Premature Mortality From General Medical Illnesses Among Persons With Bipolar Disorder: A Review
Although several studies have found evidence that major depression is associated with increased risk of early mortality from general medical illnesses, fewer studies have investigated premature mortality from such illnesses among individuals with bipolar disorder ( 1 ). In the past, excess deaths associated with bipolar illness were attributed mostly to unnatural causes, such as suicide, homicide, and accidents ( 2 , 3 , 4 , 5 ). Over the past decade, there is increasing evidence that patients with bipolar illness may be at higher risk of premature death from general medical disorders.
Emerging data indicate that although standardized mortality ratios are higher for unnatural causes (that is, suicide and accidents), the majority of excess deaths among persons with bipolar disorder are secondary to comorbid general medical conditions. The causes of this excess mortality may include unhealthy diet ( 6 ), obesity ( 6 , 7 ), binge eating ( 8 , 9 ), sedentary lifestyle ( 6 , 10 ), smoking ( 11 , 12 , 13 ), social deprivation ( 14 , 15 ), living alone or being homeless or single ( 15 , 16 ), poor access to and less effective use of health services ( 17 , 18 , 19 , 20 , 21 ), biased attitudes among health care providers ( 18 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 ), failure of psychiatric providers to ask about or address medical problems ( 31 , 32 , 33 ), the "competing needs theory" (that is, health care providers might give precedence to conditions that need immediate attention while management of other conditions is delayed or forgotten) ( 34 , 35 , 36 ), and comorbid substance use disorders ( 37 , 38 , 39 , 40 ). Biologic factors associated with bipolar illness, such as stress-related effects on the immune system ( 41 , 42 , 43 , 44 , 45 ) and on the hypothalamic-pituitary axis ( 41 , 46 , 47 ), increased activity of the sympathetic nervous system ( 48 , 49 ), and metabolic side effects of pharmacologic treatments, may also increase the risk of mortality ( 50 , 51 , 52 , 53 , 54 , 55 ).
The association of psychopharmacologic agents with obesity and type 2 diabetes has also helped stimulate research about general medical outcomes of patients with severe mental illness. Olanzapine, which was approved in 2000, was the first second-generation antipsychotic to gain approval by the Food and Drug Administration for the treatment of acute mania and subsequently for maintenance treatment. Since then four other second-generation antipsychotics have been introduced and approved for the treatment of bipolar disorder ( 56 , 57 ). Although second-generation antipsychotics have less risk than first-generation agents of side effects, such as extrapyramidal symptoms, tardive dyskinesia, and hyperprolactinemia, evidence shows that some of these medications are associated with obesity and metabolic abnormalities, which may increase morbidity and mortality resulting from diabetes and vascular disease ( 58 , 59 ). Many patients with bipolar disorder are treated with mood stabilizers that are also associated with increased risks of obesity and metabolic syndrome.
The aim of this study was to review the literature in regard to excess mortality attributable to medical problems among patients with bipolar spectrum disorders. Verification that bipolar disorder is associated with premature mortality from general medical illnesses could lead to development of health services models that integrate preventive medical interventions into community mental health settings. Given that most of the studies reviewed did not include structured psychiatric interviews, the following diagnoses were included in the definition of bipolar spectrum disorders used in this study: bipolar disorder, schizoaffective disorder, affective psychosis, and affective disorder severe enough to require inpatient admission or treatment with lithium.
Methods
We searched the MEDLINE database from 1959–2007 using combinations of the following terms: bipolar, mania, or affective disorder and mortality, outcome, or follow-up. We also located additional studies by searching the citations from all the retrieved and other relevant articles. In addition, e-mails were sent to authors of selected articles if more information was needed. Several inclusion criteria were used for this review. Only English-language reports of studies with more than 100 patients were included. All studies included cause-specific standardized mortality ratios (SMRs) or mortality ratios (MRs) or SMRs or MRs due to natural causes as a whole, or the studies provided sufficient information to calculate these measures. SMRs measure the excess or deficit in mortality in the selected study population compared with the age- and gender-specific death rates of a standard population. MRs measure the number of deaths per specific study population compared with a control group. All studies measured mortality rates among patients with bipolar disorder (either type I or II), schizoaffective disorder, affective disorder (severity level sufficient to require inpatient psychiatric admission or treatment with lithium), affective psychosis, or any combination of these. If there was more than one report describing results from the same study sample over time, the report with the longest follow-up period was included.
Results
Through the electronic and manual searches, we identified 44 English-language articles. A total of 27 were excluded for the following reasons: eight articles reported studies that included fewer than 100 patients, seven articles presented data from studies that included patients with several psychiatric diagnoses (that is, we could not differentiate patients with bipolar spectrum illnesses), five articles reported on samples already included in another paper, five articles reported studies that did not separate suicide and nonsuicide mortality, and two articles reported studies that did not include a comparison group.
