Adjunctive Divalproex and Hostility Among Patients With Schizophrenia Receiving Olanzapine or Risperidone
Abstract
OBJECTIVE: This study compared the specific antihostility effects of atypical antipsychotic monotherapy (olanzapine or risperidone) with that of combination treatment with divalproex sodium among patients with schizophrenia experiencing an acute psychotic episode. METHODS: A total of 249 inpatients with schizophrenia were randomly assigned to receive olanzapine plus placebo, olanzapine plus divalproex, risperidone plus placebo, or risperidone plus divalproex in a double-blind, 28-day multicenter trial. The target daily dose was 15 milligrams for olanzapine, 6 milligrams for risperidone, and up to 30 milligrams per kilogram (minimum, 15 milligrams per kilogram) for divalproex. The hostility item of the Positive and Negative Syndrome Scale (PANSS) was the principal outcome measure. Covariates included the PANSS items reflecting positive symptoms of schizophrenia (delusions, suspiciousness/persecution, grandiosity, unusual thought content, conceptual disorganization, and hallucinatory behavior). RESULTS: Combination treatment with risperidone or olanzapine plus divalproex was associated with different scores on the hostility item of the PANSS compared with antipsychotic monotherapy. Combination therapy had a significantly greater antihostility effect at days 3 and 7 than monotherapy. This result was not seen beyond the first week of treatment, but there was a trend toward a difference in effect for the entire treatment period. The effect on hostility appears to be statistically independent of antipsychotic effect on other PANSS items reflecting delusional thinking, a formal thought disorder, or hallucinations. CONCLUSIONS: Divalproex sodium may be useful as an adjunctive agent in specifically reducing hostility in the first week of treatment with risperidone or olanzapine among patients with schizophrenia experiencing an acute psychotic episode.
Some patients with a diagnosis of schizophrenia are occasionally hostile and violent. Violent or threatening behavior is a frequent reason for admission to a psychiatric inpatient facility. Moreover, if such behaviors continue after admission, they can prolong hospitalization and interfere with discharge.
Risperidone was shown to have a selective effect on hostility that was superior to that of haloperidol among patients with acute schizophrenia (1), as measured by the Positive and Negative Syndrome Scale (PANSS) (2) among patients enrolled in the double-blind randomized multicenter trial of risperidone conducted in the United States (3). However, there have also been negative reports (4,5).
Olanzapine was shown to result in greater improvement in agitation—as defined by the Brief Psychiatric Rating Scale (BPRS) agitation score, comprising the items of anxiety, tension, hostility, uncooperativeness, and excitement—than that achieved with haloperidol among patients with schizophrenia enrolled in a six-week, double-blind, randomized multicenter clinical trial (6). However, whether this improvement was independent of the improvement in hallucinations, delusions, and thought disorder was not reported.
Neither risperidone nor olanzapine showed superiority over haloperidol in their effects on hostility in a 14-week, double-blind, randomized clinical trial of patients with schizophrenia or schizoaffective disorder (7). In that study, clozapine was associated with a significantly greater antihostility effect than either haloperidol or risperidone. The effect on hostility was independent of antipsychotic effect on delusional thinking, formal thought disorder, or hallucinations and was independent of sedation. However, all patients in the study had a history of suboptimal response to conventional antipsychotics. Thus it may not be possible to generalize the results to patients whose illness is not treatment refractory.
It is generally expected that adjunctive mood stabilizers can reduce aggressive and impulsive behavior (8). Expert consensus guidelines suggest the use of adjunctive valproate—the active moiety of valproic acid and divalproex sodium—for patients with schizophrenia who exhibit agitation, excitement, aggression, or violence (9). The use of valproate among patients with schizophrenia is extensive (10,11). However, the literature on the use of valproate specifically for aggressive behavior in schizophrenia is limited.
Four patients with treatment-resistant schizophrenia were given valproate in addition to their antipsychotic medication, resulting in a reduction of positive symptoms as measured by the BPRS and a reduction of hostile or disruptive behavior (12). Two patients with schizophrenia and one with schizoaffective disorder were given valproic acid in addition to antipsychotics in an effort to control severe antipsychotic-resistant psychotic symptoms, with good results (13).
