Benzodiazepine Use and Abuse Among Patients With Severe Mental Illness and Co-occurring Substance Use Disorders
Abstract
OBJECTIVES: Because use of benzodiazepines may exacerbate existing substance use disorders or become abused substances, prescription of benzodiazepines for patients with severe mental illness (schizophrenia or bipolar disorder) and co-occurring substance use disorders (abuse or dependence) is controversial. The authors examined benzodiazepine use and associated psychiatric, substance abuse, and institutional outcomes in a six-year longitudinal study of patients with co-occurring disorders. METHODS: At baseline and yearly follow-up for six years, 203 patients with co-occurring severe mental illness and substance use disorder were prospectively assessed for medication use, substance use, psychiatric symptoms, use of hospitalization, and quality of life. RESULTS: Almost one-half of the patients (43 percent) reported taking prescribed benzodiazepines at the time of at least one assessment. Patients taking prescribed benzodiazepines were more likely to have high scores on measures of overall symptoms and affective symptoms (anxiety and depression) and low ratings for general quality of life throughout the study. Benzodiazepine use was unrelated to remission of substance use disorder or hospitalization, but a greater proportion of patients who were prescribed benzodiazepines developed benzodiazepine abuse, compared with those who were not prescribed benzodiazepines (15 percent compared with 6 percent). CONCLUSIONS: Prescription benzodiazepine use was common among patients with co-occurring severe mental illness and a substance use disorder and was not associated with any of the measured outcomes other than increasing the likelihood of benzodiazepine abuse. Physicians should consider other treatments for anxiety in this population.
Benzodiazepines are commonly prescribed for patients with severe mental illness, such as schizophrenia and bipolar disorder, but their use in the context of co-occurring substance use disorders is highly controversial. On one hand, benzodiazepines are widely prescribed because they are effective in managing psychiatric symptoms and medication side effects (1,2,3,4). On the other hand, because benzodiazepines may exacerbate existing substance use disorders or become abused substances (5), many experts recommend avoiding the prescription of benzodiazepines for patients with co-occurring disorders (6,7,8,9).
The research evidence regarding benzodiazepine abuse among persons with substance use disorders in the general population is limited (10,11), and some experts argue that these medications can be used safely with close supervision (10). We were unable to find research on use of prescribed benzodiazepines by patients with co-occurring severe mental illness and a substance use disorder.
To address this gap in knowledge, we examined benzodiazepine use, substance use and abuse, psychiatric symptoms, hospitalization, and quality of life among 203 patients with co-occurring disorders who were followed prospectively for six years. We addressed the following questions: How frequently are benzodiazepines prescribed for patients with severe mental illness and substance use disorders? Is benzodiazepine use related to positive or negative outcomes? When benzodiazepines are prescribed for persons with co-occurring disorders, how often are they abused? What factors predict abuse of prescribed benzodiazepines among persons with co-occurring disorders?
Methods
Study group
The analysis reported here, which is part of a prospective, longitudinal follow-up study, examined data for 203 outpatients with severe mental illness (schizophrenia or bipolar disorder) and co-occurring substance abuse or dependence who were receiving treatment in a state community mental health system between 1990 and 1997. Although 223 outpatients initially entered the study, 20 were lost to attrition early in the follow-up period and were therefore excluded from this longitudinal analysis. The remaining 203 patients, who were in the study for at least three years, constituted the study group for the longitudinal analyses reported here. At the four-year follow-up, 182 of the 203 patients (90 percent) were assessed; at five years, 178 patients (88 percent) were assessed; and at six years, 170 patients (84 percent of the longitudinal study group) were assessed. At the end of the six years, the attrition of 33 patients from the 203 patients in the study group was due to 17 patients' refusal to be interviewed, the death of six patients, and our inability to locate ten patients.
According to the baseline research interviews, 107 patients (53 percent) had a diagnosis of schizophrenia, 45 (22 percent) had schizoaffective disorder, and 51 (25 percent) had bipolar disorder. In addition, 145 (71 percent) received a diagnosis of alcohol use disorder, and 87 (43 percent) received a diagnosis of drug use disorder (predominantly cannabis or cocaine use disorders, or both). Demographically, the study group was predominantly male (N=151, or 74 percent), white (N=197, or 97 percent), and never married (N=124, or 61 percent). The mean±SD age at study entry was 33.7±8.1 years. The majority of the patients (N=161, or 80 percent) had completed high school or attained a graduate equivalency dipolma. The mean±SD baseline score on the Expanded Brief Psychiatric Rating Scale (BPRS) (12) at study entry was 45.8±13.6, indicating moderate symptoms in this group.
