Systematic Use of Patient-Rated Depression Severity Monitoring: Is It Helpful and Feasible in Clinical Psychiatry?
Depression is highly prevalent, is often chronic and recurrent, and is the most common diagnosis among patients of U.S. psychiatrists, accounting for over 50% of patient visits ( 1 , 2 , 3 ). Although evidence suggests greater adequacy of depression treatment in psychiatric settings, compared with primary care practices ( 4 , 5 , 6 ), there is significant room for improvement. Kessler and colleagues ( 4 ) found that only 64% of patients with depression who were treated in specialty mental health settings received "minimally adequate" guideline-based care; other studies found that only 49%–67% of patients received three months of treatment ( 7 , 8 , 9 ). These simple "adequacy of treatment" guidelines, however, mask the fact that despite availability of several well-studied instruments, psychiatrists do not use measurement tools to monitor patient progress and treatment response. In a study in the United Kingdom, a majority of psychiatrists did not utilize standardized measures for depression case identification, functioning, clinical audit, or measuring clinical change over time ( 10 ); we have no reason to suspect that utilization rates would be higher in the Unites States.
More recently, the Texas Medication Algorithm Project (TMAP) and the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) effectiveness trials employed simple-to-use methods in measurement-based care and examined their impact on depression care in primary care settings and in psychiatric practice settings ( 11 , 12 , 13 ). The core of measurement-based care consisted of a depression treatment algorithm and use of severity measures to evaluate symptoms at critical decision points. Although there is no direct evidence that the use of measures led to improved depression outcomes in STAR*D, investigators hypothesized that the use of such measures facilitated change in provider behavior, which may have contributed to improved patient outcomes and to achieving response and remission rates that were comparable to rates found in efficacy trials ( 14 ). Kashner and colleagues ( 15 ) reported on the implementation of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) that was administered by psychiatric nurses in outpatient public mental health clinics; the study found that implementing SCID was feasible and clinically useful, influencing patients' diagnosis as well as the prescribing pattern and dosing.
Patient-rated depression assessments have been found to be equivalent to clinician-rated depression severity assessments ( 16 , 17 , 18 ). This finding has facilitated adoption of these assessments by lessening the barriers of clinician training and administration time, which limit routine use of depression severity measures in clinical practice. Several patient-rated instruments are currently available, including the Quick Inventory of Depressive Symptomatology-Self-Rated (QIDS-SR) ( 19 ) and the Nine-Item Patient Health Questionnaire (PHQ-9) ( 20 ).
The PHQ-9 parallels the DSM-IV symptom criteria for depressive disorders. Each symptom is measured on a 4-point scale for frequency over a two-week period; scores range from 0, not at all, to 3, nearly every day, with an overall score ranging between 0 and 27. Thresholds for levels of severity are as follows: 0–4, none; 5–9, mild; 10–14, moderate; 15–19, moderately severe; and 20–27, severe. [A copy of the PHQ-9 is available as an online supplement at ps.psychiatryonline.org.] Because of its brevity, ease of administration and interpretation, and dual capability of assessing DSM-IV criteria and symptom severity, the PHQ-9 has been used increasingly in primary care for screening as well as in the routine follow-up of patients with depression ( 20 , 21 , 22 , 23 ). Many psychiatrists, however, do not consider a simple instrument such as the PHQ-9, which includes items they routinely ask, to provide added benefit to their practice.
The overarching aims of this pilot study are to assess the helpfulness of PHQ-9 in psychiatric practice and to test office-based management strategies that optimize the monitoring and treatment of depression. Specific questions addressed include the following: Do psychiatrists find PHQ-9 scores helpful in their practice? What percentage of treatment decisions are altered on the basis of the PHQ-9 score? What percentage of patients with depression who are treated by psychiatrists complete initial and follow-up assessments with the PHQ-9? And what office-based changes need to be implemented to support PHQ-9–based proactive monitoring in solo and group psychiatric practices and other systems of care?
Methods
The National Depression Management Leadership Initiative is a collaborative effort of the American Academy of Family Physicians, the American College of Physicians, and the American Psychiatric Institute for Research and Education (APIRE), an affiliate of the American Psychiatric Association (APA). The data reported pertains to the sample of psychiatrists and their patients. The study design at the practice level is quasi-experimental one-group, pretest-posttest; design at the patient-level is longitudinal observational. The program began in March 2005 and concluded by April 2006.
