Heterogeneity in Aripiprazole Diffusion for Bipolar Disorder Treatment in the Veterans Health Administration
Abstract
Objectives
The objectives of this study were to ascertain the relative importance of scientific “approval” versus U.S. Food and Drug Administration (FDA) regulatory approval regarding changes in aripiprazole prescribing rates for treating bipolar disorder and to identify system-level covariates associated with faster regional uptake of aripiprazole.
Methods
Medication use data for 2002–2009 were obtained from the Veterans Health Administration (VHA) National Psychosis Registry for 106,547 patients with diagnoses of bipolar disorder, aggregated at the level of the Veterans Integrated Service Network (VISN). VISN-level independent variables were obtained from several VHA organizational databases. Interrupted time-series analysis was used to examine changes in rates of prescribing aripiprazole, and logistic regression was used to model above- versus below-median growth in aripiprazole prescribing across VISNs.
Results
Three inflections were observed, corresponding to the publication of two positive studies and FDA approval of aripiprazole for the treatment of bipolar mania. No significant VISN-level policy, administrative, or staffing predictors of the growth rate in aripiprazole prescribing were identified. Exploratory analyses showed that access to care may play a role in uptake, whereas competing demands, such as substance abuse treatment needs, may retard adoption.
Conclusions
Early published evidence may have a strong impact on practice for low-barrier innovations, such as newly marketed medications or changes in indication for approved medications. Regional targeting of prescriber behavior interventions may maximize efficiency in efforts to change prescribing; further delineation of factors associated with regional heterogeneity in prescribing would support such efforts.
Second-generation antipsychotics are the most widely used antipsychotics within the Veterans Health Administration (VHA). In 2009, four of the top ten drugs in terms of expenditure were second-generation antipsychotics, with these medications accounting for over $365 million in pharmacy costs (>8% of all VHA pharmacy costs) (TS Semla, personal communication to MSB, December 14, 2011). From 2000 to 2004 the U.S. Food and Drug Administration (FDA) approved five second-generation antipsychotics for monotherapy or adjuvant treatment of bipolar disorder. Although FDA approval has been associated with increases in second-generation antipsychotic use for bipolar disorder (1), little is known about the relative influence of clinical versus administrative factors affecting changes in prescribing practices. Moreover, these drugs are expensive and can cause serious side effects, including weight gain and glucose intolerance, potentially contributing to preventable morbidity in this group. Thus information concerning the prescription of these medications is of substantial importance to policy and quality of care for the VHA, as it is for other payers (including Medicare and Medicaid) (2–4).
Patient, provider, and health system characteristics are important factors in the adoption of health care innovations—both for new chemical entities and changes in prescribing when the FDA approves new indications (5–11). It is also well known that “off-label” use of medications precedes official FDA approval by years (12–16). Faster uptake is associated with perceived clinical effectiveness, influence of opinion leaders (17,18), and pharmaceutical “detailing” (19–22). However, the literature is surprisingly sparse in regard to the impact of publishing clinical research results on the uptake of new practice behaviors. It is commonly argued that empirically supported treatments (evidence-based) take ten to 20 years to be fully adopted by the health care system (23–28). However, new medications may be adopted much faster (13,14,29,30). It thus becomes of interest to determine the relationships between the adoption of medications and their scientific and clinical or regulatory support.
Aripiprazole is of interest because of its relatively recent FDA approval. It entered the field when the field was already well populated with first- and second-generation antipsychotics but provided the potential advantages of not being associated with high rates of weight gain, metabolic problems, cardiac conduction abnormalities, or tardive dyskinesia (31,32). Therefore, our objective in this study was to identify spatial-temporal patterns of diffusion for the prescribing of aripiprazole for bipolar disorder across Veterans Integrated Service Networks (VISNs)—large, geographically discrete regional administrative units within the VHA—over an eight-year period. Investigating aripiprazole use for bipolar disorder allowed us to investigate the effects of a new FDA indication on a medication already available in the U.S. marketplace.
The uniformity of new prescribing practices across the country has yet to be determined. Available evidence regarding regional differences in prescribing behavior indicates that regional factors are often significant (33–36). Regional (VISN) factors in the VHA include, for example, level of academic affiliation and mental health clinician supply per veteran. Significant regional differences have been demonstrated in diffusion of ziprasidone, another second-generation antipsychotic (29), as well as off-label use of prazosin for posttraumatic stress disorder (37). The social-spatial contagion of information is one mechanism by which geographic disparities arise (38–43). However, it is not yet clear the degree to which such provider-level factors explain differences (6) compared with administrative and other organizational factors (44,45).
