Racial-Ethnic Differences in Patterns of Discontinuous Medication Treatment Among Medicaid-Insured Youths With ADHD
Abstract
Objective:
This study examined the association between race-ethnicity and patterns of medication gaps and discontinuities among Medicaid-insured children initiating pharmacotherapy for attention-deficit hyperactivity disorder (ADHD).
Methods:
Medicaid claims data from nine states were used to identify racial-ethnic differences in patterns of ADHD medication treatment among 102,669 children initiating ADHD medication. Multinomial logistic regression with state indicators was used to estimate these differences, with adjustment for individual and contextual confounders.
Results:
Approximately three-fifths of the sample did not receive continuous medication treatment as defined by HEDIS guidelines; among them, one-fifth discontinued treatment with no subsequent reinitiation (early termination), less than one-tenth reinitiated pharmacotherapy following a single medication gap, more than three-tenths experienced discontinuous pharmacotherapy with two gaps, and more than four-tenths experienced discontinuous pharmacotherapy with three or more gaps. Compared with white children, black children had a 25% relative increase in the likelihood of early termination and Hispanic children had a 21% relative increase (p<.001); their relative increases in the likelihood of two medication gaps were 41% and 29%, respectively (p<.001), and for three or more gaps they were 56% and 40%, respectively (p<.001).
Conclusions:
Black and Hispanic children were much more likely than white children to be classified as discontinuing ADHD medication treatment, according to HEDIS. The differences predominantly occurred because youths from minority groups were more likely to experience multiple medication gaps, rather than complete discontinuation. Future studies should examine reasons for these multiple gaps to inform interventions to improve ADHD treatment continuity.
Attention-deficit hyperactivity disorder (ADHD) is one of the most common mental disorders among young children (1,2), and racial-ethnic disparities have been documented in the receipt of ADHD treatment (3–6). Medicaid is the largest insurer of youths, providing health insurance coverage to 36 million children in 2014 (7). Studies have found that Medicaid-enrolled youths from racial-ethnic minority groups are less likely to initiate and more likely to discontinue ADHD treatment compared with non-Hispanic whites (3–6,8).
Pharmacotherapy is a common type of treatment received by youths with ADHD and has been shown to reduce core ADHD symptoms (9–11). However, high rates of medication nonadherence may prevent many youths from realizing the potential benefits of these medications (12,13). Nonadherence to medication may include periods of medication gaps or early termination without consultation with a provider (14).
Prior studies exploring racial-ethnic differences in ADHD medication discontinuation have typically examined the period from medication initiation until medication is first discontinued (5,6,14). However, these analyses cannot shed light on the patterns of medication use after the initial discontinuation, including differences in reinitiation or subsequent treatment gaps. Other studies have examined racial-ethnic differences in the number of days that patients should have optimally received medication (14–16). Nevertheless, these aggregated measures of medication adherence represent average medication gaps over the entire follow-up period and provide no information about when and how frequently medication gaps occur or whether subsequent reinitiation takes place. Furthermore, prior research shows that most children who discontinue medication do not receive any psychotherapy services (including behavioral therapy) and disengage from ADHD treatment entirely (8). When medication gaps are followed by subsequent reinitiation, providers have additional opportunities at subsequent visits to intervene and work with families to improve medication continuity. Therefore, it is important to understand the patterns of medication gaps and discontinuities and whether these patterns differ across racial-ethnic groups.
Although ADHD medication gaps may indicate a break from medication that has been planned by parents in consultation with their child’s treating provider (for example, a weekend or summer “drug holiday”) (17), gaps can also indicate an unplanned medication break due to various child-, family-, and health system–level factors. Whether gaps are planned or unplanned, symptoms associated with ADHD quickly return once the child is no longer taking the medication (18). In addition, unplanned periodic medication breaks can create challenges for prescribers to identify efficacy and evaluate the necessity of dose or medication adjustment (19,20). Unplanned breaks are also concerning because of the physiological tolerance that can develop with chronic use of stimulant medication for ADHD; in this case, disruption and discontinuation of medication may produce side effects, including depression, lethargy, and impaired cognition (18,21).
We used Medicaid data from nine states to examine patterns of medication discontinuities among a racially diverse sample of children receiving pharmacotherapy for ADHD. To capture planned medication breaks, we defined discontinuous medication treatment according to the HEDIS guidelines, which allow children to be off medication for a specified period. Our analysis provides the first step toward understanding patterns of medication gaps and discontinuities after initiation of pharmacotherapy and the racial-ethnic differences in these patterns.