Participants and sites
At the outset of the study, 19 psychiatric practices were recruited nationally: six practices from the South, five from the mid-Atlantic, four from the Midwest, and four from the West and Southwest. Practices represented a variety of organizational structures—that is, six group multispecialty practices, six group mental health specialty practices, four departmental practices that were part of a larger system of care, one outpatient public clinic, and two solo private practitioners with minimal office assistance.
Psychiatric practices were enrolled in the project upon agreeing to identify two representatives from their practice to participate in three learning sessions during the 12-month project period; to complete the baseline and follow-up questionnaires and clinically detailed visit-based questionnaires; and to form an improvement team within their practice to identify and implement improvements for depression care. In return, practice representatives were reimbursed for their travel costs to project learning sessions and received a $1,500 stipend per practice for participation in the project.
Two practices dropped out of the project: one was part of a group mental health specialty practice; the other practice belonged to a larger system of care. Both practices were unable to fulfill data collection requirements of the project.
The intervention
Modeled after the Institute for Health Care Improvement Breakthrough Series ( 24 ), this study recruited practices to volunteer for this pilot project. The intervention was spread over a year. It entailed a series of three weekend-long face-to-face learning sessions—each session was followed by an action phase, which consisted of testing improvements and conference calls where practices shared their experiences and lessons learned. At the outset of the project, each practice identified a lead psychiatrist and a nonphysician coleader, who were charged with attending the learning sessions and implementing the project at their respective practice sites. Lead psychiatrists who participated in the project included 16 APA members and three nonmembers. Project coleaders consisted of six nursing staff, four social workers, three psychologists, two clinical research coordinators, two office assistants, one health care administrator, and one case manager.
The learning sessions were structured to introduce practices to strategies for improving depression care through application of the chronic care model ( 25 , 26 ). Practices were introduced to the Plan-Do-Study-Act cycle to test small changes in a rapid fashion for implementation of incremental improvements. Learning sessions demonstrated the use of PHQ-9 to facilitate the monitoring of depression severity, the implementation of a registry for tracking patients with depression, and the systematic planning for and documentation of self-management. A more detailed description of a similar intervention has been described previously ( 27 ).
Data collection
During the course of the study, both practice-level and patient-level data were collected. Data on race-ethnicity of psychiatrists, coleaders, and their patients were not collected.
Practice level. Project psychiatrists were asked to complete two surveys at baseline (after practices were recruited) and at the conclusion of the study (one year after the baseline). The first survey was the 21-item Assessment of Clinician Depression Management (ACDM), adapted for psychiatric care from the Assessment of Chronic Illness Care ( 28 ). The second survey was the Practice Information Form (PIF). These surveys obtained information about general characteristics of each practice and its office system's capacity to provide depression care. Similarly, project co-leaders were asked to complete the ten-item Assessment of Depression-Practice (ADM-P) at baseline and the 12-month follow-up, to provide assessment of practices' depression care from their perspective.
The improvement team roster was completed by each practice to identify improvement team members and their roles. Monthly reports provided qualitative data on the process of implementing change and successes or barriers encountered by the practices. At the conclusion of the project, a program evaluation was completed by both the lead psychiatrist and the co-leader.
Patient level. Project psychiatrists were asked to administer the PHQ-9 each time a new or existing patient age 18 years or older with a primary or secondary axis I diagnosis of a depressive disorder (even patients in remission) had a medical visit at his or her selected outpatient practice site. Patients with bipolar disorder, schizophrenia, and other psychotic disorders were excluded from this study. Lead psychiatrists evaluated patients in their caseload; patient-level data from other clinicians in the selected practice sites were not gathered. For patients who met the aforementioned inclusion criteria, the project psychiatrist completed the following set of information for each encounter on the Depression Monitoring Flow Sheet (DMFS): patient's overall PHQ-9 score for that encounter; the helpfulness of PHQ-9 in clinical decision making; and the type of treatment changes made, if any, based on the PHQ-9 assessments. The deidentified DMFSs were forwarded to APIRE on a monthly basis. The DMFS was pretested by clinicians before its implementation.
Institutional review board
On the basis of the decision of the APIRE Institutional Review Board (IRB) and the IRBs of participating practices, lead psychiatrists and their coleaders were required to sign consent forms. The deidentified patient-level data reported by the psychiatrists via DMFSs, however, were exempt; practices were not required to obtain patients' informed consent.