A retrospective review of 147 hospitalized patients concluded that valproate was helpful as a calmative adjunct for aggressive patients with schizophrenia (14). A one-year open-label prospective trial of adjunctive valproate with olanzapine among ten patients with paranoid schizophrenia demonstrated statistically significant reductions in hostility (15). A recent review of the use of valproate to address violence and aggressive behaviors among patients with a variety of diagnoses (16) revealed a 77 percent response rate (defined by a 50 percent reduction in target behavior) based on 17 reports (164 patients), but only 16 patients with schizophrenia were included.
The antiaggressive effect of adjunctive valproate and that of antipsychotic monotherapy have not yet been directly compared in a controlled prospective study. The data reported in this article were obtained during the conduct of a large-scale, multicenter, prospective, double-blind trial designed to examine the safety and efficacy of atypical antipsychotic monotherapy with risperidone or olanzapine compared with that of combination therapy with divalproex (17).
In this article we present the results of the secondary hypothesis (post hoc) that a reduction of the PANSS hostility item would occur during combination therapy and that the reduction would be greater than that observed during monotherapy. We hypothesized that the reduction of hostility would be selective in the sense that the effect on hostility would be independent of the effect on other positive items as measured by the PANSS.
Methods
The study was a prospective four-week, randomized, double-blind, parallel-group clinical trial conducted at 29 centers in the United States. Institutional review board approval was obtained, and written informed consent was required. Data were collected from January 26, 2000, to February 20, 2001. Further details can be found in the original report (17). The main entry criteria were that patients had to be men or nonpregnant, nonlactating women between the ages of 18 and 65 years; had to have a current DSM-IV diagnosis of schizophrenia (based on the Structured Clinical Interview for DSM-IV Axis I Disorders); had to be hospitalized for acute exacerbation of schizophrenia; had to have a minimum total score of 60 on the PANSS (items based on a scale of 1 to 7; the theoretical range for the total score is 30 to 210); had to have a sum of at least 8 on any two of the following four PANSS items: hallucinatory behavior, unusual thought content, conceptual disorganization and suspiciousness/persecution; had to have a score of at least 6 on one of the following two PANSS item pairs: hostility and uncooperativeness, or excitement and tension; had to have no current diagnosis of schizoaffective disorder, drug-induced psychosis, manic episode, or major depressive episode; and had to have a positive response to antipsychotics in the previous two years.
On day 1, all patients started taking oral antipsychotic medication, and their dosages were titrated during the first six days to fixed daily doses of 6 milligrams of risperidone at night or 15 milligrams of olanzapine at night. Divalproex (delayed release) was initiated (day 1) at 15 milligrams per kilogram per day (dosed twice daily) and titrated at the physician's discretion to a maximum of 30 milligrams per kilogram per day (dosed twice daily) over 12 days. For divalproex and olanzapine, the mean modal daily divalproex sodium dose was 2,363 milligrams (range, 500 to 3,500); for divalproex and risperidone the mean modal daily dose was 2,258 milligrams (range, 1,000 to 3,500). Resultant plasma concentrations of valproate were approximately 100 micrograms per milliliter.
Lorazepam was allowed at a dosage of up to 4 milligrams a day during week 1 and up to 2 milligrams a day during weeks 2 and 3. Restricted use of chloral hydrate and zolpidem was allowed during the first three weeks. Propranolol and benztropine could be used for extrapyramidal symptoms and akathisia. PANSS ratings were done by blinded raters at baseline and on days 3, 5, 7, 10, 14, 21, and 28.
We tested the hypothesis (post hoc) that reduction in scores on the PANSS hostility item would occur during combination therapy, that the reductions would be greater than those observed during monotherapy, and that the reductions in hostility would be selective—that is, independent of the effect on other positive items as measured by the PANSS. Covariates included the sum of the PANSS measures of positive psychotic symptoms (excluding excitement and hostility) and unusual thought content. Hostility is defined by the PANSS as verbal and nonverbal expressions of anger and resentment, including sarcasm, passive-aggressive behavior, verbal abuse, and assaultiveness (2). The rating is based on the interpersonal behavior observed during an interview and on reports by primary care staff or family members. Ratings range from 1, indicating that hostility is absent, to 7, indicating extreme hostility that includes marked anger resulting in extreme uncooperativeness, precluding other interactions, or in an episode of physical assault on others.