The VHA is a particularly informative system, because it has a standardized national formulary with no mandatory fail-first policies or formulary restrictions on available second-generation antipsychotics (TS Semla, personal communication to MSB, December 14, 2011). Given the availability of national pharmacy data, a standard formulary, and its prominence in training the next generation of prescribers (residents, advanced practice nurses, and physician assistants), the VHA is an ideal setting in which to conduct this type of analysis. We were particularly interested in identifying whether regional groups of clinicians (aggregated by VISNs) were more likely to be “early adopters” of aripiprazole for treating bipolar disorder based on the relative influence of scientific “approval” (in the form of peer-reviewed published manuscripts) versus FDA regulatory approval.
Methods
This study had three components: a VISN-level description of the uptake of aripiprazole, an interrupted time-series analysis of prescribing rates, and a cross-sectional analysis of VISN-level correlates associated with the uptake of aripiprazole. This study was reviewed by the Group Health Research Institute and VHA Central institutional review boards.
Data and population
Data concerning second-generation antipsychotic medication use were obtained from the VHA National Psychosis Registry. The registry contains administrative data on diagnosis, utilization, and cost for all VHA patients with an ICD-9-CM diagnosis of schizophrenia, bipolar disorder, or other psychotic disorder (46,47).
The analyses in this study were restricted to patients with a diagnosis of bipolar disorder. This population grew from 70,345 in 2002 to 106,547 in 2008. This cohort of patients was 88% male and 59% white. Approximately 8% were <35 years old; 35% were ages 35–50 years; 42% were 50–65 years; 13% were 65–80 years; and 2% were >80 years.
Annual time series
Time series for the 21 VISNs were constructed for all aripiprazole prescriptions dispensed to patients with bipolar disorder between October 2002 and September 2009. Crude rates of aripiprazole use were calculated on an annual basis. The numerator of annual rates included all individuals who had at least one prescription for aripiprazole during the fiscal year. The denominator included all individuals with at least one outpatient or inpatient encounter during the same fiscal year that included an ICD-9-CM diagnosis of bipolar disorder.
We ranked VISNs by the absolute change in the aripiprazole utilization rate between 2004 and 2005. This period captured the two dates of FDA approval for use of aripiprazole in treating bipolar disorder. We constructed two time series based on the median split in change in the utilization rate between 2004 and 2005. That is, one time series for VISNs with increases in utilization above the median 2004–2005 increase and one for VISNs below the median increase. Thus the final descriptive analysis involved two groups with seven annual observations.
Monthly time series
Crude rates of aripiprazole use by month were calculated between October 2002 and September 2009 with the same approach for the annual series described above.
Monthly rates were plotted and examined visually. Independently of the annual analysis, we aggregated VISNs into three groups according to the first observed upward inflection points in the time series. The three observed first inflection points occurred in September 2003, May 2004, and September 2004 and correspond, respectively, to the publication of the results of the first placebo-controlled, double-blind study of the efficacy and safety of aripiprazole for patients with acute bipolar mania (48), the results of the first randomized controlled trial of aripiprazole for bipolar disorder (49), and the FDA approval of aripiprazole for the treatment of bipolar mania in September 2004 (50,51). These three dates were used as independent variables to demarcate periods where we tested changes in the trend of prescribing aripiprazole.
Time-series analysis
We used segmented linear regression to test for changes in the time series at the three study dates. The analysis involved calibrating three separate regression models, each with 84 observations (7 years × 12 months). The interrupted time-series analysis models included parameters for time, occurrence of the event (three dates above), time since the event, and any autoregressive parameters (to remove spurious inflation of significance). The specification of these models allowed the testing of immediate changes in the level of prescribing (specifically, shifts in the intercept) and changes in the slope (the inflection points).
VISN-level correlates of prescribing rates
Our third, exploratory analysis focused on regional covariates of heterogeneity in the adoption of aripiprazole for bipolar disorder. We used the cross-sectional classification of VISN membership based on the median split described above. We created an indicator variable, with 1 indicating that a VISN belonged to the above–median increase group and 0 indicating that a VISN belonged to the below–median increase group. The sample size for cross-sectional analyses was 21.