Analytic methods
All analyses were performed with SAS software. Basic frequencies, cross-tabulations, and their respective chi square tests, as well as paired t tests for subscales and total scores, are reported in the following section.
Results
Nineteen practices (including 19 psychiatrists and 19 coleaders) enrolled in the project, two practices withdrew during the course of the project, and one practice's local IRB review process delayed completion of baseline practice-level information. Therefore, 16 out of 17 practices that continued with the project completed both baseline and follow-up practice-level data that were collected via ACDM, ADM-P, and PIF.
Seven out of 16 lead psychiatrists who completed the ACDM reported a caseload consisting of 25% or more patients from a racial or ethnic minority group. Two psychiatrists provided care to a caseload consisting of 25% or more Medicaid patients.
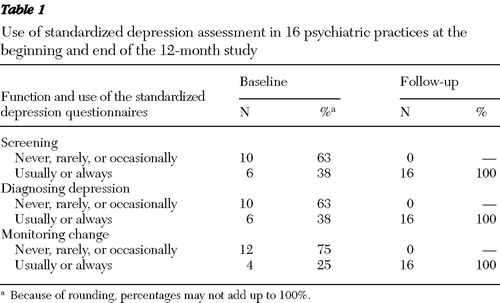
Use of standardized depression assessment
Table 1 displays various ways in which practices utilized standardized depression assessments. At baseline, 16 out of 17 practices reported on their use of standardized depression assessments: six used these assessments for screening or diagnostic purposes, and four used these assessments to monitor change. The types of standardized depression assessments used at baseline included the PHQ-9, QIDS-SR, the Beck Depression Inventory, the Center for Epidemiologic Studies Depression Scale, and the Zung Self-Rating Depression Scale. By the end of 12 months, all 16 practices were usually or always using PHQ-9 for screening, diagnosis, and monitoring change among patients with depression. [An appendix showing a comparison between practices with prior experience in using standardized depression assessments at baseline and those without such experience is available as an online supplement at ps.psychiatryonline.org.]
 |
Characteristics of patients with depressive disorders
By the conclusion of the study, all 17 lead psychiatrists who continued with the study provided patient-level data on 6,363 clinical contacts for 1,763 patients with a diagnosis of depressive disorder. The sample was predominantly female (N=1,174, or 67%) with a median patient age of 49. The primary diagnosis included major depressive disorder (N=1,326, or 75%), depression not otherwise specified (N=190, or 11%), dysthymia (N=165, or 9%), anxiety disorder (N=54, or 3%), other mood disorders (N=7, or <1%), and other psychiatric diagnoses (N=22, or 1%). Forty-two percent of patients (N=740) had one or more co-occurring psychiatric conditions. The most common co-occurring axis I diagnoses were anxiety disorder (N=336, or 19%), a second depressive disorder (N=186, or 11%), and substance use disorder (N=65, or 4%). One percent of patients (N=23) had a diagnosis of co-occurring personality disorder. At the initial visit, 54% (N=960) of patients had PHQ-9 scores ≥10, indicating the presence of moderate symptoms, with 33% (N=575) having PHQ-9 scores ≥15, considered to represent moderately severe or severe symptoms of depression.
Percentage of patients who completed initial and follow-up PHQ-9
According to data obtained from practices' monthly reports, on average across all practices 78% of lead psychiatrists' caseload of adult outpatients with depressive disorders in a given month completed at least one PHQ-9; the range varied across practices from 42% to 100%. On the basis of the DMFS data, of the 1,763 patients who completed the PHQ-9 during an initial visit, a total of 1,378 (78%) completed at least one PHQ-9 in follow-up visits (range varied across practices from 51% to 100%).
Psychiatrists' ratings of helpfulness of the PHQ-9
Reporting via DMFS, lead psychiatrists rated the PHQ-9 score as helpful for treatment decisions in 93% of patient visits (5,663 of 6,096 visits) for 97% of patients with depressive disorders (1,717 of 1,763 patients). Moreover, according to results of the program evaluation that was completed at the conclusion of the study, a majority of the 17 psychiatrists and co-leaders regarded the PHQ-9 to be extremely or very helpful for diagnosing depression (N=12, or 71%, for psychiatrists, and N=13, or 76%, for coleaders, respectively), determining depression severity (N=16, or 94%, and N=16, or 94%, respectively), monitoring response (N=17, or 100%, and N=15, or 88%, respectively), tailoring treatment (N=14, or 82%, and N=13, or 76% respectively), monitoring risk of suicide (N=12, or 71%, and N=13, or 76%, respectively), and for therapeutic alliance (N=9, or 53%, and N=12, or 71%, respectively). No statistically significant differences were observed between the psychiatrists' and coleaders' reports with respect to the factors listed above.
