=.01 for each test. Means for four of the five groups (all but the youngest age group, 18 to 24) were significantly lower than expected, indicating that four age groups from this population reported poorer physical functioning than the norm, even when the analysis adjusted for age. We also examined differences among age groups with post hoc pairwise contrasts by using Dunnett's T3 procedure because of the unequal variances among the groups (47). We found that even though our sample reported worse physical functioning than the relevant normative groups, participants aged 35 to 44 years and 45 to 54 years reported significantly poorer physical functioning than participants in the 18- to 24-year-old group. Participants aged 25 to 34 and 55 to 64 also reported poorer physical functioning than the youngest group; however, the differences were not statistically significant. Finally, we tested whether the mean deviation scores indicated increasingly poorer physical functioning across age groups and found a significant, decreasing linear trend (F=10.51, df=1, 304, p=.001, eta squared=.03).
Discussion
The demographic characteristics of this sample are comparable to those of adults with severe mental illness described in other mental health systems, with mean ages generally in the late 30s to early 40s (21,23,48,49,50). The high ratio of men to women, along with the considerable ethnic diversity and high rates of residential instability and substance abuse, indicate that study participants are similar to clients in programs in urban areas for high-risk subgroups, such as persons with dual diagnoses (50). It is therefore reassuring that most study participants reported going to a regular place for health care. However, only half saw one provider consistently, and more than half rated their overall health as fair to poor. These global self-ratings are mirrored by their scores for physical functioning.
Determinants of physical functioning scores that were identified in the regression model include demographic factors that are well known to affect health—gender, age, and earned income—in addition to smoking and a psychiatric diagnosis. The fact that we did not identify effects of lifetime substance abuse is puzzling and may reflect the ubiquitous character of abuse histories in this sample. In contrast, smoking has a significant impact on current functioning and probably reflects the relationship between smoking-related problems, such as shortness of breath, and items in the physical functioning scale, which included walking, lifting, and vigorous activities. Diagnoses of depressive disorders and of schizophrenia spectrum disorders (nonaffective psychoses) had the most adverse effects on physical functioning, and the affects did not differ significantly from each other. The comparatively superior physical functioning scores among participants with bipolar disorder may indicate effects of mood on SF-36 self-ratings (43). The small number of participants in the "other" group had varied diagnoses that may have had less physical impact than schizophrenia and major mood disorders.
The more compelling finding with regard to physical functioning scores in this sample is the magnitude of deviations from national norms for specific age groups. Significant deviations from norms were observed among relatively young adults—those in the third decade of life. Forty-eight percent of our study participants were older than 40 years, and they reported levels of functioning comparable to the elderly in the general population. The mean score for the sample as a whole resembles scores reported anecdotally in other studies of adults with severe mental illness (34,36,37). The mean score is also comparable or slightly better than normative scores for persons with diagnoses of chronic medical illnesses that are prevalent among persons with severe mental illness, including hypertension and type II diabetes (33). However, these norms are based on chronically ill people who were considerably older than our sample, as indicated by mean ages and the proportion older than 55.
The cross-sectional self-reported nature of these data precludes any conclusions about accelerated physical decline in the population represented by the study participants. Conditions that influenced the health of 35-year-olds in this sample may not be the same factors that produce physical limitations among persons over age 50. Nevertheless, the limitations observed at early ages and the consistent trend for each successive age group suggest decline. Although it is imprecise, we came informally to refer to this phenomenon as "premature aging."
This study had other important limitations. Persons who agree to participate in a clinical trial may differ from other persons with mental illness. Diagnoses were abstracted from clinical charts, and their validity is unknown. Normative data lacked information on sociodemographic factors that often accompany mental disorders. The relatively small contribution of diagnostic factors to the final regression model suggests that these social and behavioral variables may be more important than mental disorders themselves. Further longitudinal studies can elucidate whether mental illness plays a causal role, in the presence of sociodemographic factors, on declines in physical functioning.