We hypothesized that growth in aripiprazole prescribing would be related to five general types of VISN-level characteristics: patient access to care, composition of the patient population, supply and composition of mental health clinicians, and mental health policies and directives. [A list of covariates is available online as a data supplement to this article.] We specifically hypothesized that greater access to care, patients with more severe illness, greater supply of mental health care providers, more prevalent nonphysician prescribing, and more control over mental health policies would be associated with higher rates of growth in prescribing.
VISN-level covariates were obtained from the 2007 Mental Health Program Survey (52) in order to model organizational characteristics specific to mental health that we hypothesized would be related to increases in the aripiprazole prescribing rate. We also used the Inventory of Organization Characteristics (53) to obtain information about VISN-level policies or directives regarding mental health. Third, we accessed the VHA Personnel and Accounting Integrated Data payroll system (54,55) to obtain VISN-level information concerning clinical and administrative staffing.
Because these analyses were considered exploratory, using regional-level data to identify possible areas for further patient- and medical center–level analyses, we investigated a relatively large number of variables related to the four domains of interest (access, population composition, mental health staffing, and policies). We calibrated separate bivariate logistic regression models for each of the candidate VISN-level covariates. Variables with a type I error <.20 were entered into a multivariate model. The final adjusted model included only those variables with a type I error <.05.
Results
VISN-level descriptive analysis
Figure 1 shows annual growth in the rate of individuals with bipolar disorder who were prescribed aripiprazole at least once during the fiscal year. Aripiprazole was first available in the United States for the treatment of schizophrenia in November 2002. Between 2002 and 2004, the growth in prescribing was uniform across VISNs. Utilization was between 3.3% and 3.9% of all patients with bipolar disorder in 2004. VISNs were separated into two groups between 2004 and 2005, with some VISNs experiencing large increases in the rate of aripiprazole use and others continuing on a more linear path. In the above–median growth group, aripiprazole use averaged 3.3% in 2004 and 12.0% in 2005. In contrast, aripiprazole use grew from 3.9% of patients with bipolar disorder in 2004 to 8.5% among VISNs in the below-median group.
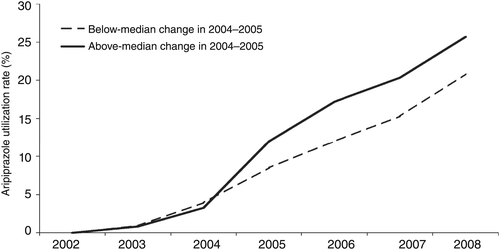
aSource: National Psychosis Registry Annual Reports: 2003–2009
VISNs in the above-median group continued to have higher utilization rates for the remaining years of the study period. Although there was an immediate, upward shift of 4%–5% in the overall utilization rate in this group, the growth rate in subsequent years was similar to that in the less responsive VISNs.
Interrupted time-series results
Figures 2, 3 and 4 show changes in the monthly rate of aripiprazole prescribing that correspond to the three catalyzing events in September 2003, May 2004, and September 2004. Each event was associated with an upward inflection in the time series.
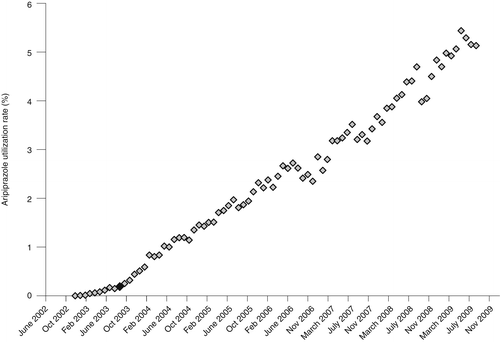
aN=4 Veterans Integrated Service Networks. The black diamond represents the publication date, September 2003.
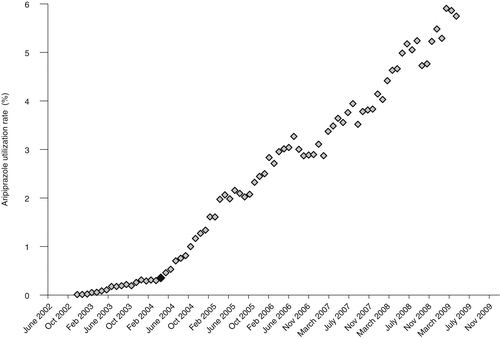
aN=12 Veterans Integrated Service Networks. The black diamond represents the publication date, May 2004.