Percentage of treatment decisions altered because of the PHQ-9 score
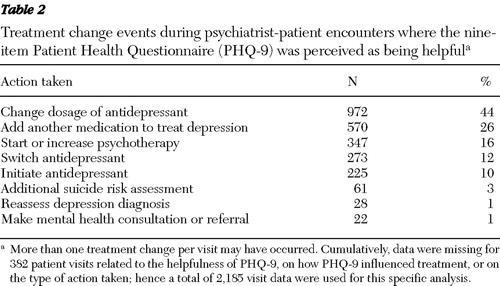
For visits where PHQ-9 was rated as helpful by the lead psychiatrist, the overall PHQ-9 score or item scores led to a treatment change during 40% of visits (2,215 of 5,578 visits), whereas for 60% of the encounters (3,363 of 5,578 visits), the score confirmed the benefits of continuing the treatment plan. During patient visits where treatment was adjusted, changing the dosage of the antidepressant or adding another medication was the most common event recorded by psychiatrists ( Table 2 ). In 3% of the contacts, using the PHQ-9 led to additional suicide risk assessment, and in 1% of the contacts, use of the instrument prompted reassessment of depression diagnosis.
 |
Changes to support implementation of PHQ-9 monitoring
To implement the PHQ-9 successfully, practices employed Plan-Do-Study-Act methodology to test various office-based strategies to support the integration of PHQ-9 in routine care of patients with depression. These incremental adjustments and shared learning experiences led to significant office-based improvements.
Table 3 displays the results of the ACDM and ADM-P analyses. Sixteen surveys were available for pre-post analysis. Survey items were grouped into six domains (described in Table 3 ) in order to study various components of chronic care management issues related to depression care.
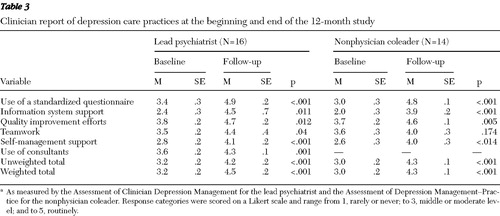 |
The data suggest that practices made substantial gains in various elements of chronic care management for depression. With the exception of the teamwork element from the co-leaders' report, all other individual subscales and the overall scores showed significant improvements from baseline. Analysis of subscales common for both the lead psychiatrist and the project coleader demonstrated no significant differences with respect to depression management capacities within each practice.
Discussion
This study is unique because it examined the helpfulness and feasibility of a simple and easy-to-use depression symptom and severity monitoring tool (that is, PHQ-9) in routine psychiatric care of patients with depression. Unlike STAR*D and TMAP effectiveness studies, where a rigorous stepwise algorithm paralleled depression severity scores, this study tracked routine treatment of patients in psychiatric care. The main focus of the intervention in this study involved the use of PHQ-9 for monitoring depression severity, systematic tracking and population-based approaches for provision of high-quality patient care for depression, and systematic planning and documentation for self-management.
Our study findings suggest that measurement-based care approaches and, more specifically, use of the PHQ-9 for routine monitoring of depression severity and patient outcome are useful clinical tools and feasible to implement in psychiatric practices. Moreover, this study demonstrates that adopting evidenced-based clinical methods is possible, even in practices with limited resources. However, strategies for implementation of PHQ-9 in routine care of patients should be pilot-tested before systemwide implementation and adoption.
At the outset of the project, a majority of practices never, rarely, or only occasionally used standardized depression assessments for screening, diagnosing, or monitoring depression severity. By the conclusion of the project, all 16 practices incorporated the use of PHQ-9 in the routine care of their patients. Project psychiatrists rated PHQ-9 as helpful in their treatment decisions for 93% of patient visits where it was used, which led to a treatment change during 40% of such contacts and confirmed the benefits of continuing a course of treatment for 60% of encounters.