Even given these limitations, our findings raise the question of where to set the threshold for receipt of services usually reserved for older adults, including home visits and other supports for personal and medical care. Our findings also underscore the need for early intervention to address the formidable health risks that contribute to poor physical functioning in this population. An extensive body of research in psychiatric rehabilitation demonstrates that adults with severe mental disorders can be actively engaged in their own psychiatric care. Mueser and colleagues (51) noted that 40 randomized clinical trials have established the value of behavioral, educational, and cognitive-behavioral interventions for selfmanagement of severe psychiatric illness. These models may have comparable value for medical risk reduction, as indicated by recent studies of smoking cessation (52) and weight control (53). At this juncture, it appears critical to integrate these approaches into community mental health programs.
Dr. Chafetz, Dr. White, Ms. Collins-Bride, and Dr. Cooper are affiliated with the community heath systems department of the School of Nursing at the University of California, San Francisco, Box 0608, San Francisco, California 94143 (email, [email protected]). Dr. Nickens is with Progress Foundation in San Francisco.
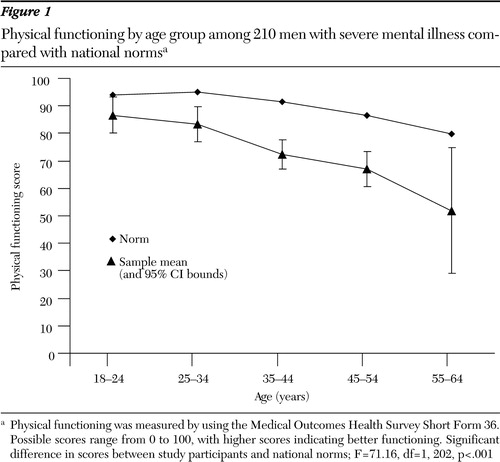
Figure 1. Physical functioning by age group among 210 men with severe mental illness compared with national normsa
a Physical functioning was measured by using the Medical Outcomes Health Survey Short Form 36. Possible scores range from 0 to 100, with higher scores indicating better functioning. Significant difference in scores between study participants and national norms; F=71.16, df=1, 202, p<.001
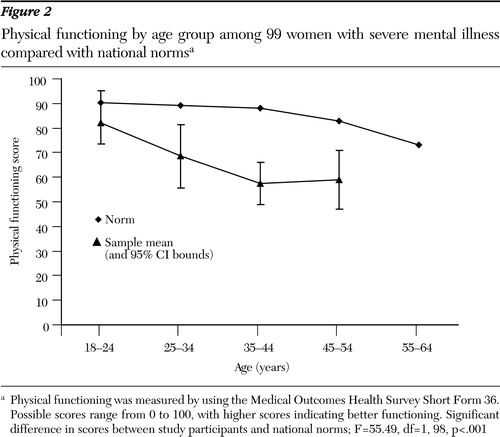
Figure 2. Physical functioning by age group among 99 women with severe mental illness compared with national normsa
a Physical functioning was measured by using the Medical Outcomes Health Survey Short Form 36. Possible scores range from 0 to 100, with higher scores indicating better functioning. Significant difference in scores between study participants and national norms; F=55.49, df=1, 98, p<.001
 |
Table 1. Characteristics of 309 adults with severe mental illness, by gendera
a Percentages do not add to 100 because of rounding.
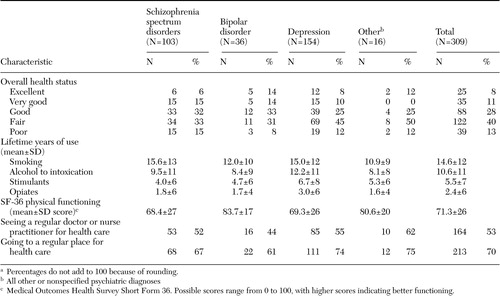 |
Table 2. Healthrelated characteristics of 309 adults with severe mental illness, by primary diagnosis on admissiona
a Percentages do not add to 100 because of rounding.