Methods
Data
We analyzed 2008–2010 Medicaid Analytic eXtract (MAX) files from Alabama, Georgia, Kentucky, Louisiana, Missouri, North Carolina, Tennessee, Texas, and Virginia, which provided sufficiently complete claims for enrollees in managed care (22–25). We merged MAX data with contextual-level measures from the Area Health Resource File and the 2008 National Survey of Mental Health Treatment Facilities (26,27).
Approval from the Emory University Institutional Review Board was obtained for this study.
Study Sample
Our analysis contained children ages six to 12 who were diagnosed as having ADHD on the basis of two inpatient or outpatient claims associated with ICD-9-CM code 314 (5,9,28–30). Our sample was restricted to children initiating ADHD medication between January 1, 2008, and February 28, 2010. Our sample was derived by using the HEDIS algorithm for quality measures of ADHD care (30). The index date was defined as the first prescription fill date for ADHD medication (30). The observation window included the 120 days prior to the index date, during which children had no prescription for ADHD, and the 300-day treatment period after the index date (30). Within this time frame, children had to be continuously enrolled in Medicaid, with an allowable enrollment gap of up to 45 days (30).
Of the 104,704 children identified, we further excluded those who had inaccurate county codes (N=290), missing information on control measures (N=649), dual Medicare eligibility (N=40), or an acute inpatient encounter for mental illness or substance abuse (N=1,056). Our final analytic sample included 102,669 children.
Measures
Patterns of medication treatment.
We first identified children who had continuous medication treatment defined by HEDIS as having an ADHD prescription filled for at least 210 days of the 300-day period. In this analysis, ADHD prescriptions included stimulant and nonstimulant medications (31). [A table in an online supplement to this article lists the HEDIS prescription medications for ADHD.] According to HEDIS, children are allowed to be off medication for at most 90 days of the 300-day period to account for drug holidays or washout periods, when refills were delayed or medications were switched (30). We divided those who did not meet the HEDIS guidelines for continuous medication into four groups: a single medication stop without subsequent reinitiation (that is, early termination), a single gap with reinitiation, two gaps, and three or more gaps. Of the children with three or more gaps, the mean±SD number of gaps was 3.4±.6, with a maximum of six gaps.
We defined a medication gap as a period in which medication was not filled for at least 14 days (32,33). This 14-day criterion further accounted for expected medication breaks, such as weekend drug holidays, and was applied only to children who did not have continuous medication (30). In sensitivity analyses that used a more stringent threshold of 30 days as an alternative definition of medication gaps, estimates for race-ethnicity were similar in direction and significance.
Race-ethnicity.
We classified race-ethnicity into five mutually exclusive categories: non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic other, and unknown race-ethnicity. Non-Hispanic other included persons of more than one race and racial-ethnic groups that were too small for separate analysis (for example, Asians).
Covariates.
We included individual-level predisposing (age in years and gender) and enabling (type of health plan) characteristics of the sample (34). To control for need-related characteristics, we created dichotomous indicators for the presence of general medical and mental comorbidities. We also used Medicaid eligibility codes to control for the basis of eligibility, a proxy for impairment (35–37). Finally, we used Current Procedural Terminology codes to create an indicator for those who received any (individual, group, or family) psychotherapy (38). These codes lack specificity to identify particular types of psychotherapy services and can include evidenced-based treatment for ADHD (that is, behavioral therapy) (39,40) and other psychotherapy that may not be evidence based. [Tables in the online supplement provide more detail on the variables described in this paragraph.]
Compared with whites, youths from racial-ethnic minority groups may have different experiences switching medications to find an effective drug with fewer side effects, and these differences may disproportionally affect treatment continuity (14,41,42). Thus we created an indicator to determine whether the main active ingredient of the child’s ADHD medication was changed during the treatment period. In addition, newly available formulations of medication may have different side-effect profiles, which may affect medication discontinuities. If there was variation in the receipt of these newer formulations across racial-ethnic groups, it would be important to control for this measure. Therefore, we derived an indicator for those receiving new medication formulations that entered the market in 2008–2010 (43).