Practices employed the Plan-Do-Study-Act approach to test what works before formal implementation of change. Through these incremental improvement processes, project practices demonstrated significant gains in major components of chronic illness management, including decision support (use of evidence-based depression monitoring assessment tools, such as the PHQ-9, and other evidence-based guidelines and tools to support clinical decision making); clinical information system and delivery system design (establishing a registry for patients with depression to support proactive follow-up); self-management (collaborative planning and documentation of individualized self-management goals with patients); community linkages (improved communication with other providers and systematic follow-up of patients in shared care arrangements); and from the lead psychiatrists' perspective, improvements in organization of health care (gaining support of senior leadership and staff and establishing improvement teams to promote a team approach for implementing change).
This study did have some limitations. First, practice-level changes observed may possibly reflect national trends, which cannot be studied because of a lack of a comparison group. Second, practices volunteered to take part in this project; consequently, findings may not be generalizable to all psychiatric practices. Additionally, over one-third of practices had familiarity with measurement-based care and several utilized standardized depression assessments in their daily practice even before starting the project. Thus participating practices may have been better positioned to adopt measurement-based care, compared with a typical practice setting. Therefore, their reports of feasibility of integrating measurement-based care into practice may not translate as readily in less enthusiastic practices. However, recruitment via self-selection is an approach championed by the Institute for Healthcare Improvement Breakthrough Series methodology, which promotes engaging early adopters to test and implement quality improvements and subsequently assume a leadership role in their dissemination. Given the variety of practice sizes and organizational structures represented in this study, the capacity of various practice types to adopt measurement-based care methods for depression care is promising.
Finally, because of lack of a control group, it will be difficult to make any inference as to whether use of PHQ-9 facilitated improved outcomes in this patient population. The STAR*D and TMAP results have demonstrated that use of depression severity measures coupled with treatment algorithms result in improved outcomes, compared with treatment as usual. This study is unique in assessing the helpfulness of PHQ-9 (as a component of measurement-based care), as part of overall clinical decision making in psychiatric practices. Similar to several primary care trials in depression where patient-rated depression instruments were utilized ( 29 , 30 , 31 , 32 ), findings suggest that PHQ-9 is helpful, despite anecdotal experiences suggesting that many psychiatrists eschew its use because of the belief that it does not provide additional value in clinical practice. With our findings, an appropriate next step is to conduct a clinical trial to determine whether use of PHQ-9 results in better outcomes for patients with depression, compared with usual psychiatric treatment.
The strengths of this study include sampling psychiatric practices from broad geographic areas with significant diversity in organizational structure, practice size, and patient populations; a large number of patients; and the longitudinal design of the study, which included patient-level outcomes (to be reported in a separate article). Moreover, patients with depressive disorders and most nonpsychotic co-occurring conditions were included, potentially providing a more generalizable sample of psychiatric outpatients.
Conclusions
Findings of this study emphasize the helpfulness of a simple and easy-to-use depression symptom and severity monitoring tool (such as PHQ-9) in facilitating clinical decision making in routine psychiatric care of patients with depression. The implementation of measurement-based care methods was achievable even in practices with limited resources. Definitive studies evaluating the magnitude of effect from measurement-based care compared with usual care are necessary next steps.
Acknowledgments and disclosures
This study benefited from generous support of the American Psychiatric Foundation (APF), and a consortium of industry supporters, including AstraZeneca International, Eli Lilly and Company, Lilly Foundation, Forest Laboratories, Pfizer, Sanofi Aventis, and Wyeth, who provided an unrestricted educational grant to the APF for this research. The authors acknowledge significant contributions of the 17 psychiatric practices that participated in this project including the following: Sheryl Ashcroft, Ph.D., R. Scott Benson, M.D., Daniel Chen, M.D., Isabel Erickson, Nadeem Haider, M.D., Elizabeth C. Henderson, M.D., James Henderson, M.S., L.C.P., Lynn Horne, M.D., Michele Howland, M.S.W., L.C.S.W., Robert H. Howland, M.D., Maria Llorente, M.D., Deborah Martz, R.N., Gabrielle Melin, M.D., M.S., Louis Moench, M.D., Ana Muller, L.C.S.W., Alicia Munoz M.A.S., R. Rodrigo A. Munoz, M.D., Susana Prieto, M.D., Cathy Reynolds, M.S., David Resch, M.D., Daniel D. Storch, M.D., Nina G. Storch, Margaret Thurmond, M.A., L.C.P., Victoria Urrutia, M.D., Pamela Van Steinburg, B.S.N., Kristin Vickers-Douglas, Ph.D., Laura Villafane, B.S.N., Paul H. Wick, M.D., Mark D. Williams, M.D., Jeannie Wohl, R.N., and other participating practice sites that took part in this project. The authors thank Liz Kershner, M.S.W., for facilitating the learning sessions, William Narrow, M.D., M.P.H., Joyce West, Ph.D., M.P.P., Maritza Rubio-Stipec, Sc.D., and Paul Sirovatka, M.S., for their contributions to the study, and Lisa Countis and Rebecca Hall, B.A. for assisting with the project implementation.