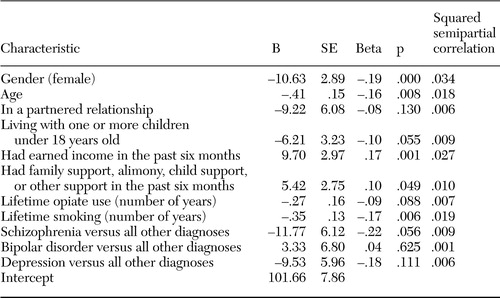 |
Table 3. Final linear regression model for physical functioning scores regressed on sociodemographic variables, substance use, and psychiatric diagnosis among 309 adults with severe mental illness
1. Harris EC, Barraclough B: Excess mortality of mental disorder. British Journal of Psychiatry 173:11–531998Crossref, Medline, Google Scholar
2. White MC, Tulsky JP, Dawson C, et al: Association between time homeless and perceived health status among the homeless in San Francisco. Journal of Community Health 22:271–282,1997Crossref, Medline, Google Scholar
3. Cohen DA, Mason K, Bedimo A, et al: Neighborhood physical conditions and health. American Journal of Public Health 93:467–471,2003Crossref, Medline, Google Scholar
4. Williams JM, Ziedonis D: Addressing tobacco among individuals with a mental illness or an addiction. Addictive Behavior 29:1067–1083,2004Crossref, Medline, Google Scholar
5. Susce MT, Villaneuva N, Diaz JD, et al: Obesity and associated complications in patients with severe mental illnesses: a crosssectional survey. Journal of Clinical Psychiatry 66:167–173,2005Crossref, Medline, Google Scholar
6. RachBeisel J, Scott J, Dixon L: Cooccurring severe mental illness and substance use disorders: a review of recent research. Psychiatric Services 50:1427–1434,1999Link, Google Scholar
7. Blank MB, Mandell DS, Aiken L, et al: Cooccurrence of HIV and serious mental illness among Medicaid recipients. Psychiatric Services 53:868–873,2002Link, Google Scholar
8. Essock SM, Dowden S, Constantine NT, et al: Risk factors for HIV, hepatitis B, and hepatitis C among persons with severe mental illness. Psychiatric Services 54:836–841,2003Link, Google Scholar
9. Osher FC, Goldberg RW, McNary SW, et al: Substance abuse and the transmission of hepatitis C among persons with severe mental illness. Psychiatric Services 54:842–847,2003Link, Google Scholar
10. Carney RM, Freedland KE, Miller GE, et al: Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. Journal of Psychosomatic Research 53:897–902,2002Crossref, Medline, Google Scholar
11. Eaton WW: Epidemiologic evidence on the comorbidity of depression and diabetes. Journal of Psychosomatic Research 53:903–906,2002Crossref, Medline, Google Scholar
12. Ryan MC, Collins P, Thakore JH: Impaired fasting glucose tolerance in firstepisode, drugnaive patients with schizophrenia. American Journal of Psychiatry 160:284–289,2003Link, Google Scholar
13. Ryan MC, Thakore JH: Physical consequences of schizophrenia and its treatment: the metabolic syndrome. Life Sciences 71:239–257,2002Crossref, Medline, Google Scholar
14. Cohn T, Prud'homme D, Streiner D, et al: Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Canadian Journal of Psychiatry 49:753–60,2004Crossref, Medline, Google Scholar
15. Schwartz TL, Nihalani N, Jindal S, et al: Psychiatric medicationinduced obesity: a review. Obesity Review 5:115–121,2004Crossref, Medline, Google Scholar
16. Fontaine KR, Heo M, Harrigan EP, et al: Estimating the consequences of antipsychotic induced weight gain on health and mortality rate. Psychiatry Research 101:277–288,2001Crossref, Medline, Google Scholar
17. Wirshing DA, Pierre JM, Erhart SM, et al: Understanding the new and evolving profile of adverse drug effects in schizophrenia. Psychiatric Clinics of North America 26:165–190,2003Crossref, Medline, Google Scholar
18. Calabrese JR, Kasper S, Johnson G, et al: International Consensus Group on Bipolar I: depression treatment guidelines. Journal of Clinical Psychiatry 65:571–579,2004Crossref, Medline, Google Scholar