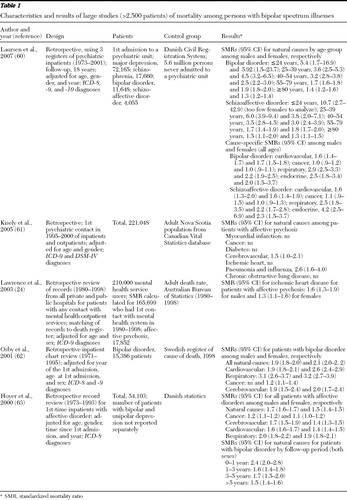
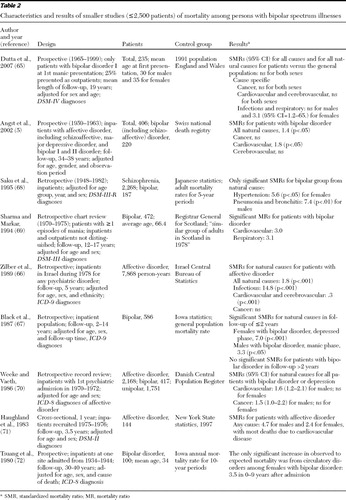
Our systematic review of the literature identified 17 studies that met the inclusion criteria. Tables 1 and 2 divide the studies into more generalizable samples, which we defined as those with more than 2,500 patients with bipolar spectrum illnesses ( Table 1 ) and smaller studies with fewer than 2,500 patients with these illnesses and with samples selected on the basis of lithium use ( Table 3 ).
 |
 |
 |
In one of the larger studies, Laursen and colleagues ( 60 ) compared mortality rates among patients with severe mental illness who were admitted to a psychiatric hospital and rates among those without a history of psychiatric hospital admission in the entire adult population of Denmark after controlling for age, gender, and calendar year. Among patients with bipolar or schizoaffective disorder, an inverse relationship was found between age group and mortality rate. Compared with persons without major psychiatric disorders, patients with either bipolar or schizoaffective disorder had significantly higher mortality risk in all age groups, but SMRs were highest in the younger age groups. In addition, SMRs were also higher for all natural causes of death (cardiovascular, respiratory, and endocrine conditions) except for cancer both among patients with bipolar disorder and among those with schizoaffective disorder.
Kisely and colleagues ( 61 ) studied mortality rates among patients who had received psychiatric treatment (inpatient and outpatient) in Nova Scotia, Canada, and compared these rates to those in the entire adult Canadian population. Controlling for age and gender, the regression analysis showed that an "affective psychosis" diagnosis was significantly associated with increased mortality risk (SMR=1.35, 95% confidence interval [CI]=1.24–1.47.) Among patients with affective psychosis, mortality risk from natural causes was not increased in most categories except for cerebrovascular disease and pneumonia or influenza.
Lawrence and colleagues ( 24 ) studied the rate of hospital admissions, revascularization procedures, and deaths from ischemic heart disease among more than 210,000 patients with mental illness and compared it with the rate in the general population of Western Australia, after adjusting for age and gender. Among patients with affective psychosis, mortality from ischemic heart disease was significantly higher for both sexes, although the hospital admission rate was not different from that in the general community. The rate of revascularization was significantly lower for psychiatric patients. Among users of mental health services, ischemic heart disease was responsible for 16% of excess deaths (foremost cause of excess deaths), compared with 8% of excess deaths caused by suicide.
Osby and colleagues ( 62 ) studied mortality rates among more than 15,000 Swedish citizens who had a hospital discharge diagnosis of bipolar disorder and compared it with the mortality rate for the Swedish population. This study controlled for sex, age at admission, and calendar year. Among patients with bipolar disorder, SMRs from all natural causes were significantly higher for both sexes. Cardiovascular disease was the most frequent cause of death. This study showed that except for cancer and central nervous system diseases among males, all other natural causes of death were higher among patients with bipolar disorder.
In another Danish study, Hoyer and colleagues ( 63 ) investigated mortality and cause of death among more than 54,000 patients with affective disorder who were admitted to a psychiatric hospital. After the analyses controlled for age, gender, duration of illness since first psychiatric hospitalization, and calendar year, SMRs from all natural causes were higher among patients with affective disorder compared with the general population of Denmark. In the bipolar disorder subgroup, SMRs for natural causes were highest in the first year after admission and decreased as duration of disease increased over the course of years.
The five studies discussed above were categorized as larger studies, that is, more than 2,500 patients. Twelve of the other studies reviewed were categorized as smaller studies with fewer than 2,500 patients (range 100 to 2,168) ( 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ). The smaller studies may have been underpowered to find differences in mortality. Most of these studies compared specific medical causes of death among patients with affective disorders and a control group; only five studies reported SMRs for deaths from all natural causes ( 5 , 64 , 65 , 66 , 67 ). In a prospective study with an average follow-up period of 19 years, Dutta and colleagues ( 65 ) studied causes of death among 235 patients with newly diagnosed bipolar disorder. The study showed no increase in mortality from natural causes compared with the 1991 population of England and Wales, except for deaths from infectious and respiratory diseases among females. In another prospective study, over a 34- to 38-year period, Angst and colleagues ( 5 ) showed that SMRs for all natural causes and for cardiovascular disease (but not cerebrovascular disease or cancer) were significantly higher among 220 inpatients with bipolar or schizoaffective disorder compared with the general population of Switzerland.
