Experience of VA Psychiatrists With Pharmaceutical Detailing of Antipsychotic Medications
There has been much recent interest in effectiveness research in the treatment of schizophrenia. Recent independent studies such as Clinical Antipsychotic Trials in Intervention Effectiveness (CATIE) ( 1 ) and Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS) ( 2 ) have raised doubts about the symptom and side effect advantages of second-generation antipsychotics and have raised questions about how these medications came to dominate the market, with over 90% of antipsychotic prescriptions being written for these drugs ( 3 ). Although the decision to prescribe one medication over another is a complex one, prescriber knowledge clearly plays a role, and an important source of information for psychiatrists is representatives of the pharmaceutical industry.
Although the relationship between physicians and the pharmaceutical industry has become a subject of increased interest and concern ( 4 ), little is known about the nature of the interaction between physicians and sales personnel on the subject of antipsychotic drugs. Billions of dollars are spent on marketing to physicians each year using a greatly expanded detailing workforce ( 5 ). Extensive product-promoting contacts occur between industry representatives and medical students at the beginning of students' careers ( 6 ), and increasingly close relationships have emerged between clinical investigators and industry ( 7 ). Questions about the role of pharmaceutical companies in continuing medical education ( 8 ) have also led physician leaders to call for reform ( 9 , 10 ). Concern about these issues has recently resulted in the promulgation of guidelines from academia ( 10 ), industry ( 11 ), the American Medical Association ( 12 ), and the federal government ( 13 ) seeking to attenuate the influence of industry on physicians' prescribing behavior. These relationships have also been discussed extensively in the lay press ( 14 ).
Although recent reviews have addressed ethical dilemmas associated with receiving gifts from pharmaceutical companies ( 15 ), the frequency of such gift giving ( 16 ), and the effect of free samples on prescribing ( 17 ), the study presented here was designed to investigate the less easily quantifiable exchange of information that occurs when physicians interact with representatives of the pharmaceutical industry. Although there have been reports examining the accuracy of pharmaceutical materials ( 18 ), types of materials ( 19 ), and the selective and overly positive information ( 20 ) distributed by company representatives, these reports have not addressed the specific kinds of information psychiatrists report as being conveyed by company representatives.
This study, accordingly, focuses on information exchanges between company sales personnel and psychiatrists regarding the performance of second-generation antipsychotics, because these medications are widely used and represent a substantial cost to consumers and health care organizations. In 2005 approximately $10 billion was spent on second-generation antipsychotics, and the $3 billion dollars spent by Medicaid made it the most costly group of medications provided by that program ( 21 ). Clozapine, with its unique side effect profile, monitoring requirements, and target population, was not included in this survey.
We surveyed psychiatrists practicing within Department of Veterans Affairs (VA) medical centers throughout the country for the purpose of characterizing the experiences of this group of physicians with pharmaceutical representatives for each of the manufacturers of second-generation antipsychotics. Assuming information is communicated in several ways, we inquired about direct speech, implied meanings, sponsoring others to speak, or providing literature to the prescribers. We first hypothesized that industry representatives would be more likely to report information included in the package insert, and thus approved by the U.S. Food and Drug Administration (FDA), and less likely to report information not included in the labeling. We further hypothesized that nonapproved communications would be less likely to be communicated by direct speech and more likely to be communicated by implication, sponsoring others to speak, or providing literature to the prescribers.
Methods
Sample
The population of interest for this study consisted of salaried psychiatrists of the VA who were listed in the current VA Personnel and Accounting Integrated Data (PAID) file, had an occupational code recorded as "psychiatrist," and had an active e-mail account in the same name on the internal VA e-mail server in June and July of 2005. Each eligible physician who fulfilled these criteria was sent an e-mail message that described the purpose of the study along with an invitation to take a Web-based survey on an Intranet site. Respondents were offered confidentiality but also provided with an opportunity to register an e-mail address to be provided with the results of this survey. Second and third e-mail reminders were sent at two-week intervals to all identified potential participants.
Sources of data
The survey consisted of a series of 18 questions that were answered by using "radio buttons" or pull-down menus. Answers were recorded and collected by a research group within the VA that was contracted to develop the e-mail list, administer the survey, and maintain the Intranet site.
Institutional review board approval for this study was obtained from the VA Connecticut Healthcare System Institutional Review Board.