Dr. Duffy and Mr. Rae have obtained unrestricted educational funds from a consortium of industry supporters, including AstraZeneca International, Eli Lilly and Company, Lilly Foundation, Forest Laboratories, Pfizer, Sanofi Aventis, and Wyeth for this research. Dr. Chung is on the speaker's bureau of and is a consultant for Pfizer. He is also on the advisory board of Jazz Pharmaceuticals and has received grants from Aetna Foundation. Dr. Trivedi has received honoraria or a consulting fee from, or has served as a speaker for, Abbott Laboratories, Abdi Ibrahim, Akzo Pharmaceuticals (Organon Pharmaceuticals), AstraZeneca, Bristol-Myers Squibb Company, Cephalon Pharmaceutical, Cyberonics, Fabre Kramer Pharmaceuticals, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica Products, Eli Lilly and Company, Meade Johnson, Neuronetics, Parke-Davis Pharmaceuticals, Pfizer, Pharmacia and Upjohn, Sepracor Pharmaceuticals, Solvay Pharmaceuticals, Vantage Support, and Wyeth Ayerst Laboratories. Dr. Trivedi has also received research support from Bristol-Myers Squibb Company, Cephalon, Corcept Therapeutics, Cyberonics, Eli Lilly and Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica Products, Merck, Novartis Pharmaceuticals, Pfizer, Pharmacia and Upjohn, Predix Pharmaceuticals, Solvay Pharmaceuticals, Targacept, and Wyeth Ayerst Laboratories. Dr. Katzelnick is on the speaker's bureau for Abbott Laboratories, Forest Pharmaceuticals, GlaxoSmithKline, Eli Lilly and Company, Novartis Pharmaceuticals, Pfizer, Pharmacia, and UCB Pharma; he has also received grant or research support from Abbott Laboratories, AstraZeneca, GlaxoSmithKline, Merck, Novartis Pharmaceuticals, Pfizer, and Solvay; he has also served as a consultant for Abbott Laboratories, Forest Pharmaceuticals, Merck, Novartis Pharmaceuticals, Organon, Pfizer, UCB Pharma, Shire, and Wyeth Ayerst Laboratories. Dr. Katzelnick is also a minor stock shareholder of Johnson and Johnson and a principal stockholder of Healthcare Technology Systems. He has also received intermittent financial support from the pharmaceutical companies listed above. Dr. Regier reports no competing interest.