In a study in Japan, Saku and colleagues ( 68 ) found that SMRs among 187 patients with manic-depressive illness were significantly higher for hypertension among females and for pneumonia and bronchitis among males compared with adults in the Japanese population. In a retrospective British follow-up study over 17 years completed by Sharma and Markar ( 69 ), the MR from cardiovascular and respiratory disorders was significantly higher among 472 patients with bipolar disorder compared with a control group. In a five-year follow-up study, Zilber and colleagues ( 66 ) examined the risk of medical mortality for more than 7,868 person-years (the actual number of patients was not reported) among Israeli patients who had at least one inpatient psychiatric treatment for affective disorder during 1978. After the analyses controlled for age, sex, and ethnicity, the SMR for natural causes among patients with affective disorder was significantly higher than that in the general population of Israel. The higher SMR for these patients was attributable to a higher mortality rate from infectious diseases, whereas the SMR attributable to cardiovascular and cerebrovascular disease together was significantly lower among patients with affective disorder.
Black and colleagues ( 67 ) compared mortality among 586 patients who had bipolar disorder with mortality in the general population of Iowa. In a follow-up period of less than two years, SMRs from natural causes were significantly higher among women with bipolar disorder who were depressed and men with bipolar disorder who were experiencing a manic episode. Using data from the Danish Central Psychiatric Register, Weeke and Vaeth ( 70 ) identified 417 patients with manic-depression and 1,751 with unipolar depression who had a first psychiatric admission between 1970 and 1972. Compared with the general population, SMRs for cardiovascular disease and cancer were both significantly higher among males, but not among females, with affective disorder. In a 3.5-year follow-up study by Haugland and colleagues ( 71 ), the SMR for all causes among 144 patients with affective disorder was significantly higher than the age-specific SMR in New York State. Most deaths were reported to be from heart disease. Tsuang and colleagues ( 72 ) studied causes of death among 525 patients with severe mental illness over 30 to 40 years. In the subgroup of 100 patients with mania, the observed to expected mortality ratio for circulatory system diseases among women, but not among men, was significantly higher than in the general population of Iowa over a nine-year follow-up period.
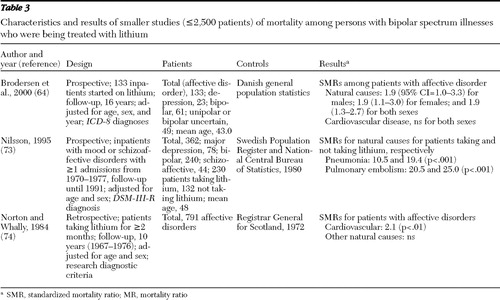
We also reviewed studies with small samples in which patients were selected because they were being treated with lithium. In a Danish prospective study by Brodersen and colleagues ( 64 ), 133 patients with affective disorder were started on lithium and followed up for 16 years. Regardless of medication adherence, trends for higher SMRs for all natural causes were found for both sexes, although the all-cause SMR was statistically significant only for female patients. The SMR for cardiovascular disease was not different from that in the general population. Nilsson ( 73 ) investigated mortality among 362 patients with a mood disorder or schizoaffective disorder who were treated with lithium for at least one year. Compared with the adult Swedish population, cause-specific SMRs were higher for pneumonia and pulmonary embolism among the patients both when they were taking lithium and when they were not taking lithium. In a Scottish retrospective study by Norton and Whalley ( 74 ), 791 patients with affective disorder who had received lithium for at least two months during the ten-year follow-up had a significantly higher SMR for cardiovascular disease compared with the general population of Scotland.
Discussion
Findings and implications
This literature review showed that individuals with bipolar spectrum disorders appear to be at significantly higher risk of premature death from natural causes compared with the general population. The review of larger studies with at least several thousand participants indicated that persons with bipolar or schizoaffective disorder or severe affective disorder had consistently higher mortality rates from natural causes. Higher mortality from natural causes among patients with bipolar spectrum disorders ranged from 35% higher than a comparison group to twofold higher. The increased mortality rate is similar to the increased risk of mortality associated with smoking. Data from the National Health and Nutrition Examination Survey found that being a current smoker was associated with a 20% to approximately twofold increase in mortality among middle-aged and older men and women in the community, depending on age group (higher risks were found in younger age groups) ( 75 ).
In the larger studies the higher mortality was attributable to almost every cause of death that was investigated, such as cardiovascular, respiratory, cerebrovascular, and endocrine disorders. Deaths from neoplasms either were not higher or were slightly elevated despite the probable higher number of risk factors for cancer (such as smoking and obesity) in this population. Among all causes of death, cardiovascular disease seemed to be responsible for the majority of excess deaths; the mortality risk was 35% to 2.5-fold higher. Studies that examined mortality from natural causes in different age groups or groups with different durations of illness found that as age or duration of illness increased, mortality from natural causes decreased ( 60 , 63 )
Review of the smaller studies with fewer than 2,500 patients generally showed findings similar to but less consistent than those of the larger studies. Most studies showed higher mortality attributable either to natural causes or to several specific medical illnesses. In several small studies, mortality from cardiovascular disease was higher among persons with bipolar spectrum disorders. However, one of these smaller studies actually showed that the combined mortality rate from cardiovascular and cerebrovascular diseases was significantly lower among patients with affective disorder ( 66 ). The inconsistent results of the small studies may be due to chance or to use of samples that were less representative of patient or control populations.