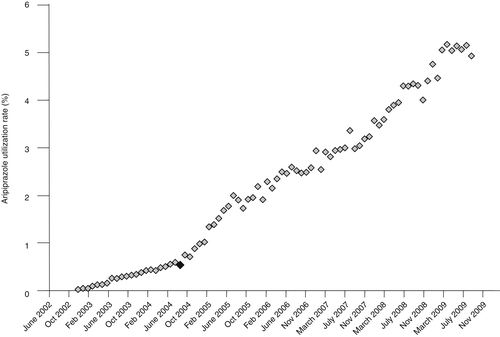
aN=5 Veterans Integrated Service Networks. The black diamond represents the approval date, September 2004.
Segmented regression results
Table 1 shows the results of the three segmented regression models that correspond to Figures 2–4. The time covariate in each model controls for the secular trend (increase) in aripiprazole prescribing rates over the entire period (to remove the effect of generally increasing rates over time). The covariates labeled “manuscript 1,” “manuscript 2,” and “FDA approval” measured immediate changes in the level of aripiprazole prescribing (that is, vertical shifts). None of the intercept covariates were statistically significant. The covariates labeled “time after manuscript 1,” “time after manuscript 2,” and “time after FDA approval” measured immediate changes in the slope of the time series in each of the study months of interest.
| Variable | Parameter | SE | tb | Pr>| t | |
|---|---|---|---|---|
| September 2003 modelc | ||||
| Intercept | –.05 | .20 | –.23 | .82 |
| Time | .01 | .02 | .65 | .52 |
| Manuscript 1 (level) | .08 | .17 | .47 | .64 |
| Time after manuscript 1 (slope) | .05 | .02 | 2.39 | .02 |
| May 2004 modeld | ||||
| Intercept | –.11 | .16 | –.68 | .50 |
| Time | .02 | .01 | 2.14 | .04 |
| Manuscript 2 (level) | .08 | .14 | .55 | .58 |
| Time after manuscript 2 (slope) | .06 | .01 | 4.95 | <.01 |
| September 2004 modele | ||||
| Intercept | –.05 | .16 | –.29 | .77 |
| Time | .03 | .01 | 3.09 | <.01 |
| FDA approval (level) | .01 | .13 | .03 | .98 |
| Time after FDA approval (slope) | .04 | .01 | 3.21 | <.01 |
The upward inflection points observed in Figures 2–4 represent statistically significant increases in the rate of growth in aripiprazole prescribing for the VISNs included in each of these groups. The first manuscript was associated with a monthly .05% increase in prescribing. Prescribing rates increased .06% per month among VISNs most responsive to the second manuscript. Similarly, the monthly increase was .04% per month in VISNs most responsive to the FDA approval of aripiprazole for treatment of bipolar mania.
Multivariate logistic regression results
Only two of the hypothesized VISN-level covariates were significantly associated with the annual growth rate in aripiprazole prescribing; both were associated with patients’ access to care. With controls for geographic access to care among patients with bipolar disorder, a unit increase in the average number of general psychiatric visits per patient with bipolar disorder was associated with a nearly fourfold greater likelihood that a VISN would belong to the above-median growth group (odds ratio [OR]=3.92, 95% confidence interval [CI]=1.21–12.69). In contrast, a unit increase in the average number of visits for substance use problems among individuals with bipolar disorder was associated with an OR=.41 (CI=.17–.19). Although the Hosmer and Lemeshow goodness-of-fit statistic indicated an adequate model fit—as demonstrated by the nonsignificant chi square test—the global fit of this model was only moderate (pseudo-R2=.35, with 61.9% correctly classified at the .5 cutoff) (56).
Discussion
We report three significant findings from this study. First, VISNs were heterogeneous in terms of the timing and speed of adoption of aripiprazole. In aggregate, some VISN-level prescribing practices responded most strongly to the publishing of scientific evidence for the efficacy of aripiprazole, whereas other VISN rates changed most after ultimate FDA approval of aripiprazole for the treatment of bipolar disorder. Second, both regulatory and scientific “approval” appeared to be important and immediate influences on prescribing patterns of second-generation antipsychotics, as demonstrated by the significant inflection points in each of the monthly time series. Third, our exploratory analyses using regional-level data showed that access to care may have had a permissive effect on uptake, whereas competing demands (such as response to substance abuse treatment needs) may have retarded adoption.