Treatments
All participants in the study received care in dual-disorder treatment programs in seven participating community mental health centers (13). The standard interventions included medication management, case management, rehabilitation services, substance abuse counseling in individual and group sessions, and linkage with substance abuse self-help groups in the community. Staff psychiatrists within the participating community mental health centers and in local hospitals made all decisions about medications on clinical grounds. No uniform guidelines for prescription of benzodiazepines were used during the study period.
Measures
Research psychiatrists established diagnoses of severe mental illness (schizophrenia, schizoaffective disorder, or bipolar disorder) and substance use disorder (abuse or dependence) using the Structured Clinical Interview for DSM-III-R (14). The Structured Clinical Interview for DSM-III-R Personality Disorders (15) was used to diagnose antisocial personality disorder. Research diagnoses of other personality disorders, anxiety disorders, and mood disorders other than bipolar and schizoaffective disorders were not made. At baseline, the research interview also included items from the Uniform Client Data Inventory (16), used to assess demographic information; the Time-Line Follow-Back (17), used to determine the number of days of alcohol and drug use over the previous six months; chronological assessment of housing history and institutional stays by means of a self-report calendar supplemented by outpatient records and hospital records (18); the Quality of Life Interview (19); and the Expanded BPRS (12), used to assess current psychiatric symptoms. The Quality of Life Interview includes a general score from 1 to 7 and subscale scores from 0 to 1 for objective measures of living situation and daily activities; family contact, social contact, and financial adequacy are measured on subscales from 1 to 5. Higer scores on this instrument indicate better quality of life. The Expanded BPRS includes subscales for affect, anergia, activation, disorganization, and thought disorder, each of which is scored on a scale from 1 to 7; it provides a total score that can range from 24 to 168, with higher scores indicating more severe symptoms. Side effects, such as akathisia and tardive dyskinesia, were not measured. The follow-up interviews contained the same instruments but did not reassess demographic and lifetime data.
Procedures
The study was approved by the Dartmouth Medical School and the New Hampshire state institutional review boards. Participants gave written informed consent yearly throughout the study. The same two trained clinical research interviewers assessed participants at baseline and yearly thereafter. The interviewers were supervised and checked yearly for reliability.
Because the most valid assessments of substance use disorder are based on multimodal data (20), an independent research team assigned substance use ratings after reviewing the patients' self-reports from the research interviews, results of urine toxicology screens, and clinicians' ratings. The research team used the Alcohol Use Scale (AUS) (21), the Drug Use Scale (DUS) (21), and the Substance Abuse Treatment Scale (SATS) (22) to rate participants' substance use. Both the AUS and the DUS measure levels of use with anchor points: 1 for abstinence, 2 for use without impairment, 3 for abuse, 4 for dependence, and 5 for dependence with institutionalization. The SATS measures progress in eight steps toward stable remission, from 1, indicating pre-engagement in treatment, to 8, indicating in remission and no longer in treatment for a substance use disorder. Interrater reliability, determined by using intraclass correlation coefficients, was excellent, with coefficients of .93 for the SATS and .94 for the AUS and the DUS (13).
The researchers assessed benzodiazepine use in several ways. At each assessment, the interviewers asked the patients about their use of all prescribed medications and all nonprescribed substances. The patients' self-reports of benzodiazepine prescriptions were validated with 1995 Medicaid records. Self-reported use of prescription benzodiazepines agreed with the presence of prescriptions that were billed through Medicaid over the month before the interview in 92 percent of cases (kappa=.77). Clinical reasons for benzodiazepine prescription were not assessed in this study. Nonprescription benzodiazepine use was identified through a combination of self-reports, urine toxicology screening results, and clinicians' ratings. Any indication of benzodiazepine use outside of prescription parameters triggered a complete review of the patient's research data and clinical records to locate information about benzodiazepine abuse or dependence, as identified according to DSM-III-R criteria.