Differences between treatment groups in the change from baseline in hostility score—that is, severity of the hostility item on the PANSS—at days 3, 5, 7, 10, 14, 21, and 28 (last observation carried forward) were assessed by a two-way analysis of variance (ANOVA) and analysis of covariance (ANCOVA) with factors for treatment and investigator. Repeated-measures ANOVA and ANCOVA were used to assess differences between groups on hostility scores from day 1 through day 7 and from day 1 through day 28, with effects for treatment, time, and investigator.
To correct for potential confounding variables, change in certain positive symptoms (sum of the PANSS items of delusions, suspiciousness/ persecution, grandiosity, unusual thought content, conceptual disorganization, and hallucinatory behavior) was introduced as a covariate. Additional analyses were conducted with baseline positive symptoms, baseline akathisia (as measured by the Barnes Akathisia Scale), presence or absence of sedation ("somnolence") as reported in the course of adverse event monitoring, use of adjunctive lorazepam, chloral hydrate or zolpidem, or benztropine or propranolol, and ethnicity, as covariates.
Results
A total of 249 study participants were randomly assigned to study groups. The sample comprised 190 men (76 percent) and 59 women (24 percent); 204 (82 percent) had a DSM-IV diagnosis of schizophrenia, paranoid type; 12 (5 percent) had schizophrenia, disorganized type; and 33 (13 percent) had schizophrenia, undifferentiated type. The average age of the participants was 38.7±10.1 years (range, 18 to 63 years), the average age at the first psychiatric hospitalization was 23.8±9 years, and the lifetime number of hospitalizations was one to five for 41 percent of participants, six to ten for 24 percent, and more than ten for 34 percent. A total of 121 participants (49 percent) were African American, 116 (47 percent) were white, five (2 percent) were Asian, three (1 percent) were native American or Alaskan, and four (2 percent) were from other ethnic groups.
Baseline mean±SD PANSS total scores were 100±15.2 (range, 67 to 149) for the participants in the antipsychotic monotherapy group and 103±15.2 (range, 73 to 155) for those in the combination therapy group. The differences among groups on the baseline mean PANSS total scores were not statistically significant, and there were no significant differences on any demographic variable.
A total of 166 participants (67 percent) completed the four-week study. The most frequent reason for premature discontinuation was withdrawal of consent (25 patients in the antipsychotic monotherapy group and 12 in the combination therapy group). Seven patients discontinued their participation because of treatment-emergent adverse effects, including two patients each in the olanzapine group (abnormal liver function test results and asthma), the olanzapine and divalproex group (hyperglycemia and rash), and the risperidone and divalproex group (dyspepsia and flank pain) as well as one patient in the risperidone group (somnolence and hypotension).
Mean scores on the PANSS hostility item are summarized in Table 1. Mean hostility scores at baseline were 2.7±1.26 for antipsychotic monotherapy and 2.8±1.33 for combination therapy with the antipsychotic and divalproex (possible scores on the item range from 1 to 7). The differences among treatment groups in baseline mean scores on the PANSS hostility item was not statistically significant. Mean changes from baseline to day 3 demonstrated an improvement of .1±.98 for the monotherapy group and .4±.95 for the combination therapy group. Mean changes from baseline to day 7 were an improvement of .3±1.04 for the monotherapy group and .7±1.26 for the combination therapy group. Combination therapy was associated with significantly greater antihostility effects at days 3 and 7 compared with antipsychotic monotherapy (ANOVA p=.028 and .007, respectively; for ANCOVA see Table 1 and explanation below).
Effect sizes for this improvement (an estimate of the magnitude of treatment effect when variability within groups is controlled for) were calculated by dividing the treatment difference for mean change from baseline by the pooled standard deviation and were .10 and .42 for monotherapy and combination treatment, respectively, at day 3 and were .29 and .56, respectively, at day 7. (Generally, a small effect size is about .2, a moderate effect size about .6, and a large effect size about 1.2). When PANSS items that reflect delusional thinking, presence of a formal thought disorder, or hallucinations at baseline were introduced as a covariate, the results remained statistically significant (p=.027 and .011, respectively).