Measures
Respondents were first asked whether they had contact with representatives of each of the pharmaceutical companies currently marketing second-generation antipsychotic medications other than clozapine in the United States: Janssen Pharmaceutica (risperidone), Eli Lilly (olanzapine), AstraZeneca (quetiapine), Pfizer (ziprasidone), and Bristol-Myers Squibb (aripiprazole). Contacts were characterized as occurring within the past year, prior years, both, or neither. Respondents were also asked to describe how many times they had met with a pharmaceutical representative or had attended either a continuing medical education (CME) course or a social event sponsored by a pharmaceutical company in the previous year.
Respondents were then questioned about whether any of the pharmaceutical representatives made each of eight assertions. These assertions were any of the following: Our second-generation antipsychotic results in greater symptom reduction than placebo, better positive symptom control than first-generation antipsychotics, better negative symptom control than first-generation antipsychotics, better positive or negative symptom control than another second-generation antipsychotic, decreased risk of tardive dyskinesia as compared to other medications, decreased risk of extrapyramidal symptoms, increased risk of the development of diabetes mellitus, or net monetary savings through decreasing hospitalizations. For each of these assertions, psychiatrists were asked to answer "yes" or "no" as to whether the company representative directly said it, implied it, presented publications supporting the assertion, presented publications that included evidence both for and against this assertion (whether a publication met this definition was left to the respondent—these are referred to as balanced publications in this article), or sponsored a speaker who said it.
Of the seven assertions that could be characterized as favorable to these medications, only one (superiority to placebo) is explicitly endorsed in the package insert material, although information on side effects clearly lends support to claims of a decreased risk of extrapyramidal symptoms in comparison with the information available on the older drugs ( 22 , 23 , 24 , 25 , 26 ). Another assertion—increased risk of diabetes—is generally endorsed by the package insert ( 22 , 23 , 24 , 25 , 26 ).
Respondents were also asked three questions concerning their own beliefs about second-generation antipsychotics—that is, whether they believed that use of second-generation antipsychotics results in greater symptom reduction than placebo, whether use results in better positive symptom control than first-generation antipsychotics, or whether they thought that any of the second-generation drugs (other than clozapine) are generally more effective than the others.
Finally, respondents were asked some details about their practice, including number of patients with schizophrenia treated in the previous year, in which of the 21 VA regions (VISNs) they were employed, their age, and number of years practicing as a psychiatrist.
Results
A total of 2,043 psychiatrists were identified in the VA PAID database. Within this group 1,919 (94%) had a listed VA e-mail address. A total of 86 e-mails (4%) "bounced back," leaving a target group of 1,833 identified psychiatrists with VA e-mail addresses who were sent the e-mail solicitation.
Of the 1,833 potential participants, 639 (35%) visited the Web site and answered the survey questions. All responses were recorded between June 20, 2005, and July 25, 2005. Of those who completed the survey, 558 (87%) identified themselves as psychiatrists who had at least one contact with pharmaceutical industry representatives. This is the sample whose answers were available for analysis.
This response rate is in keeping with other e-mail-based surveys performed within the VA on other subjects, such as one targeting all physicians (15% response rate) and one specifically addressing primary care physicians (43% response rate) (Goff C, private communication, 2007).
Demographic data and experience
Responses indicated that the psychiatrists in the sample had treated a mean±SD of 120±449 patients (range of 0–1,045) with a diagnosis of schizophrenia in the year before the survey. During that same time they also reported meeting with pharmaceutical representatives an average of 14±26 times (range of 1–300) and having attended a continuing medical education event or a social event sponsored by a pharmaceutical company 5±7 times (range of 0–50). This average reported rate of contact with industry representatives can be compared with one found in a summary of 29 studies which found an average reported frequency of contact among physicians of three or four times per month ( 16 ). Forty-seven percent (N=265) of respondents were between 30 and 50 years old, another 34% (N=188) were between 51 and 60 years old, and 19% (N=105) were older than 60. Most (325 respondents, or 58%) had been practicing for ten to 30 years, whereas another 15% (N=85) had more than 30 years experience.
Experience with pharmaceutical sales representatives
Survey respondents were asked to characterize their contacts in the past year and in previous years with representatives from the various pharmaceutical companies offering antipsychotic medications in the United States. In the past year the percentage of respondents reporting contact with each particular company varied from 58% to 70%. Reported contact in the years before the current one was somewhat higher and ranged from 65% to 89% among the five companies.