Data analyses
We assessed baseline differences between benzodiazepine users and nonusers with chi square and t tests. We used generalized estimating equation (GEE) procedures (23) in SAS Proc GENMOD (24) to assess differences between the two groups over time. An extension of the generalized linear model, GEE offers an alternative strategy to mixed-effects models for correlated longitudinal or clustered data. One advantage of GEE is that both discrete and continuous variables can be modeled in the same framework with appropriate link functions. In addition, GEE gives consistent estimation for model parameters and adjusts the intraclass correlation with robust variance estimation. We used logistic regression to assess the relationship between benzodiazepine use and attainment of stable remission from a substance use disorder, taking into account the duration of benzodiazepine use. Finally, we used logistic regression to predict risk factors for benzodiazepine abuse among benzodiazepine users.
Results
Benzodiazepine use
On the basis of the seven assessments over six years, 88 of the 203 patients (43 percent) reported prescription benzodiazepine use at least once. At each of the follow-up assessments, between 18 percent and 21 percent of the patients were using prescribed benzodiazepines. Among those for whom benzodiazepines were prescribed, 55 (63 percent) used prescribed benzodiazepines at more than one time point, 31 (35 percent) used more than one type of benzodiazepine over the six years, and eight (9 percent) used more than one type of benzodiazepine simultaneously.
Benzodiazepine users did not differ from nonusers on measures of age, gender, marital status, psychiatric diagnosis, and substance use disorder; however, benzodiazepine users were less likely than nonusers to have a high school education (N=25, or 28 percent and N=16, or 14 percent, respectively; χ2=6.34, df=1, p=.01). During the study, only seven of the 115 patients (6 percent) for whom benzodiazepines were not prescribed reported taking benzodiazepines.
Remission and other outcomes
The study participants in general made steady progress over six years toward attaining full remission of their substance use disorders (defined as at least six months without evidence of abuse or dependence). At the final assessment (at the six-year follow-up or the final interview), 96 of the 203 participants (47 percent) had attained remission from their substance use disorder. The users and nonusers of prescription benzodiazepines had similar rates of full remission—43 of the 88 users (48.9 percent) and 52 of 108 nonusers (48.1 percent). Participants who abused nonprescribed benzodiazepines had a low rate of attaining remission—one of seven participants, or 14 percent of the nonusers who abused. Although large, these differences were not statistically significant.
Of the 43 users of prescribed benzodiazepines who had experienced remission from substance use disorders at the last assessment, less than half (N=19, or 44 percent) were still taking prescribed benzodiazepines at the last assessment. No relationship was found between the number of times participants reported use of prescribed benzodiazepines and attainment of stable remission at the final assessment.
We next examined associations between use of prescribed benzodiazepines and outcomes at each follow-up. Patients were classified on the basis of medication status at each assessment—users and nonusers of prescription benzodiazepines. We examined 19 outcomes in the domains of substance abuse, psychiatric symptoms, quality of life, days in psychiatric hospitals, and proportion of days in the community. Age, gender, and education were used as covariates. Because the plots of group means against time for outcomes revealed no clear functional forms for some variables, we examined the relationships with benzodiazepine use in terms of group mean differences (rather than regression coefficients), time trends, and group-by-time interactions. To control for multiple tests, we set the alpha level at .01.
The results of the GEE analysis over the six years of the study are summarized in Table 1. Significant differences between groups were found for three variables: BPRS affect subscore, BPRS total score, and general life satisfaction score. The group who used prescribed benzodiazepines scored significantly higher (more severe symptoms) than the nonuser group on the BPRS affect subscale and total score and significantly lower on the general quality-of-life rating. Significant time effects were found for the total BPRS score, the BPRS anergia subscore, the BPRS thought disorder subscore, the BPRS disorganization subscore, the number of days hospitalized, the SATS score, the AUS score, the DUS score, the Quality of Life Interview general life satisfaction score, and the Quality of Life Interview financial support adequacy subscore. Both groups improved over time, and no interactions between group and time were significant, suggesting that the users of prescribed benzodiazepines had outcomes that were similar to those of the nonuser group.