Excess mortality from natural causes among patients with bipolar spectrum disorders may result from several mechanisms. Patients with bipolar spectrum disorders are more likely to smoke and to smoke heavily and might have higher exposure to second-hand smoke ( 11 , 12 , 13 ). In addition, bipolar disorder is highly comorbid with alcohol and other substance abuse ( 6 , 37 ). These patients are more likely to have a poor diet and sedentary lifestyle with resultant weight gain and obesity, which is more prominent during the depressive phase of the disorder and can further jeopardize health and increase mortality ( 6 , 7 , 8 , 9 , 10 ).
Several biologic mechanisms may contribute to the increased mortality risk from natural causes found among patients with bipolar disorder. Chronic stress is associated with increased cortisol levels, lack of cortisol suppression, and changes in the hypothalamic-pituitary-adrenal axis responses. Both phases of bipolar disorder act as chronic stressors and lead to dysregulation of the hypothalamic-pituitary-adrenal axis and increases in cortisol levels ( 76 , 77 ). Chronic dysregulation of the hypothalamic-pituitary-adrenal axis and high levels of cortisol may increase insulin resistance, which can lead to hyperglycemia, increased oxidative stress, metabolic syndrome, and atherosclerosis ( 46 , 47 ). In addition, increased activity of the hypothalamic-pituitary-adrenal axis may be associated with hyperactivity of the sympathetic nervous system, a finding that is commonly observed among patients with bipolar disorder ( 48 , 49 ) Dysregulation of the autonomic nervous system may also lead to insulin resistance and may worsen metabolic syndrome ( 78 ) and also lead to an increased risk of sudden cardiac death. Interleukin-6 is one of several inflammatory markers that are higher among patients with bipolar disorder ( 43 ). Interleukin-6, by stimulating corticotrophin-releasing factor, can lead to hypothalamic-pituitary-adrenal axis hyperactivity and hypercortisolemia ( 79 ), which can lead to increased morbidity and mortality ( 41 , 42 , 43 , 44 , 45 ). Medications used to treat bipolar disorder may also increase the risk of diabetes and cardiovascular disorder.
Most patients with bipolar disorder are treated with mono- or polypharmacy, including at least one mood stabilizer with or without a second-generation antipsychotic ( 80 ). Most mood stabilizers are associated with weight gain ( 50 , 51 , 52 ). Most second-generation antipsychotics (with the exception of ziprasidone and aripiprazole) are also associated with increased risk of weight gain, diabetes mellitus, and impaired glucose and lipid metabolism, all of which can increase mortality from cardiovascular disease ( 53 , 54 , 55 ). Increased weight gain and change in body fat distribution can lead to insulin resistance, type 2 diabetes mellitus, and dyslipidemia, all of which may result in increased mortality from cardiovascular disease. Second-generation antipsychotics can also increase triglycerides via 5-HT2 receptor blockade and further impair lipid metabolism ( 81 ).
Emerging data have shown that patients with severe mental disorders often receive lower-quality medical care, including post-myocardial infarction cardiovascular procedures and care ( 24 , 25 , 29 , 30 ), diabetes care ( 21 , 82 , 83 , 84 , 85 ), preventive medical care ( 86 , 87 ), treatment of hypertension ( 85 , 88 ), and treatment for dyslipidemia ( 85 ). Disparities in the quality of medical care of patients with mental illnesses can be ascribed to diverse factors. At one level, a person with mental illness may not be able to effectively communicate with providers and express concerns because of cognitive disturbance or affective instability ( 89 , 90 ). At the provider level, the non-psychiatrist physician's bias against persons with mental illness can adversely affect medical management of the patient and lead to poor-quality care ( 22 , 23 ). Psychiatrists and other mental health providers may also prioritize psychiatric issues and neglect medical problems ( 35 , 36 ) They may also feel uncomfortable treating certain medical problems or lack experience in treating certain problems ( 91 ). At the national level, lack of private insurance, poor access to health services, and the fragmented mental and physical health care systems are additional factors that may lead to disparities in medical care for individuals with mental illness ( 20 , 24 , 29 , 83 , 92 )
New models of care may be necessary to improve general medical and psychological outcomes of patients with mental illness. Collaborative care models that integrate depression care coordinators and mental health specialists into primary care have been shown in 37 studies to be practical, cost-effective models to improve quality of depression care, patient satisfaction, treatment adherence, and depression outcomes ( 93 ). Recent studies that have tested integrating primary care clinicians into community mental health settings to provide preventive medical interventions have shown improved physical health of patients with chronic general medical illness and substance use disorders who were treated with these new models of care ( 94 ). More research needs to be planned to test ways to improve general medical outcomes of patients with chronic mental illnesses such as bipolar disease.