Figure 1 shows the five types of communications as well as communications by any of these methods. The most common assertions made at any time in the past through any modality of communication were that second-generation antipsychotics yielded greater symptom reduction than placebo (N=525, 94%), better negative symptom control than first-generation antipsychotics (N=506, 91%), decreased risk of extrapyramidal symptoms (N=501, 90%), and decreased risk of tardive dyskinesia compared with other medications" (N=472, 85%). Only half of these statements, those concerning placebo and extrapyramidal symptoms, were suggested by the package insert.
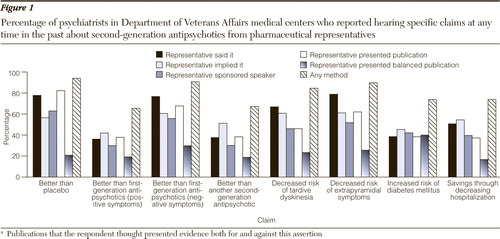
The least frequent assertions were that second-generation antipsychotics afford better positive symptom control than first-generation antipsychotics (N=365, 65%), afford better positive or negative symptom control than another second-generation antipsychotic (N=375, 67%), result in an increased risk of the development of diabetes mellitus (N=412, 74%), and result in a net monetary savings through decreasing hospitalizations (N=412, 74%). Of these four assertions, only one was suggested by the package insert—that second-generations increase the risk of diabetes.
These data support our primary hypothesis because among the most common assertions, half were consistent with the package insert, while among the least common assertions only one-fourth were consistent with the package insert. Because the assertion consistent with the package insert in the least frequent group detailed a risk, not a benefit, none of the positive statements that were least frequently asserted were endorsed by the package insert.
The three assertions most commonly reported to have been made through direct speech ( Figure 1 ) were that the second-generation antipsychotic marketed by the representative's company resulted in a decreased risk of extrapyramidal symptoms (N=440, 79%), yielded greater symptom reduction than placebo (N=434, 78%), or offered better negative symptom control than first-generation antipsychotics (N=430, 77%), two-thirds of which (decreased risk of extrapyramidal symptoms and superiority to placebo) were consistent with the package insert. Of statements least likely to be reported directly—better positive symptom control than first-generation antipsychotics (N=202, 36%), better positive or negative symptom control than another second-generation antipsychotic (N=210, 38%), and increased risk of the development of diabetes mellitus (N=215, 39%)—only the decreased risk of diabetes was consistent with the package insert. Consistent with our second hypothesis, statements commonly made by direct speech were more likely to be consistent with the package insert, and among the least frequent assertions made directly, the only one consistent with the package insert detailed a risk, not a benefit.
In further consideration of our second hypothesis, we expected to see increased communication of statements not supported by the package insert using indirect means of communication. However, statements not supported by the package insert such as the assertions of superiority to first-generation antipsychotics on negative symptoms and tardive dyskinesia all showed less frequency of indirect communication than direct communication and did not support the second hypothesis.
Prescriber beliefs
Survey respondents were also asked about what they themselves believed. Ninety-eight percent (N=547) agreed that the use of second-generation antipsychotics results in greater symptom reduction than placebo, whereas only 23% (N=130) agreed that the use of second-generation antipsychotics results in better positive symptom control than first-generation antipsychotics and only 30% (N=170) agreed that any of the second-generation antipsychotics (other than clozapine) is generally more effective than any other.
Discussion
In this survey of psychiatrists practicing within VA medical centers, more than four out of every five respondents reported having had at least one contact with representatives of the pharmaceutical companies that sell second-generation antipsychotic medication, and among this group, contact occurred quite frequently, at an average of 14 times per year. Respondents also frequently attended CME courses and other events supported by these companies. There was some variability as to the degree of contact with representatives of individual companies, but nearly two-thirds of these psychiatrists, a group that reported extensive experience in treating patients with schizophrenia, reported meeting with representatives of even the company least often encountered.