Benzodiazepine abuse
Evidence of benzodiazepine abuse or dependence, based on DSM-III-R criteria, was detected among 15 percent of the 88 users of prescribed benzodiazepines (N=13), compared with 6 percent (N=7) of the 107 persons for whom benzodiazepines were not prescribed (χ2=4.23, df=1, p=.04), indicating that benzodiazepine abuse was significantly more likely among the patients for whom benzodiazepines were prescribed. In a comparison of the 13 patients who abused prescribed benzodiazepines with the 75 patients who were users but not abusers of prescribed benzodiazepines, logistic regression showed that the patients with higher baseline BPRS affect subscale scores were more likely to become benzodiazepine abusers (odds ratio=2.8, 95 percent confidence interval=1.09 to 7.23). Otherwise, baseline demographic, clinical, and diagnostic variables did not predict benzodiazepine abuse.
Discussion
This naturalistic follow-up study clarified several issues regarding benzodiazepine use and abuse by patients with co-occurring disorders. First, the high rate of use of prescribed benzodiazepines (43 percent) suggests that many physicians are willing to prescribe benzodiazepines for patients with co-occurring disorders. Benzodiazepines were prescribed throughout the study, primarily to patients who had high levels of symptoms in general and affective symptoms (anxiety and depression) in particular as well as lower quality of life. This high rate of benzodiazepine prescription for persons with severe mental illness and substance use disorder was recently replicated in a much larger study, which showed that 62.5 percent (384 of 614 persons with schizophrenia and substance use disorders) received benzodiazepine prescriptions (25).
Second, use of prescribed benzodiazepines was unrelated to substance abuse and hospitalization outcomes. Patients taking prescribed benzodiazepines generally improved and had outcomes similar to those of patients for whom benzodiazepines were not prescribed, although neither group improved in terms of affective symptoms and the benzodiazepine group experienced consistently worse affective and overall symptoms. Although controlled trials would be needed to definitively resolve the issue of benzodiazepine effects on outcomes for persons with co-occurring disorders, the results reported here contrasted markedly with those of our previous analysis of the effects of clozapine in the same study group (26), in which we found that clozapine was associated with dramatic reductions in substance abuse—a finding that has been replicated in other naturalistic studies (27) and that has led to controlled trials.
Third, patients with dual diagnoses for whom benzodiazepines were prescribed were more than twice as likely to develop benzodiazepine abuse as those who did not use prescribed benzodiazepines (15 percent compared with 6 percent). Thus exposure to prescribed benzodiazepines may increase the risk of benzodiazepine abuse, or put another way, physician prescriptions may create another abuse problem for this group of already highly vulnerable individuals. Among those for whom benzodiazepines were prescribed, high levels of affective symptoms were the only predictor of the development of benzodiazepine abuse. Other potential risk factors, such as gender, drug use disorder, or antisocial personality disorder (10,28,29), were not associated with benzodiazepine abuse in this study group.
Fourth, affective symptoms both predicted and continued to covary with benzodiazepine use, and affective symptoms did not improve over the course of the follow-up, suggesting that the use of benzodiazepines did not lead to reductions of anxiety and depression among these patients. Additionally, total symptoms were higher in this group, suggesting that benzodiazepines did not improve symtoms in general. The lack of evidence for effectiveness and the significant risk of benzodiazepine abuse suggest that other psychotherapeutic and pharmacologic approaches to managing anxiety, depression, and psychosis should be considered for this population, although very little research has been done to assess the impact of these types of interventions on symptoms in this population (30). A previous study found that abstinence leads to decreased affective symptoms among similar patients (31).
This study was limited by several factors. Because the study was a naturalistic follow-up designed to document the long-term course of substance use disorders among patients with co-occurring disorders, medication dosages and symptoms were not evaluated frequently enough to assess the short-term relationships between benzodiazepine use and symptom severity. In addition, medication side effects were not measured at all, so the relationship between benzodiazepine use, side effects, and outcomes could not be assessed. Because assessments were done yearly, we may have missed intermittent benzodiazepine use. In addition, benzodiazepine abuse may have been underreported because of the stigma and denial associated with substance abuse. However, we used multiple methods to ascertain substance abuse, the same interviewers conducted the assessments throughout the study, and little indication of underreporting was found on the basis of toxicology and collateral reports after the first year. Most of the study group was Caucasian and male. The analyses controlled for gender and did not find gender differences. However, because we could not control for race, the findings may not generalize to non-Caucasian groups. Finally, the study took place in a small, rural state where community mental health care and prescribing practices may differ from those in other settings. Thus the results may not generalize to routine practice in the United States.