No statistically significant differences were noted at days 5, 10, 14, 21, or 28. When repeated measures were used for the period day 1 through day 7, the advantage of combination therapy over monotherapy remained statistically significant when no covariates were used (p=.002) or when the covariates of baseline delusional thinking, presence of a thought disorder and hallucinations (p=.005), or change over time in delusional thinking, thought disorder, and hallucinations (p=.027) were used. Similarly, the advantage of combination therapy over monotherapy remained statistically significant when the covariates of baseline Barnes Akathisia Scale scores (p=.002), somnolence (p=.005), ethnicity (p=.002), or use of lorazepam, chloral hydrate, benztropine or propranolol (p=.002 to .003) were used. Statistical significance for the antihostility effect of adjunctive divalproex was not seen beyond the first week of treatment, but there was a trend toward a difference between combination treatment and antipsychotic monotherapy when repeated measures were used over the period day 1 through day 28 (p=.078).
Of note, the frequency of use of lorazepam, chloral hydrate, and zolpidem did not differ between the monotherapy and combination treatment groups (62 percent and 59 percent, respectively, used at least one of these agents during the course of the study). Lorazepam was used by 62 patients (52 percent) in the monotherapy group and by 60 patients (49 percent) in the combination therapy group, at an average dosage (in milligrams) of 1.8±.9 and 1.8±.6, respectively, for an average of 5.8±5.8 and 5.3±4.8 times, respectively, during the course of the trial. None of these differences was statistically significant.
Discussion
Although the literature contains a number of retrospective reports, this is the first double-blind randomized clinical trial to demonstrate the advantage of adjunctive divalproex for schizophrenia and hostility. The effect of adjunctive divalproex on hostility appears to be independent of antipsychotic effect on other PANSS items that reflect delusional thinking, presence of a formal thought disorder, or hallucinations. Moreover, the findings were essentially unchanged when the analysis was repeated to assess the possible confounds of akathisia; sedation; adjunctive use of lorazepam, chloral hydrate, or zolpidem; and ethnicity. The method used in this study is similar to that described in a report on the effects of clozapine, risperidone, olanzapine, and haloperidol on hostility (7), the results of which were validated when a separate analysis of overt acts of aggression was conducted (18). In that study, clozapine was associated with a significantly greater antihostility effect than either haloperidol or risperidone and with a lower total aggression score, as measured by summing up weighted scores obtained by using the Overt Aggression Scale for all incidents over time.
Although a statistically significant difference was observed between monotherapy and combination therapy at days 3 and 7, this effect was not evident at day 5. We used the statistical technique of repeated measures to demonstrate the statistically significant advantage of combination therapy over monotherapy for the period of baseline through day 7. The repeated-measures technique has the advantage of controlling for subject heterogeneity (individual differences). Nevertheless, the mean difference in decrease in the PANSS hostility item between the combination and monotherapy treatment groups at day 7 was small (.4).
An important limitation of this study was that the study participants were not specifically selected because of a history of severe aggressive and hostile behavior, although participants had to meet certain minimum requirements regarding acuity of symptoms at baseline; their average baseline scores were accordingly low. Another limitation was that we had only one outcome measure for a complex behavior. This approach is different from using PANSS factors comprising several different PANSS items (19), but similar results (statistically significant changes from baseline levels detected at days 3 and 7) were obtained when we measured the effect of time on changes in the sum of the PANSS items for poor impulse control, tension, hostility, uncooperativeness, and excitement.
In addition, the atypical antipsychotics used in the trial may be associated with antihostility effects of their own, leaving the possibility that the effects of the combination of typical antipsychotics and divalproex may be more pronounced than what was demonstrated in this trial with the combination of olanzapine or risperidone with divalproex. Such differences can be tested in future studies by directly comparing the effect of olanzapine (or risperidone) plus divalproex with that of haloperidol plus divalproex. Unexplored are any differences between the effects of olanzapine and of risperidone. The study was powered to detect differences between monotherapy and combination therapy but not differences among the four separate treatment groups.