The Federal Food, Drug, and Cosmetic Act (1906) and the Kefauver-Harris Drug Amendments (1962) require that drugs be proven safe and effective before their marketing in the United States and designate the FDA with enforcing these acts. One of the important functions of the FDA is to negotiate with the manufacturer about the information that will appear in the package insert and that may be used in advertising. This document (along with balanced, peer-reviewed articles) provides the basis for what representatives of the manufacturer are authorized by the FDA to state when speaking with health care providers about their product. The package inserts for each of the second-generation antipsychotics are easily accessed at Web sites via the Internet ( 22 , 23 , 24 , 25 , 26 ) or in the Physicians' Desk Reference, and each insert specifies approved indications and side effects.
The package insert materials also assert that "epidemiological studies have suggested treatment-emergent hyperglycemia-related adverse events in patients treated with atypical antipsychotics"—although it is noted that ziprasidone and aripiprazole were not marketed at the time these studies were performed.
Perhaps the most striking observation is that when all modes of interactions are combined, all of the assertions studied were commonly made. Consistent with our first hypothesis we found that of the most common assertions made through any means, half were consistent with the package insert, whereas among the least common assertions only one-fourth were consistent with the package insert. Thus, although the most commonly made assertions were more often consistent with the package insert than the least commonly made ones, these differences do not appear substantial. Our second hypothesis, that statements not supported by the package insert would be made through indirect means, was not consistently supported, because these types of statements were often made and often made directly. In other words, whether a statement was consistent with the package insert did not predict the mode of assertion. It is worth noting, however, that the one adverse statement we asked about concerning risk of diabetes—which was also consistent with the package insert—was among the least frequently endorsed and was more often made indirectly than directly
When asked about their own professional opinions, respondents were nearly unanimous in their belief, consistent with FDA-approved information, that these medications are superior to placebo, but less than a third agreed with either of the two assertions not approved by the FDA—that these drugs are superior to first-generation antipsychotics or to other second-generation antipsychotics. They thus concurred far more often with FDA-approved statements than with the nonapproved statements, just as the industry sales personnel were more likely to make the approved statements than the nonapproved statements. Nonetheless, as discussed in the introduction, the self-reported independence of practitioners may not be nearly as great as they believe it to be ( 27 ).
Several limitations of this study deserve mention. First, these observations are the result of voluntary participation in a survey. Whether the third of the eligible psychiatrists who participated differ either from other VA psychiatrists or psychiatrists in general is unknown. Second, the nature of VA practice, especially in regard to the type of psychiatrists who work in this salary-based, commonly academically affiliated practice and the degree to which some hospitals restrict access to pharmaceutical representatives may limit generalizability to other practice situations. Third, this survey relied on the recall of respondents, and there are no objective data documenting the information that was communicated.
Nevertheless, respondents in this study clearly described extensive contact with representatives from the manufacturers of second-generation antipsychotics in which information about these medications was communicated by direct conversation, by sponsored speakers, and by informational handouts. Half of the most commonly reported assertions were consistent with the FDA-approved prescribing information, compared with only one-quarter of less commonly made assertions. Self-reported clinical beliefs were consistent in providing more support for the approved statements and less support for the nonapproved statements.
Conclusions
These findings suggest that there have been substantial discrepancies between information clinicians report having received in their contacts with industry representatives and FDA-approved information. It is some consolation that among the most common communications, FDA-approved statements are more frequent and consistent with prescriber beliefs. Nevertheless, many assertions are not consistent with the package insert, but the observation that these are less often endorsed by clinicians suggests at least some exercise of independent judgment among professionals.
Acknowledgments and disclosures
Dr. Sernyak has received honoraria from and served as a consultant to Pfizer. Dr. Rosenheck has received research support from Eli Lilly, Janssen Pharmaceutica, AstraZeneca, and Wyeth. He has also been a consultant to GlaxoSmithKline, Bristol-Myers Squibb, and Janssen Pharmaceutica. He has been retained as an expert witness in United Food and Commercial Workers Union Local 1776 and Participating Employers, Health and Welfare Fund, Eric Tayag and Mid-West National Life Insurance Company of Tennessee on behalf of themselves and similarly situated Plaintiffs v. Eli Lilly and Company.