The advantages of the study include the relatively large number of patients with co-occurring disorders who participated, the prospective long-term design, the use of standardized measures, the assessment of substance use disorder from multiple perspectives, and the relatively high rate of follow-up. We believe that this is the first study to assess the rate, correlates, and outcomes of benzodiazepine use and abuse among patients with co-occurring disorders. Further studies are needed to confirm the safety and abuse liability of benzodiazepine use by patients with co-occurring disorders, but this study provides preliminary evidence suggesting minimal benefit and substantial risk.
Conclusions
A high rate of use of prescribed benzodiazepines (43 percent) was found in this study group of patients with severe mental illness and co-occurring substance use disorders, and a significant number of patients for whom benzodiazepines were prescribed abused them (15 percent). We found little evidence that benzodiazepine use was helpful to these patients, either for relief of target symptoms of anxiety and depression or for attaining remission from substance use disorder. We therefore recommend that clinicians and researchers consider other approaches to managing symptoms among patients with severe mental illness and co-occurring substance use disorders.
The authors are affiliated with the department of psychiatry at Dartmouth Medical School in Concord, New Hampshire. Address correspondence to Dr. Brunette at the New Hampshire-Dartmouth Psychiatric Research Center, State Office Park South, Main Building, 105 Pleasant Street, Concord, New Hampshire 03301 (e-mail, [email protected]).
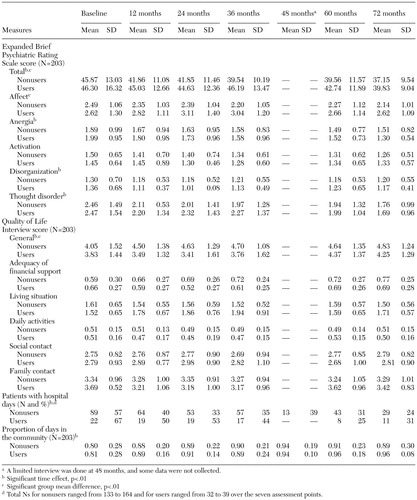 |
Table 1. Scores on measures of psychiatric and institutional outcomes over a 72-month period among patients with severe mental illness who were users or nonusers of prescription benzodiazepines
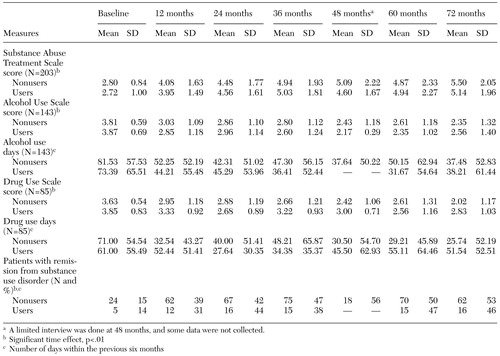 |
Table 2. Scores on measures of substance abuse outcomes over a 72-month period among patients with severe mental illness who were users or nonusers of prescription benzodiazepines
1. Licht RW: Drug treatment of mania: a critical review. Acta Psychiatrica Scandinavica 97:387–397, 1998Crossref, Medline, Google Scholar
2. Kupfer DJ: Pathophysiology and management of insomnia during depression. Annals of Clinical Psychiatry 11:267–276, 1999Crossref, Medline, Google Scholar
3. Simpson GM: The treatment of tardive dyskinesia and tardive dystonia. Journal of Clinical Psychiatry 61(4 suppl):39–44, 2000Google Scholar
4. Stimmel GL: Benzodiazepines in schizophrenia. Pharmacotherapy 16(6 Pt 2):148S-151S, 1996Google Scholar
5. Roache JD, Weisch RA: Findings from self-administration research on the addiction potential of benzodiazepines. Psychiatric Annals 25:153–157, 1995Crossref, Google Scholar
6. Johnson B, Longo LP: Considerations in the physician's decision to prescribe benzodiazepines to patients with addiction. Psychiatric Annals 28:160–165, 1998Crossref, Google Scholar
7. Miller NS: Liability and efficacy from long-term use of benzodiazepines: documentation and interpretation. Psychiatric Annals 25:166–173, 1995Crossref, Google Scholar
8. Mueser KT, Noordsy DL, Drake RE, et al: Integrated Treatment for Dual Disorders: A Guide to Effective Practice. New York, Guilford, 2003Google Scholar
9. Sowers W, Golden S: Psychotropic medication management in persons with co-occurring psychiatric and substance use disorders. Journal Psychoactive Drugs 31:59–70, 1999Crossref, Medline, Google Scholar
10. Ciraulo DA, Nace EP: Benzodiazepine treatment of anxiety or insomnia in substance abuse. American Journal of Addictions 9:276–279, 2000Crossref, Medline, Google Scholar