19. McElroy SL, Frye MA, Suppes T, et al: Correlates of overweight and obesity in 644 patients with bipolar disorder. Journal of Clinical Psychiatry 63:207–213,2002Crossref, Medline, Google Scholar
20. Himmelhoch S, Lehman H, Kreyenbuhl J, et al: Prevalence of chronic obstructive pulmonary disease among those with serious mental illness. American Journal of Psychiatry 161:2317–2319,2004Link, Google Scholar
21. Daumit GL, Pratt LA, Crum RM, et al: Characteristics of primary care visits for individuals with severe mental illness in a national sample. General Hospital Psychiatry 24:391–395,2002Crossref, Medline, Google Scholar
22. Curkendal SM, Mo J, Glasse DB, et al: Cardiovascular disease in patients with schizophrenia in Saskatchewan, Canada. Journal of Clinical Psychiatry 65:715–720,2004Crossref, Medline, Google Scholar
23. Dickey B, Normand SL, Weiss RD, et al: Medical morbidity, mental illness, and substance use disorders. Psychiatric Services 53:861–867,2002Link, Google Scholar
24. Chafetz L, White MC, CollinsBride G, et al: The poor health of the severely mentally ill. Community Mental Health Journal 41:169–184,2005Crossref, Medline, Google Scholar
25. Hannerz H, Borga P: Mortality among persons with a history as psychiatric inpatients with functional psychosis. Social Psychiatry Psychiatric Epidemiology 35:380–387,2000Crossref, Medline, Google Scholar
26. Osby U, Correia N, Brandt L, et al: Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophrenia Research 45:21–28,2000Crossref, Medline, Google Scholar
27. Hoyer EH, Mortensen PB, Olesen AV: Mortality and causes of death in a total national sample of patients with affective disorders admitted for the first time between 1973 and 1993. British Journal of Psychiatry 176:76–82,2000Crossref, Medline, Google Scholar
28. Hansen V, Jacobsen BK, Arnesen E: Causespecific mortality in psychiatric patients after deinstitutionalisation. British Journal of Psychiatry 179:438–443,2001Crossref, Medline, Google Scholar
29. Lawrence DM, Holman CD, Jablensky AV, et al: Death rate from ischaemic heart disease in Western Australian psychiatric patients 1980-1998. British Journal of Psychiatry 182:31–36,2003Crossref, Medline, Google Scholar
30. Brown S, Inskip H, Barraclough B: Causes of the excess mortality of schizophrenia. British Journal of Psychiatry 177:212–217,2000Crossref, Medline, Google Scholar
31. Fields SL: Progress Foundation, San Francisco, in Alternatives to the Hospital for Acute Psychiatric Treatment. Edited by Warner R: Washington, DC, American Psychiatric Press, 1995Google Scholar
32. McLellan AT, Luborsky L, Cacciola J, et al: New data from the Addiction Severity Index: reliability and validity in three centers. Journal of Nervous and Mental Disease 173:412–423,1985Crossref, Medline, Google Scholar
33. Ware JE Jr, Snow KK, Kosinski M: SF36(R) Health Survey: Manual and Interpretation Guide. Lincoln, RI, QualityMetric, 2000Google Scholar
34. Sciolla A, Patterson TL, Wetherell JL, et al: Functioning and wellbeing of middleaged and older patients with schizophrenia: measurement with the 36item ShortForm (SF36) Health Survey. American Journal of Geriatric Psychiatry 11:629–637,2003Medline, Google Scholar
35. Pyne JM, Sullivan G, Kaplan R, et al: Comparing the sensitivity of generic effectiveness measures with symptom improvement in persons with schizophrenia. Medical Care 41:208–217,2003Medline, Google Scholar
36. Strassnig M, Brar JS, Ganguli R: Body mass index and quality of life in communitydwelling patients with schizophrenia. Schizophrenia Research 62:73–76,2003Crossref, Medline, Google Scholar