This study advances our understanding of how antipsychotic medications diffuse in large health care systems such as the VHA, particularly during the period preceding FDA approval of new indications. A previous study of the diffusion of ziprasidone (29) reported that patient-level and medical center–level factors were significant predictors of uptake. However, the study investigated uptake for the approved indication (schizophrenia) and covered the three-year period after the initial marketing of ziprasidone. Thus that study was not designed to examine the impact of changes in the extant evidence base for the use of ziprasidone or changes in prescribing with new FDA-approved indications for ziprasidone. Similarly, Pinarella and colleagues (1) studied the impact of FDA approval of olanzapine for treating bipolar disorder but did not evaluate events that occurred before approval.
Similar to system-level investigations in Medicaid-insured populations (13), we did not identify any significant VISN-level policy, administrative, or staffing predictors of the growth rate in aripiprazole (including the level of academic affiliation). Lack of association may point to identifying the levers or characteristics associated with more diffusion or less diffusion from an understanding of bottom-up decision making, at the individual provider level, consistent with classic diffusion theory (6).
Administrative factors not evident from VISN-level analyses may yet play an important role. In their systematic review, Greenhalgh and colleagues (57) noted several limitations concerning the applicability of individual-level diffusion theory to modern health care. Dearing (44,45) proposed reorienting diffusion theory to emphasize societal sectors, defined as “collections of focal organizations operating in the same topical domain, regardless of physical proximity . . . identified by the similarity of their services, products, or functions, together with those organizations that critically influence the performance of the focal organizations” (44). These sectors may be constituted at the organizational level and tied together by administrative structures that define and support specific functions such as managing innovation spread. Further research incorporating patient, clinician, and health system covariates simultaneously is needed to identify the relative importance of each of these factors.
Our analysis was limited by the use of regional data but not by data at the patient or medical center levels. Our goal was to identify system-level covariates, and VISNs are an appropriate unit of analysis for this goal. However, our ability to identify system-level covariates was limited by the small sample (N=21 VISNs). Further research at the medical center and prescriber levels would provide a more powerful approach to identifying administrative, policy, and other local factors in prescribing as well as their interaction. We also had no information on pharmaceutical detailing to providers and advertising to consumers. Our study cannot distinguish between scientific communication between scientist and clinician—via peer-reviewed articles, for example—and promotional communication from pharmaceutical industry to clinician that makes use of scientific data.
We could not parse the impact of research reporting the reduced cardiometabolic side effects of aripiprazole from research reporting harms associated with other second-generation antipsychotic medications. Eli Lilly and the FDA issued a warning and required a label change in March 2004 regarding the increased risk of diabetes and hyperglycemia for patients taking these medications. The FDA also issued a black box warning in April 2005 regarding the increased risk of mortality among elderly patients with dementia who were taking these medications. Thus the inflections observed in our times series for aripiprazole likely reflect the cumulative impact of available evidence. Nevertheless, the impact of this information was heterogeneous across VISNs.
Finally, our study focused on a single psychotropic agent within one therapeutic class. It is unclear whether other pharmaceutical agents or innovative medical devices would show a similar pattern of adoption over time among the same VISNs.
Conclusions
Early published evidence may have a strong impact on prescribing practices for low-barrier innovations such as newly marketed medications or changes in indication for approved medications. Results also suggest that it may be useful to establish early prescriber behavior interventions that target certain geographic or administratively defined populations of clinicians, thereby maximizing efficiency, particularly if certain regions are found to be “early adopters” across a range of new medications and practices. Similarly, delineating administrative factors that influence prescriber behavior, and their interaction with diffusion-based factors (36,37), will provide the most comprehensive model of prescriber behavior to date. Identifying such factors will permit prospectively targeted interventions when new medications and practices are being developed.