11. Ciraulo DA, Sands BF, Shader RI: Critical review of liability for benzodiazepine abuse among alcoholics. American Journal of Psychiatry 145:1501–1506, 1988Link, Google Scholar
12. Lukoff D, Nuechterlein KH, Ventura J: Manual for the expanded Brief Psychiatric Rating Scale (BPRS). Schizophrenia Bulletin 12:594–602, 1986Google Scholar
13. Drake RE, McHugo GJ, Clark RE, et al: Assertive community treatment for patients with co-occurring severe mental illness and substance use disorder: a clinical trial. American Journal of Orthopsychiatry 68:201–215, 1998Crossref, Medline, Google Scholar
14. Spitzer RL, Williams JBW, Gibbon M, et al: Instruction Manual for the Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1988Google Scholar
15. Spitzer RL, Williams JBW, Gibbon M, et al: Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Washington, DC, American Psychiatric Press, 1990Google Scholar
16. Tessler RC, Goldman HH: The Chronically Mentally Ill: Assessing Community Support Programs. Cambridge, Mass, Ballinger, 1982Google Scholar
17. Sobell MB, Maisto SA, Sobell LC, et al: Developing a prototype for evaluating alcohol treatment effectiveness, in Evaluating Alcohol and Drug Treatment Effectiveness. Edited by Sobell LC, Sobell MB, Ward E. New York, Pergamon, 1980Google Scholar
18. Clark RE, Ricketts SK, McHugo GJ: Measuring hospital use without claims: a comparison of patient and provider reports. Health Services Research 31:153–169, 1996Medline, Google Scholar
19. Lehman AF: A quality of life interview for the chronically mentally ill. Evaluation and Program Planning 11:51–62, 1988Crossref, Google Scholar
20. Wolford GL, Rosenberg SD, Oxman TE, et al: Evaluating current methods for detecting substance use disorder in persons with severe mental illness. Psychology of Addictive Behaviors 13:313–326, 1999Crossref, Google Scholar
21. Drake RE, Osher FC, Noordsy DL, et al: Diagnosis of alcohol use disorders in schizophrenia. Schizophrenia Bulletin 16:57–67, 1990Crossref, Medline, Google Scholar
22. McHugo GJ, Drake RE, Burton HL, et al: A scale for assessing the stage of substance abuse treatment in persons with severe mental illness. Journal of Nervous and Mental Disease 183:762–767, 1995Crossref, Medline, Google Scholar
23. Liang KY, Zeger S: Longitudinal data analysis using generalized linear models. Biometrika 73:13–22, 1986Crossref, Google Scholar
24. SAS/STAT User's Guide, Version 8, vols 1, 2, and 3. Cary, NC, SAS Institute, 2002Google Scholar
25. 25,Clark RE, Xie H, Brunette MF: Benzodiazepine prescription practices and substance abuse in persons with severe mental illness. Journal of Clinical Psychiatry, in pressGoogle Scholar
26. Drake RE, Xie H, McHugo GJ, et al: The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophrenia Bulletin 26:441–449, 2000Crossref, Medline, Google Scholar
27. Zimmet SV, Strous RD, Burgess ES, et al: Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. Journal of Clinical Psychopharmacology 20:94–98, 2000Crossref, Medline, Google Scholar
28. Ross HE: Benzodiazepine use and anxiolytic abuse and dependence in treated alcoholics. Addiction 88:209–218, 1993Crossref, Medline, Google Scholar
29. Graham K, Wilsnack SC: The relationship between alcohol problems and use of tranquilizing drugs: longitudinal patterns among American women. Addictive Behaviors 25:13–28, 2000Crossref, Medline, Google Scholar
30. Noordsy DL, Green AI: Pharmacotherapy for schizophrenia and co-occurring substance use disorders. Current Psychiatry Reports, in pressGoogle Scholar
31. Cuffel BJ, Chase P: Remission and relapse of substance use disorder in schizophrenia: results from a one-year prospective study. Journal of Nervous and Mental Disease 182:342–348, 1994Crossref, Medline, Google Scholar