Limitations
In this literature review, limitations were noted in three categories: the systematic review process, methodologic problems in large studies, and methodologic problems in small studies. The systematic review was limited in that we had to combine literature on bipolar spectrum disorders (affective psychosis, affective disorder, schizoaffective disorder, manic-depressive disorder, and bipolar disorder) because of the paucity of studies in which the sample included only persons with bipolar disorder. We did not include articles that were in languages other than English. In most of the studies included in the review, structured psychiatric interviews were not used to establish diagnoses. Use of the different inclusion and exclusion criteria made it difficult to compare studies or standardize findings from the studies we reviewed. We were unable to measure whether exposure to second-generation antipsychotic agents was associated with increased mortality rates. Inclusion of patients with schizoaffective disorder and affective disorder may have led to overestimation or underestimation, respectively, of the association of bipolar illness with mortality.
In the second category, limitations of the larger studies included the fact that all used retrospective designs, limiting evaluation of causality. Larger studies (as well as small retrospective studies) collected administrative data or reviewed medical records, and thus information about many potential confounders, such as smoking or obesity, was not available. Use of medical records or administrative data is also subject to recording bias because the more severe medical disorders are likely to have been diagnosed. In addition, the diagnoses were made by individual psychiatrists in different parts of the world at different times and with different diagnostic and coding systems. The lack of standardized research criteria and the ongoing modification of the diagnostic criteria for bipolar disorder over time can challenge the reliability and validity of the diagnosis in studies that are based on record linkage and registers. Future large prospective studies should be planned with representative community populations of respondents with and without a diagnosis of bipolar illness (a diagnosis that is based on a structured psychiatric interview) and with controls for socioeconomic factors and medical and psychiatric comorbidity; such studies should also examine behavioral risk factors (smoking, lack of exercise, obesity, and use of psychiatric medications) and quality of medical and psychiatric care as potential mediators of premature medical mortality.
In the third category, unique limitations of the smaller studies (fewer than 2,500 patients) included the fact that except for three small longitudinal studies ( 5 , 67 , 76 ), all other studies used retrospective designs that limited evaluation of causality. Also, because of small samples, these studies were probably underpowered to detect the differences in MRs that have been shown in larger studies, and only five of the 12 smaller studies measured mortality rates from all natural causes.
Conclusions
This review strongly suggests that patients with bipolar spectrum disorders are at increased risk of premature death from general medical conditions. Given the increasing concern about premature mortality from diabetes and heart disease among patients with chronic mental illness, a 2004 consensus panel that included psychiatrists, endocrinologists, and internists made detailed recommendations for psychiatrists to monitor the risk of metabolic syndrome among patients taking second-generation antipsychotics and, more recently, mood stabilizers ( 95 ). These recommendations may help decrease the risk of obesity, diabetes, and vascular disease resulting from psychiatric medications among patients with chronic mental illness. However, patients with severe mental illness often have adverse health habits (such as poor diet, smoking, and lack of exercise), adhere poorly to medical regimens, and experience disparities in quality of medical care. New models of care that integrate primary care physicians into clinics that treat patients with chronic medical illness need to be developed and tested.
Acknowledgments and disclosures
Dr. Katon has received honoraria from Eli Lilly and Company, Pfizer, Forest Laboratories, and Wyeth. He is also on an advisory board for Eli Lilly. Dr. Roshanei-Moghaddam reports no competing interests.
1. Harris EC, Barraclough B: Excess mortality of mental disorder. British Journal of Psychiatry 173:11–53, 1998Google Scholar
2. Guze SB, Robins E: Suicide and primary affective disorders. British Journal of Psychiatry 117:437–438, 1970Google Scholar
3. Bratfos O, Haug JO: The course of manic-depressive psychosis: a follow up investigation of 215 patients. Acta Psychiatrica Scandinavica 44:89–112, 1968Google Scholar
4. Eastwood MR, Stiasny S, Meier HM, et al: Mental illness and mortality. Comprehensive Psychiatry 23:377–385, 1982Google Scholar
5. Angst F, Stassen HH, Clayton PJ, et al: Mortality of patients with mood disorders: follow-up over 34–38 years. Journal of Affective Disorders 68:167–181, 2002Google Scholar