1 : Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998–2009. Psychiatric Services 63:83–86, 2012Link, Google Scholar
2 : Benefits and risks of increasing restrictions on access to costly drugs in Medicaid. Health Affairs 23(1):135–146, 2004Crossref, Google Scholar
3 : Use of atypical antipsychotic drugs for schizophrenia in Maine Medicaid following a policy change. Health Affairs 27:w185–w195, 2008Crossref, Google Scholar
4 : Unintended impacts of a Medicaid prior authorization policy on access to medications for bipolar illness. Medical Care 48:4–9, 2010Crossref, Medline, Google Scholar
5 : Delays in adopting evidence-based dosages of conventional antipsychotics. Psychiatric Services 52:1242–1244, 2001Link, Google Scholar
6. : Diffusion of Innovations, 5th ed. New York, Free Press, 2003Google Scholar
7 : Variation in implementation and use of computerized clinical reminders in an integrated healthcare system. American Journal of Managed Care 10:878–885, 2004Medline, Google Scholar
8 : Initial experience with patient-clinician secure messaging at a VA medical center. Journal of the American Medical Informatics Association 16:267–270, 2009Crossref, Medline, Google Scholar
9 : Apples don’t fall far from the tree: influences on psychotherapists’ adoption and sustained use of new therapies. Psychiatric Services 60:671–676, 2009Link, Google Scholar
10 : Initiation of assertive community treatment among veterans with serious mental illness: client and program factors. Psychiatric Services 60:196–201, 2009Link, Google Scholar
11 : Prevalence and factors associated with off-label antidepressant prescriptions for insomnia. Journal of Drug, Healthcare and Patient Safety 3:27–36, 2011Crossref, Medline, Google Scholar
12 : Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Affairs 28:w770–w781, 2009Crossref, Medline, Google Scholar
13 : Spatio-temporal clusters of new psychotropic medications among Michigan children insured by Medicaid. Pharmacoepidemiology and Drug Safety 18:531–539, 2009Crossref, Medline, Google Scholar
14 : Pediatric uptake of a newly available antipsychotic medication. Pediatrics 125:475–482, 2010Crossref, Medline, Google Scholar
15 : Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiology and Drug Safety 20:177–184, 2011Crossref, Medline, Google Scholar
16 : Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA 306:1359–1369, 2011Crossref, Medline, Google Scholar
17 : Accelerating the diffusion of innovations using opinion leaders. Annals of the American Academy of Political and Social Science 566:55–67, 1999Crossref, Google Scholar
18 : Identifying opinion leaders to promote behavior change. Health Education and Behavior 34:881–896, 2007Crossref, Medline, Google Scholar
19 : Characteristics and impact of drug detailing for gabapentin. PLoS Medicine 4:e134, 2007Crossref, Medline, Google Scholar
20 : Eli Lilly pays record $1.4bn for promoting off-label use of olanzapine. BMJ 338:b217, 2009Crossref, Medline, Google Scholar
21 : The promotion of olanzapine in primary care: an examination of internal industry documents. Social Science and Medicine 69:14–20, 2009Crossref, Medline, Google Scholar
22 : Bitter pills for drug companies. BMJ 341:c5095, 2010Crossref, Medline, Google Scholar
23 Mental Health: A Report of the Surgeon General. Washington, DC, US Department of Health and Human Services, 1999. Available at www.surgeongeneral.gov/library/mentalhealth/home.htmlGoogle Scholar
24 : Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC, National Academies Press, 2001Google Scholar
25 : Strategies for disseminating evidence-based practices to staff who treat people with serious mental illness. Psychiatric Services 52:1598–1606, 2001Link, Google Scholar
26 : Policy implications for implementing evidence-based practices. Psychiatric Services 52:1591–1597, 2001Link, Google Scholar
27 Achieving the Promise: Transforming Mental Health Care in America. Pub no SMA-03-3832. Rockville, Md, Department of Health and Human Services, President’s New Freedom Commission on Mental Health, 2003Google Scholar
28 : The decision to adopt evidence-based and other innovative mental health practices: risky business? Psychiatric Services 57:1153–1161, 2006Link, Google Scholar
29 : Patient- and facility-level factors associated with diffusion of a new antipsychotic in the VA health system. Psychiatric Services 57:70–76, 2006Link, Google Scholar
30 : Use of surveillance data in developing geographic dissemination strategies: a study of the diffusion of olanzapine to Michigan children insured by Medicaid. Clinical Therapeutics 29:357–370, 2007Crossref, Google Scholar