The effect on hostility appeared to differentiate combination therapy from monotherapy only during the first week of treatment. The use of adjunctive rescue medications such as lorazepam, zolpidem, and chloral hydrate was similar among the treatment groups (including dosage, number of days used, and percentage of patients using these agents) (17), and when controlled for in our analysis—by presence or absence—did not affect our findings.
Untested is the possibility that a higher dosage of olanzapine would have resulted in a stronger effect of monotherapy, as suggested by a recent report of a rapid escalating dosage strategy for olanzapine (20).
It needs to be noted that it is during the initial week of treatment that assaults can commonly take place. Speed is of the essence in the control of hostile behaviors. Violent or threatening behavior is a frequent reason for admission to a psychiatric inpatient facility, and that behavior may continue after the admission. Binder and associates (21) reported that during the first 24 hours after admission to a locked university-based psychiatric inpatient unit, nine (10 percent) of 87 patients with schizophrenia physically attacked another person, and 37 (43 percent) engaged in fear-inducing behavior, such as verbal attacks, threats to attack, or attacks on objects. The age and gender distributions of these patients were not dissimilar to those of the patients enrolled in our study. Lengths of stay and behaviors occurring after 24 hours were not reported.
Ideally, studies of putative antiaggressive agents should be conducted among persons who have been specially selected on the basis of their aggressive behavior and in a double-blind manner with randomized treatment assignment. This approach is operationally difficult because of the relative rarity of aggressive events and subsequent need for a large sample and lengthy baseline and trial periods, selection and consent bias, and practical barriers such as the need for a specialized inpatient unit designed to manage this type of challenging patient population (22).
Our clinical trial used the delayed-release formulation of divalproex sodium. Aside from tolerability issues, the valproic acid formulation may be expected to provide similar efficacy. The average dosages of divalproex achieved were approximately 2,300 mg a day, resulting in plasma concentrations of valproate of approximately 100 micrograms per milliliter. It is not known whether lower dosages would be as efficacious in reducing hostility or whether higher dosages would be more efficacious. The appropriate dosage for an individual patient may also vary and may require further adjustment in the event of side effects. It is anticipated that future studies will use an extended-release formulation of divalproex sodium that can be administered once a day (23).
Conclusions
To our knowledge, this is the first study to compare the antihostility effects of adjunctive divalproex among patients with schizophrenia in a randomized, double-blind clinical trial. The pattern of results from this post hoc analysis indicates that adjunctive divalproex may be a helpful augmentation strategy in reducing hostility. This antihostility effect appears to be independent of the effects on other symptoms of psychosis. However, this differential effect was seen only in the first week. Further double-blind, controlled research on the use of divalproex in reducing hostility among patients with schizophrenia is needed.
Dr. Citrome is affiliated with the clinical research and evaluation facility of the Nathan S. Kline Institute for Psychiatric Research in Orangeburg, New York, and with the department of psychiatry at New York University in New York City. Dr. Casey is with the department of psychiatry of Oregon Health and Science University in Portland. Dr. Daniel is with the department of psychiatry of George Washington University in Washington, D.C., and with Bioniche Development, Inc., in Falls Church, Virginia. Dr. Wozniak and Dr. Tracy are with Abbott Laboratories in Abbott Park, Illinois. Dr. Kochan was with Abbot Laboratories at the time of writing. Send correspondence to Dr. Citrome at Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, New York 10962 (e-mail, [email protected]).