1. Lieberman JA, Stroup S, McEvoy J, et al: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia: primary efficacy and safety outcomes of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial. New England Journal of Medicine 353:1209–1223, 2005Google Scholar
2. Jones PB, Barnes TRE, Davies L, et al: Randomized controlled trial of effect on quality of life of second generation versus first generation antipsychotic drugs in schizophrenia (CUtLASS 1). Archives of General Psychiatry 63:1079–1087, 2006Google Scholar
3. Lieberman JA: Comparative effectiveness of antipsychotic drugs: a commentary on: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) and Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). Archives of General Psychiatry 63:1069–1072, 2006Google Scholar
4. Please hold the free lunches. New York Times, Aug 4, 2006, p A16Google Scholar
5. Brodkey A: The role of the pharmaceutical industry in teaching psychopharmacology: a growing problem. Academic Psychiatry 29:222–229, 2005Google Scholar
6. Sierles FS, Brodkey AC, Cleary LM, et al: Medical students' exposure to and attitudes about drug company interactions: a national survey. JAMA 294:1034–1042, 2005Google Scholar
7. Bodenheimer T: Uneasy alliance: clinical investigators and the pharmaceutical industry. New England Journal of Medicine 342:1539–1544, 2000Google Scholar
8. Relman A: Separating continuing medical education from pharmaceutical marketing. JAMA 285:2009–2012, 2001Google Scholar
9. Angell M: Is academic medicine for sale? New England Journal of Medicine 342:1516–1518, 2000Google Scholar
10. Brennan T, Rothman D, Blank L, et al: Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA 295:429–433, 2006Google Scholar
11. The PhRMA Code on Interactions With Healthcare Providers. Pharmaceutical Research and Manufacturers of America. Available at www.phrma.org/files/phrma%20code.pdfGoogle Scholar
12. The Communication of Ethical Guidelines for Gifts to Physicians From Industry. American Medical Association. Available at www.ama-assn.org/ama/pub/category/8405.htmlGoogle Scholar
13. OIG compliance program guidance for pharmaceutical manufacturers. Federal Register 68:23731–23743, 2003. Available at oig.hhs.gov/authorities/docs/03/050503FRCPGpharmac.pdfGoogle Scholar
14. Elliott C: The drug pushers. Atlantic Monthly, Apr 2006, pp 82–93Google Scholar
15. Dana J, Lowenstein G: A social science perspective on gifts to physicians from industry. JAMA 290:252–255, 2003Google Scholar
16. Wazana A: Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA 283:373–380, 2000Google Scholar
17. Adair R, Holmgren L: Do drug samples influence resident prescribing behavior? A randomized trial. American Journal of Medicine 118:881–884, 2005Google Scholar
18. Ziegler M, Pauline L, Singer B: The accuracy of drug information from pharmaceutical sales representatives. JAMA 273:1296–1298, 1995Google Scholar
19. Stryer D, Bero L: Characteristics of materials distributed by drug companies: an evaluation of appropriateness. Journal of General Internal Medicine 11:575–583, 1996Google Scholar
20. Lexchin J: What information do physicians receive from pharmaceutical representatives? Canadian Family Physician 43:941–945, 1997Google Scholar
21. Sharfstein S: Antipsychotics, economics, and the press. Psychiatric News 40:3, 2005Google Scholar
22. Zyprexa (olanzapine) prescribing information. Indianapolis, Ind, Eli Lilly and company. Available at pi.lilly.com/us/zyprexa-pi.pdfGoogle Scholar
23. Risperdal (risperidone) prescribing information. Titusville, NJ, Janssen. Available at www.risperdal.com/risperdal/shared/pi/risperdal.pdfGoogle Scholar
24. Seroquel (quetiapine) prescribing information. Wilmington, Del, AstraZeneca Pharmaceuticals. Available at www.astrazeneca-us.com/pi/seroquel.pdfGoogle Scholar
25. Abilify (aripiprazole) prescribing information. Princeton, NJ, Bristol-Myers Squibb Company. Available at www.bms.com/cgi-bin/anybin.pl?sql=select%20PPI%20from%20TBPRODUCTPPI%20where%20PPISEQ=101&key=PPIGoogle Scholar
26. Geodon (ziprasidone) prescribing information. New York, Pfizer. Available at www.pfizer.com/pfizer/download/uspigeodon.pdfGoogle Scholar
27. Avorn J, Chen M, Hartley R: Scientific versus commercial sources of influence on the prescribing behavior of physicians. American Medical Journal 73:4–8, 1982Google Scholar