31 : Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clinical Therapeutics 26:649–666, 2004Crossref, Medline, Google Scholar
32 : Aripiprazole versus typical antipsychotic drugs for schizophrenia. Cochrane Database of Systematic Reviews 3:CD006617, 2008Google Scholar
33 : Prescribing conventional antipsychotics at two Veterans Administration hospitals: are there geographical differences? CNS Spectrums 6:894–896, 2001Crossref, Medline, Google Scholar
34 : Differential adoption of pharmacotherapy recommendations for type 2 diabetes by generalists and specialists. Medical Care Research and Review 60:178–200, 2003Crossref, Medline, Google Scholar
35 : Geographic variation in the quality of prescribing. New England Journal of Medicine 363:1985–1988, 2010Crossref, Medline, Google Scholar
36 : Geographic variation in cardioprotective antihypertensive medication usage in dialysis patients. American Journal of Kidney Diseases 58:73–83, 2011Crossref, Medline, Google Scholar
37 : Tracing the flow of knowledge: geographic variability in the diffusion of prazosin use for the treatment of posttraumatic stress disorder nationally in the Department of Veterans Affairs. Archives of General Psychiatry 66:417–421, 2009Crossref, Medline, Google Scholar
38 : Social contagion and innovation: cohesion versus structural equivalence. American Journal of Sociology 92:1287–1335, 1987Crossref, Google Scholar
39 : Social network thresholds in the diffusion of innovations. Social Networks 18:69–89, 1996Crossref, Google Scholar
40 : Medical innovation revisited: social contagion versus marketing effort. American Journal of Sociology 106:1409–1435, 2001Crossref, Google Scholar
41 : Social contagion and income heterogeneity in new product diffusion: a meta-analytic test. Marketing Science 23:530–544, 2004Crossref, Google Scholar
42 : Innovation diffusion in heterogeneous populations: contagion, social influence, and social learning. American Economic Review 99:1899–1924, 2009Crossref, Google Scholar
43 : Opinion leadership and social contagion in new product diffusion. Marketing Science 30:195–212, 2011Crossref, Google Scholar
44 : Evolution of diffusion and dissemination theory. Journal of Public Health Management and Practice 14:99–108, 2008Crossref, Medline, Google Scholar
45 : Applying diffusion of innovation theory to intervention development. Research on Social Work Practice 19:503–518, 2009Crossref, Medline, Google Scholar
46 : Characteristics of patients with bipolar disorder managed in VA primary care or specialty mental health care settings. Psychiatric Services 61:500–507, 2010Link, Google Scholar
47 : Quality of general medical care among patients with serious mental illness: does colocation of services matter? Psychiatric Services 62:922–928, 2011Link, Google Scholar
48 : A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. American Journal of Psychiatry 160:1651–1658, 2003Link, Google Scholar
49 : Aripiprazole: in acute mania associated with bipolar I disorder. CNS Drugs 18:367–378, 2004Crossref, Medline, Google Scholar
50 Supplemental New Drug Application Approval Letter re: NDA 21-436/S-005. Washington, DC, Food and Drug Administration, March 1, 2005. Available at www.accessdata.fda.gov/drugsatfda_docs/appletter/2005/21436s005,008,21713s003ltr.pdfGoogle Scholar
51 Supplemental New Drug Application Approval Letter re: NDA 21-436/S-002. Washington, DC, Food and Drug Administration, Sept 29, 2004. Available at www.accessdata.fda.gov/drugsatfda_docs/appletter/2004/21436s002ltr.pdfGoogle Scholar
52 : Financial incentives and accountability for integrated medical care in Department of Veterans Affairs mental health programs. Psychiatric Services 61:38–44, 2010Link, Google Scholar
53 : Job characteristics and job satisfaction among physicians involved with research in the Veterans Health Administration. Academic Medicine 86:938–945, 2011Crossref, Medline, Google Scholar
54 : Experience of VA psychiatrists with pharmaceutical detailing of antipsychotic medications. Psychiatric Services 58:1292–1296, 2007Link, Google Scholar
55 : Knowledge and use of finasteride for the prevention of prostate cancer. Cancer Epidemiology, Biomarkers and Prevention 19:2164–2171, 2010Crossref, Medline, Google Scholar
56 : Applied Logistic Regression, 2nd ed. New York, Wiley, 2000Crossref, Google Scholar
57 : Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Quarterly 82:581–629, 2004Crossref, Medline, Google Scholar