6. Kessler RC, Rubinow DR, Holmes C, et al: The epidemiology of DSM-III-R bipolar I disorder in a general population survey. Psychological Medicine 27:1079–1089, 1997Google Scholar
7. McElroy SL, Frye MA, Suppes T, et al: Correlates of overweight and obesity in 644 patients with bipolar disorder. Journal of Clinical Psychiatry 63:207–213, 2002Google Scholar
8. Elmslie JL, Silverstone JT, Mann JI, et al: Prevalence of overweight and obesity in bipolar patients. Journal of Clinical Psychiatry 61:179–184, 2000Google Scholar
9. Kruger S, Shugar G, Cooke RG: Comorbidity of binge eating disorder and the partial binge eating syndrome with bipolar disorder. International Journal of Eating Disorders 19:45–52, 1996Google Scholar
10. Strassnig M, Brar JS, Ganguli R: Self-reported body weight perception and dieting practices in community-dwelling patients with schizophrenia. Schizophrenia Research 75:425–432, 2005Google Scholar
11. Grant BF, Hasin DS, Chou SP, et al: Nicotine dependence and psychiatric disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry 61:1107–1115, 2004Google Scholar
12. Gonzalez-Pinto A, Gutierrez M, Ezcurra J, et al: Tobacco smoking and bipolar disorder. Journal of Clinical Psychiatry 59:225–228, 1998Google Scholar
13. Tanskanen A, Viinamiiki H, Koivumaa-Honkanen H, et al: Smoking among psychiatric patients. European Journal of Psychiatry 11:179–188, 1997Google Scholar
14. Harrow M, Goldberg JF, Grossman LS, et al: Outcome in manic disorders: a naturalistic follow-up study. Archives of General Psychiatry 47:665–671, 1990Google Scholar
15. Tsuchiya KJ, Byrne M, Mortensen PB: Risk factors in relation to an emergence of bipolar disorder: a systematic review. Bipolar Disorder 5:231–242, 2003Google Scholar
16. Dittmann S, Biedermann NC, Grunze H, et al: The Stanley Foundation Bipolar Network: results of the naturalistic follow-up study after 2.5 years of follow-up in the German centres. Neuropsychobiology 46 (suppl 1):2–9, 2002Google Scholar
17. Cradock-O'Leary J, Young AS, Yano EM, et al: Use of general medical services by VA patients with psychiatric disorders. Psychiatric Services 53:874–878, 2002Google Scholar
18. Desai MM, Rosenheck RA, Druss BG, et al: Mental disorders and quality of care among postacute myocardial infarction outpatients. Journal of Nervous and Mental Disease 190:51–53, 2002Google Scholar
19. Druss BG, Rosenheck RA: Use of medical services by veterans with mental disorders. Psychosomatics 38:451–458, 1997Google Scholar
20. Druss BG, Bradford DW, Rosenheck RA, et al: Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA 283:506–511, 2000Google Scholar
21. Jones LE, Clarke W, Carney CP: Receipt of diabetes services by insured adults with and without claims for mental disorders. Medical Care 42:1167–1175, 2004Google Scholar
22. Lawrence D, Coghlan R: Health inequalities and the health needs of people with mental illness. NSW Public Health Bulletin 13:155–158, 2002Google Scholar
23. Jackson JL, Kroenke K: Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Archives of Internal Medicine 159:1069–1075, 1999Google Scholar
24. Lawrence DM, Holman CD, Jablensky AV, et al: Death rate from ischaemic heart disease in Western Australian psychiatric patients 1980–1998. British Journal of Psychiatry 182:31–36, 2003Google Scholar
25. Druss BG, Hoff RA, Rosenheck RA: Underuse of antidepressants in major depression: prevalence and correlates in a national sample of young adults. Journal of Clinical Psychiatry 61:234–237, 2000Google Scholar
26. Druss BG, Bradford WD, Rosenheck RA, et al: Quality of medical care and excess mortality in older patients with mental disorders. Archives of General Psychiatry 58:565–572, 2001Google Scholar
27. Haghighat R: A unitary theory of stigmatisation: pursuit of self-interest and routes to destigmatisation. British Journal of Psychiatry 178:207–215, 2001Google Scholar
28. Graber MA, Bergus G, Dawson JD, et al: Effect of a patient's psychiatric history on physicians' estimation of probability of disease. Journal of General Internal Medicine 15:204–206, 2000Google Scholar
29. Petersen LA, Normand SL, Druss BG, et al: Process of care and outcome after acute myocardial infarction for patients with mental illness in the VA health care system: are there disparities? Health Services Research 38:41–63, 2003Google Scholar
30. Young JK, Foster DA: Cardiovascular procedures in patients with mental disorders. JAMA 283:3198, 2000Google Scholar
31. Hall RC, Popkin MK, Devaul RA, et al: Physical illness presenting as psychiatric disease. Archives of General Psychiatry 35:1315–1320, 1978Google Scholar
32. McIntyre JS, Romano J: Is there a stethoscope in the house (and is it used)? Archives of General Psychiatry 34:1147–1151, 1977Google Scholar
33. Koran LM, Sox HC Jr, Marton KI, et al: Medical evaluation of psychiatric patients: I. results in a state mental health system. Archives of General Psychiatry 46:733–740, 1989Google Scholar
34. Rost K, Nutting P, Smith J, et al: The role of competing demands in the treatment provided primary care patients with major depression. Archives of Family Medicine 9:150–154, 2000Google Scholar
35. Desai MM, Rosenheck RA: Unmet need for medical care among homeless adults with serious mental illness. General Hospital Psychiatry 27:418–425, 2005Google Scholar