37. Yatham LN, Lecrubier Y, Fieve RR, et al: Quality of life in patients with bipolar I depression: data from 920 patients. Bipolar Disorders 6:379–385,2004Crossref, Medline, Google Scholar
38. Leidy NK, Palmer C, Murray M, et al: Healthrelated quality of life assessment in euthymic and depressed patients with bipolar disorder; psychometric performance of four selfreport measures. Journal of Affective Disorders 48:207–214,1998Crossref, Medline, Google Scholar
39. Arnold LM, Witzeman KA, Swank ML, McElroy SL, Keck PE: Health related quality of life using the SF36 in patients with bipolar disorder compared with patients with chronic back pain and the general population. Journal of Affective Disorders 57:235–239,2000Crossref, Medline, Google Scholar
40. Kruijshaar ME, Hoeymans N, Bijl RV, et al: Levels of disability in major depression: findings from the Netherlands Mental Health Survey and Incidence Study. Journal of Affective Disorders 77:53–64,2003Crossref, Medline, Google Scholar
41. Gleason OC, Yates WR, Isbell MD, et al: An openlabel trial of citalopram for major depression in patients with hepatitis C. Journal of Clinical Psychiatry 63:194–198,2003Crossref, Google Scholar
42. Wittchen HU, Carter RM, Pfister H, et al: Disabilities and quality of life in pure and comorbid generalized anxiety disorder and major depression in a national survey. International Clinical Psychopharmacology 15:319–328,2000Crossref, Medline, Google Scholar
43. Simon GE, Revicki DA, Grothaus LMA, et al: SF 36 summary scores: are physical and mental health truly distinct? Medical Care 36:567–572,1998Google Scholar
44. Pukrop R, Schlaak V, MollerLeimkuhler AM, et al: Reliability and validity of Quality of Life assessed by the ShortForm 36 and the Modular System for Quality of Life in patients with schizophrenia and patients with depression. Psychiatry Research 119:63–79,2003Crossref, Medline, Google Scholar
45. Ware J: SF36 Update. Spine 25:3130–3139,2000Crossref, Medline, Google Scholar
46. Russo J, Trujillo CA, Wingerson D, et al: The MOS 36Item Short Form Health Survey: reliability, validity, and preliminary findings in schizophrenic outpatients. Medical Care 36:752–756,1998Crossref, Medline, Google Scholar
47. Maxwell SE, Delaney HD: Designing Experiments and Analyzing Data: A Model Comparison Perspective, 2nd ed. Mahwah, NJ: Erlbaum, 2004Google Scholar
48. Lehman AF, Goldberg R, Dixon LB, et al: Improving employment outcomes for persons with severe mental illness. Archives of General Psychiatry 59:165–172,2002Crossref, Medline, Google Scholar
49. Barrio C, Yamada AM, Hawthorne W, et al: Ethnic disparities in use of public mental health case management services among patients with schizophrenia. Psychiatric Services 54:1264–1270,2003.Link, Google Scholar
50. Mueser KT, Essock SM, Drake RE, et al: Rural and urban differences in patients with dual diagnosis. Schizophrenia Research 48:93–107,2001Crossref, Medline, Google Scholar
51. Mueser KT, Sengupta A, Schooler NR, et al: Illness management and recovery: a review of the research. Psychiatric Services 53:1272–1284,2002Link, Google Scholar
52. Evins AE, Cather C, Rigotti NA, et al: Twoyear followup of a smoking cessation trial in patients with schizophrenia: increased rates of smoking cessation and reduction. Journal of Clinical Psychiatry 65:307–311,2004Crossref, Medline, Google Scholar
53. Faulkner G, Soundy AA, Lloyd K: Schizophrenia and weight management: a systematic review of interventions to control weight. Acta Psychiatrica Scandinavica 108:324–332,2003Crossref, Medline, Google Scholar