1. Olfson M, Marcus SC, Pincus HA: Trends in office-based psychiatric practice. American Journal of Psychiatry 153:451–457, 1999Google Scholar
2. Olfson M, Marcus SC, Pincus HA, et al: Antidepressant prescribing practices of outpatient psychiatrist. Archives of General Psychiatry 55:310–316, 1998Google Scholar
3. Pincus HA, Tanielian TL, Marcus SC, et al: Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA 279:526–531, 1998Google Scholar
4. Kessler RC, Berglund P, Demler O, et al: The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication. JAMA 289:3095–3105, 2003Google Scholar
5. Wang PS, Lane M, Olfson M, et al: Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Archives of General Psychiatry 62:590–592, 2005Google Scholar
6. Young AS, Klap R, Sherbourne CD, et al: The quality of care for depressive and anxiety disorders in the United States. Archives of General Psychiatry 58:55–61, 2001Google Scholar
7. Simon GE, Von Korff M, Rutter CM, et al: Treatment process and outcomes for managed care patients receiving new antidepressant prescriptions from psychiatrists and primary care physicians. Archives of General Psychiatry 58:395–401, 2001Google Scholar
8. Katzelnick DJ, KobakKA, JeffersonJW, et al: Prescribing patterns of antidepressant medications for depression in a HMO. Formulary 31:374–378, 1996Google Scholar
9. Fairman KA, Drevets WC, Kreisman JJ, et al: Course of antidepressant treatment drug type, and prescriber's specialty. Psychiatric Services 49:1180–1186, 1998Google Scholar
10. Gilbody SM, House AO, Sheldon TA: Psychiatrists in the UK do not use outcomes measures: national survey. British Journal of Psychiatry 180:101–103, 2002Google Scholar
11. Trivedi MH, Rush AJ, Crismon ML, et al: Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Archives of General Psychiatry 61:669–680, 2004Google Scholar
12. Trivedi MH, Daly EJ: Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug and Alcohol Dependence 88(suppl 2):S61–S71, 2007Google Scholar
13. Trivedi, MH, Rush AJ, Gaynes BN, et al: Maximizing the adequacy of medication treatment in controlled trials and clinical practice: STAR*D measurement-based care. Neuropsychopharmacology 32:2479–2489, 2007Google Scholar
14. Trivedi MH, Rush AJ, Wisniewski SR, et al: Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implication for clinical practice. American Journal of Psychiatry 163: 28–40, 2006Google Scholar
15. Kashner TM, Rush AJ, Suris A, et al: Impact of structured clinical interviews on physicians' practices in community mental health settings. Psychiatric Services 54: 712–718, 2003Google Scholar
16. Trivedi MH, Rush AJ, Ibrahim HM, et al: The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychological Medicine 34:73–82, 2004Google Scholar
17. Rush AJ, Bernstein IH, Trivedi MH, et al: An evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: a Sequenced Treatment Alternatives to Relieve Depression trial report. Biological Psychiatry 59:493–501, 2006Google Scholar
18. Rush AJ, Carmody TJ, Ibrahim HM, et al: Comparison of self-report and clinician ratings on two inventories of depressive symptomatology. Psychiatric Services 57:829–837, 2006Google Scholar
19. Rush AJ, Trivedi MH, Ibrahim HM, et al: The 16-item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry 54:573–583, 2003Google Scholar
20. Kroenke K, Spitzer RL: The PHQ-9: a new depression diagnostic and severity measure. Psychiatric Annals 32:1–7, 2002Google Scholar
21. Kroenke K, Spitzer RL, Williams JB: The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 16:606–613, 2001Google Scholar
22. Spitzer RL, Kroenke K, Williams JB: Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study: Primary Care Evaluation of Mental Disorders: Patient Health Questionnaire. JAMA 282:1737–1744, 1999Google Scholar
23. Dietrich AJ, Oxman TE, Burns MR, et al: Application of a depression management office system in community practice: a demonstration. Journal of the American Board of Family Practice 16:107–114, 2003Google Scholar
24. Institute for Healthcare Improvement Breakthrough Series College. Boston, Mass, Institute for Healthcare Improvement, 2004Google Scholar
25. Wagner EH: Chronic disease management: what will it take to improve care for chronic illness? Effective Clinical Practice 1:2–4, 1998Google Scholar
26. Wagner EH, Austin BT, Davis C, et al: Improving chronic illness care: translating evidence into action. Health Affairs 20(6): 64–78, 2001Google Scholar
27. Katzelnick DJ, Von Korff M, Chung H, et al: Applying depression-specific change concepts in a collaborative breakthrough series. Joint Commission Journal on Quality and Patient Safety 31:386–397, 2005Google Scholar
28. Bonomi AE, Wagner EH, Glasgow RE, et al: Assessment of Chronic Illness Care (ACIC): a practical tool to measure quality improvement. Health Services Research 37:791–820, 2002Google Scholar
29. Dietrich AJ, Oxman TE, Williams JW, et al: Going to scale: re-engineering systems for primary care treatment of depression. Annals of Family Medicine 2:301–304, 2004Google Scholar
30. Alexopoulos GS, Katz IR, Bruce ML, et al: Remission in depressed geriatric primary care patients: a report from the PROSPECT study. American Journal of Psychiatry 162:718–724, 2005Google Scholar
31. Unützer J, Katon W, Callahan CM, et al: Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 288: 2836–2845, 2002Google Scholar
32. Unützer J, IMPACT Investigators: IMPACT Intervention Manual. Los Angeles, UCLA Neuropsychiatry Institute, Center for Health Services Research, 1999Google Scholar