36. Gelberg L, Gallagher TC, Andersen RM, et al: Competing priorities as a barrier to medical care among homeless adults in Los Angeles. American Journal of Public Health 87:217–220, 1997Google Scholar
37. Ostacher MJ, Sachs GS: Update on bipolar disorder and substance abuse: recent findings and treatment strategies. Journal of Clinical Psychiatry 67:e10, 2006Google Scholar
38. Levin FR, Hennessy G: Bipolar disorder and substance abuse. Biological Psychiatry 56:738–748, 2004Google Scholar
39. Dickey B, Normand SL, Weiss RD, et al: Medical morbidity, mental illness, and substance use disorders. Psychiatric Services 53:861–867, 2002Google Scholar
40. Brady K, Lydiard RB: Bipolar affective disorder and substance abuse. Journal of Clinical Psychopharmacology 12:17–22, 1992Google Scholar
41. McEwen BS: Mood disorders and allostatic load. Biological Psychiatry 54:200–207, 2003Google Scholar
42. Appels A, Bar FW, Bar J, et al: Inflammation, depressive symptomatology, and coronary artery disease. Psychosomatic Medicine 62:601–605, 2000Google Scholar
43. Ridker PM, Hennekens CH, Buring JE, et al: C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine 342:836–843, 2000Google Scholar
44. Miller AH: Neuroendocrine and immune system interactions in stress and depression. Psychiatric Clinics of North America 21:443–463, 1998Google Scholar
45. Miller GE, Stetler CA, Carney RM, et al: Clinical depression and inflammatory risk markers for coronary heart disease. American Journal of Cardiology 90:1279–1283, 2002Google Scholar
46. Brindley DN, Rolland Y: Possible connections between stress, diabetes, obesity, hypertension and altered lipoprotein metabolism that may result in atherosclerosis. Clinical Sciences (London) 77:453–461, 1989Google Scholar
47. Rosmond R, Bjorntorp P: The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. Journal of Internal Medicine 247:188–197, 2000Google Scholar
48. Zahn TP, Nurnberger JI, Jr., Berrettini WH, et al: Concordance between anxiety and autonomic nervous system activity in subjects at genetic risk for affective disorder. Psychiatry Research 36:99–110, 1991Google Scholar
49. Lake CR, Pickar D, Ziegler MG, et al: High plasma norepinephrine levels in patients with major affective disorder. American Journal of Psychiatry 139:1315–1318, 1982Google Scholar
50. Isojarvi JI, Laatikainen TJ, Knip M, et al: Obesity and endocrine disorders in women taking valproate for epilepsy. Annals of Neurology 39:579–584, 1996Google Scholar
51. Vestergaard P, Poulstrup I, Schou M: Prospective studies on a lithium cohort: 3. tremor, weight gain, diarrhea, psychological complaints. Acta Psychiatrica Scandinavica 78:434–441, 1988Google Scholar
52. Rattya J, Pakarinen AJ, Knip M, et al: Early hormonal changes during valproate or carbamazepine treatment: a 3-month study. Neurology 57:440–444, 2001Google Scholar
53. Cohn TA, Sernyak MJ: Metabolic monitoring for patients treated with antipsychotic medications. Canadian Journal of Psychiatry 51:492–501, 2006Google Scholar
54. Bergman RN, Ader M: Atypical antipsychotics and glucose homeostasis. Journal of Clinical Psychiatry 66:504–514, 2005Google Scholar
55. Haupt DW, Kane JM: Metabolic risks and effects of atypical antipsychotic treatment. Journal of Clinical Psychiatry 68:e24, 2007Google Scholar
56. Perlis RH: Treatment of bipolar disorder: the evolving role of atypical antipsychotics. American Journal of Managed Care 13:S178–S188, 2007Google Scholar
57. Vieta E, Goikolea JM: Atypical antipsychotics: newer options for mania and maintenance therapy. Bipolar Disorders 7(suppl 4):21–33, 2005Google Scholar
58. Newcomer JW: Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. Journal of Clinical Psychiatry 68(suppl 1):20–27, 2007Google Scholar
59. Haupt DW: Differential metabolic effects of antipsychotic treatments. European Neuropsychopharmacology 16(suppl 3): S149–S155, 2006Google Scholar
60. Laursen TM, Munk-Olsen T, Nordentoft M, et al: Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia. Journal of Clinical Psychiatry 68:899–907, 2007Google Scholar
61. Kisely S, Smith M, Lawrence D, et al: Mortality in individuals who have had psychiatric treatment: population-based study in Nova Scotia. British Journal of Psychiatry 187:552–558, 2005Google Scholar
62. Osby U, Brandt L, Correia N, et al: Excess mortality in bipolar and unipolar disorder in Sweden. Archives of General Psychiatry 58:844–850, 2001Google Scholar
63. Hoyer EH, Mortensen PB, Olesen AV: Mortality and causes of death in a total national sample of patients with affective disorders admitted for the first time between 1973 and 1993. British Journal of Psychiatry 176:76–82, 2000Google Scholar
64. Brodersen A, Licht RW, Vestergaard P, et al: Sixteen-year mortality in patients with affective disorder commenced on lithium. British Journal of Psychiatry 176:429–433, 2000Google Scholar
65. Dutta R, Boydell J, Kennedy N, et al: Suicide and other causes of mortality in bipolar disorder: a longitudinal study. Psychological Medicine 37:839–847, 2007Google Scholar
66. Zilber N, Schufman N, Lerner Y: Mortality among psychiatric patients: the groups at risk. Acta Psychiatrica Scandinavica 79:248–256, 1989Google Scholar