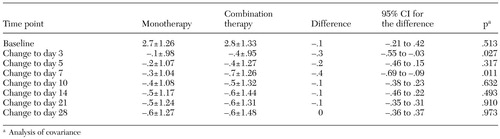 |
Table 1. Mean±SD baseline scores and changes from baseline on the hostility item of the Positive and Negative Syndrome Scale
1. Czobor P, Volavka J, Meibach RC: Effect of risperidone on hostility in schizophrenia. Journal Clinical Psychopharmacology 15:243–249, 1995Crossref, Medline, Google Scholar
2. Kay SR, Opler LA, Lindenmayer JP: The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. British Journal of Psychiatry 155(suppl):59–65, 1989Google Scholar
3. Marder SR, Meibach RC: Risperidone in the treatment of schizophrenia. American Journal of Psychiatry 151:825–835, 1994Link, Google Scholar
4. Buckley PF, Ibrahim ZY, Singer B, et al: Aggression and schizophrenia: efficacy of risperidone. Journal of the American Academy of Psychiatry and the Law 25:173–181, 1997Medline, Google Scholar
5. Beck NC, Greenfield SR, Gotham H, et al: Risperidone in the management of violent, treatment-resistant schizophrenics hospitalized in a maximum security forensic facility. Journal of the American Academy of Psychiatry and the Law 25:461–468, 1997Medline, Google Scholar
6. Kinon BJ, Roychowdhury SM, Milton DR, et al: Effective resolution with olanzapine of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia. Journal of Clinical Psychiatry 62(suppl 2):17–21, 2001Medline, Google Scholar
7. Citrome L, Volavka J, Czobor P, et al: Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility in patients with schizophrenia and schizoaffective disorder. Psychiatric Services 52:1510–1514, 2001Link, Google Scholar
8. Citrome L: Use of lithium, carbamazepine, and valproic acid in a state-operated psychiatric hospital. Journal of Pharmacy Technology 11:55–59, 1995Crossref, Medline, Google Scholar
9. McEvoy JP, Scheifler PL, Frances A: The Expert Consensus Guideline Series: treatment of schizophrenia 1999. Journal of Clinical Psychiatry 60(suppl 11):43, 1999Google Scholar
10. Citrome L, Levine J, Allingham B: Changes in use of valproate and other mood stabilizers for patients with schizophrenia from 1994 to 1998. Psychiatric Services 51:634–638, 2000Link, Google Scholar
11. Citrome L, Jaffe A, Levine J: Use of mood stabilizers among patients with schizophrenia, 1994–2001. Psychiatric Services 53:1212, 2002Link, Google Scholar
12. Morinigo A, Martin J, Gonzalez S, et al: Treatment of resistant schizophrenia with valproate and neuroleptic drugs. Hillside Journal of Clinical Psychiatry 11:199–207, 1989Medline, Google Scholar
13. Wassef A, Watson D, Morrison P, et al: Neuroleptic-valproic acid combination in treatment of psychotic symptoms: a three-case report. Journal of Clinical Psychopharmacology 9:45–48, 1989Crossref, Medline, Google Scholar
14. Afaq I, Riaz J, Sedky K, et al: Divalproex as a calmative adjunct for aggressive schizophrenic patients. Journal of the Kentucky Medical Association 100(1):17–22, 2002Google Scholar
15. Littrell KH, Petty RG, Hilligoss NM, et al: Valproate for hostility in schizophrenia patients. Journal of Clinical Psychiatry 65:134, 2004Crossref, Medline, Google Scholar
16. Lindenmayer JP, Kotsaftis A: Use of sodium valproate in violent and aggressive behaviors: a critical review. Journal of Clinical Psychiatry 61:123–128, 2000Crossref, Medline, Google Scholar
17. Casey DE, Daniel DG, Wassef AA, et al: Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology 28:182–192, 2003Crossref, Medline, Google Scholar
18. Volavka J, Czobor P, Nolan KA, et al: Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. Journal of Clinical Psychopharmacology, in pressGoogle Scholar
19. Lindenmayer JP, Bernstein-Hyman R, Grochowski S, et al: Psychopathology of schizophrenia: initial validation of a 5-factor model. Psychopathology 28:22–31, 1995Crossref, Medline, Google Scholar
20. Baker RW, Kinon BJ, Maguire GA, et al: Effectiveness of rapid initial dose escalation of up to forty milligrams per day of oral olanzapine in acute agitation. Journal of Clinical Psychopharmacology 23:342–348, 2003Crossref, Medline, Google Scholar
21. Binder RL, McNiel DE: Effects of diagnosis and context on dangerousness. American Journal of Psychiatry 145:728–732, 1988Link, Google Scholar
22. Volavka J, Citrome L: Atypical antipsychotics in the treatment of the persistently aggressive psychotic patient: methodological concerns. Schizophrenia Research 35:S23-S33, 1999Google Scholar
23. Citrome L: Schizophrenia and valproate. Psychopharmacology Bulletin, 37(3 suppl 2):74–88, 2003Google Scholar