67. Black DW, Winokur G, Nasrallah A: Mortality in patients with primary unipolar depression, secondary unipolar depression, and bipolar affective disorder: a comparison with general population mortality. International Journal of Psychiatry in Medicine 17:351–360, 1987Google Scholar
68. Saku M, Tokudome S, Ikeda M, et al: Mortality in psychiatric patients, with a specific focus on cancer mortality associated with schizophrenia. International Journal of Epidemiology 24:366–372, 1995Google Scholar
69. Sharma R, Markar HR: Mortality in affective disorder. Journal of Affective Disorders 31:91–96, 1994Google Scholar
70. Weeke A, Vaeth M: Excess mortality of bipolar and unipolar manic-depressive cardiovascular death and manic-depressive psychosis. Journal of Affective Disorders 11:227–234, 1986Google Scholar
71. Haugland G, Craig TJ, Goodman AB, et al: Mortality in the era of deinstitutionalization. American Journal of Psychiatry 140:848–852, 1983Google Scholar
72. Tsuang MT, Woolson RF, Fleming JA: Causes of death in schizophrenia and manic-depression. British Journal of Psychiatry 136:239–242, 1980Google Scholar
73. Nilsson A: Mortality in recurrent mood disorders during periods on and off lithium: a complete population study in 362 patients. Pharmacopsychiatry 28:8–13, 1995Google Scholar
74. Norton B, Whalley LJ: Mortality of a lithium-treated population. British Journal of Psychiatry 145:277–282, 1984Google Scholar
75. Davis MA, Neuhaus JM, Moritz DJ, et al: Health behaviors and survival among middle-aged and older men and women in the NHANES I Epidemiologic Follow-up Study. Preventive Medicine 23:369–376, 1994Google Scholar
76. Cassidy F, Ritchie JC, Carroll BJ: Plasma dexamethasone concentration and cortisol response during manic episodes. Biological Psychiatry 43:747–754, 1998Google Scholar
77. Schmider J, Lammers CH, Gotthardt U, et al: Combined dexamethasone/corticotropin- releasing hormone test in acute and remitted manic patients, in acute depression, and in normal controls: I. Biological Psychiatry 38:797–802, 1995Google Scholar
78. Taylor V, MacQueen G: Associations between bipolar disorder and metabolic syndrome: a review. Journal of Clinical Psychiatry 67:1034–1041, 2006Google Scholar
79. Dentino AN, Pieper CF, Rao MK, et al: Association of interleukin-6 and other biologic variables with depression in older people living in the community. Journal of the American Geriatrics Society 47:6–11, 1999Google Scholar
80. Algorithm for Treatment of BDI: Currently Hypomanic/Manic. Austin, Texas Department of State Health Services, 2005. Available at www.dshs.state.tx.us/mhprograms/pdf/timabdalgos2005.pdf Google Scholar
81. Diebold K, Michel G, Schweizer J, et al: Are psychoactive-drug-induced changes in plasma lipid and lipoprotein levels of significance for clinical remission in psychiatric disorders? Pharmacopsychiatry 31:60–67, 1998Google Scholar
82. Desai MM, Rosenheck RA, Druss BG, et al: Mental disorders and quality of diabetes care in the Veterans Health Administration. American Journal of Psychiatry 159:1584–1590, 2002Google Scholar
83. Frayne SM, Halanych JH, Miller DR, et al: Disparities in diabetes care: impact of mental illness. Archives of Internal Medicine 165:2631–2638, 2005Google Scholar
84. Kreyenbuhl J, Dickerson FB, Medoff DR, et al: Extent and management of cardiovascular risk factors in patients with type 2 diabetes and serious mental illness. Journal of Nervous and Mental Disease 194:404–410, 2006Google Scholar
85. Nasrallah HA, Meyer JM, Goff DC, et al: Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophrenia Research 86:15–22, 2006Google Scholar
86. Druss BG, Rosenheck RA, Desai MM, et al: Quality of preventive medical care for patients with mental disorders. Medical Care 40:129–136, 2002Google Scholar
87. Thorpe JM, Kalinowski CT, Patterson ME, et al: Psychological distress as a barrier to preventive care in community-dwelling elderly in the United States. Medical Care 44:187–191, 2006Google Scholar
88. Wang PS, Avorn J, Brookhart MA, et al: Effects of noncardiovascular comorbidities on antihypertensive use in elderly hypertensives. Hypertension 46:273–279, 2005Google Scholar
89. Birdwell BG, Herbers JE, Kroenke K: Evaluating chest pain: the patient's presentation style alters the physician's diagnostic approach. Archives of Internal Medicine 153:1991–1995, 1993Google Scholar
90. Bowie CR, Harvey PD: Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatric Clinics of North America 28:613–633,626, 2005Google Scholar
91. Lester H, Tritter JQ, Sorohan H: Patients' and health professionals' views on primary care for people with serious mental illness: focus group study. BMJ 330:1122, 2005Google Scholar
92. Rothman AA, Wagner EH: Chronic illness management: what is the role of primary care? Annals of Internal Medicine 138: 256–261, 2003Google Scholar
93. Gilbody S, Bower P, Fletcher J, et al: Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Archives of Internal Medicine 166:2314–2321, 2006Google Scholar
94. Druss BG, von Esenwein SA: Improving general medical care for persons with mental and addictive disorders: systematic review. General Hospital Psychiatry 28:145–153, 2006Google Scholar
95. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al: Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 27:596–601, 2004Google Scholar