Finally, because there may be important differences in sociodemographic characteristics and health care resources across communities in which racial-ethnic minority groups live, compared with whites (8,44–48), we included several measures to assess whether differences in the community context accounted for racial-ethnic differences in medication discontinuities.
Analysis
We first provided descriptive information for medication patterns and model covariates and conducted bivariate analyses to test whether these measures differed across racial-ethnic groups by using Wald tests. We then performed multinomial logistic regression analyses to examine the racial-ethnic differences in the likelihood that children experienced continuous medication, early termination, a single medication gap followed by reinitiation, two gaps, or three or more gaps. Our modeling differentiated multiple gap periods from complete treatment withdrawal, facilitating the clinical interpretation of our findings.
Our first regression model did not include any covariates. Our second model included individual-level predisposing, enabling, and need-related covariates. In the final, fully specified model, we also included county-level confounders and the individual-level measures of medication switching and new medication use. In models 2 and 3, we also included state indicators to adjust for state-level differences that may have influenced the delivery of mental health care for Medicaid-enrolled children. In all models, standard errors were clustered at the county level. Because there was little difference between models 2 and 3, we present the results from the first model and the fully specified model below. [The results for model 2 are presented in a table in the online supplement.] Results from these models were presented as marginal effects for youths from racial-ethnic minority groups (versus white youths) (49,50). Marginal effects were calculated at the observed values of other covariates in the model using the “margins” command in Stata Statistical Software (51). Marginal effects can be interpreted as the percentage-point difference in the model-adjusted likelihood of each minority group that had a specific pattern of medication use, compared with whites (the latter is represented by the model intercept).
Results
Sample Characteristics
Nearly half of the youths in our sample were white (47.8%), 32.1% were black, and 13.7% were Hispanic. Data on other descriptive characteristics are presented in Table 1. In the overall sample, 39.1% had continuous medication according to HEDIS. Of those experiencing discontinuous treatment, 21.6% terminated medication without reinitiation, 7.0% reinitiated medication following a single gap that averaged 152 days, 31.0% experienced two gaps, and 40.3% had three or more gaps (Figure 1). Among those with three or more gaps, the average length of a gap was 43 days.
| Total sample (N=102,669) | Non-Hispanic white (N=49,046) | Non-Hispanic black (N=32,929) | Hispanic (N=14,035) | Non-Hispanic otherb (N=837) | Unknown (N=5,822) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | N | % | N | % | N | % |
| Medication treatment | ||||||||||||
| Continuous | 40,129 | 39.1 | 24,317 | 49.6 | 8,894 | 27.0*** | 4,381 | 31.2*** | 283 | 33.8*** | 2,254 | 38.7*** |
| Early termination | 13,527 | 13.2 | 5,690 | 11.6 | 4,818 | 14.6*** | 2,151 | 15.3*** | 141 | 16.9*** | 727 | 12.5 |
| 1 gap with reinitiation | 4,384 | 4.3 | 2,106 | 4.3 | 1,365 | 4.2 | 600 | 4.3 | 42 | 5.0 | 271 | 4.7 |
| 2 gaps | 19,391 | 18.9 | 7,661 | 15.6 | 7,326 | 22.3*** | 3,105 | 22.1*** | 176 | 21.0*** | 1,123 | 19.3*** |
| ≥3 gaps | 25,238 | 24.6 | 9,272 | 18.9 | 10,526 | 32.0*** | 3,798 | 27.1*** | 195 | 23.3** | 1,447 | 24.9*** |
| Age (M±SD) | 8.0±1.8 | 7.9±1.8 | 8.1±1.8*** | 8.1±1.8 | *** | 8.1±1.9** | 7.9±1.8 | |||||
| Female | 32,866 | 32.0 | 17,086 | 34.8 | 9,941 | 30.2*** | 3,943 | 28.1*** | 258 | 30.8* | 1,638 | 28.1*** |
| Plan type | ||||||||||||
| Fee-for-service only | 7,264 | 7.1 | 3,477 | 7.1 | 1,894 | 5.8*** | 998 | 7.1 | 65 | 7.8 | 830 | 14.3*** |
| Any behavioral health care carve-out plan | 9,940 | 9.7 | 6,429 | 13.1 | 2,613 | 7.9*** | 527 | 3.8*** | 57 | 6.8*** | 314 | 5.4*** |
| Comprehensive managed care plan (no carve-out plan) | 42,108 | 41.0 | 20,766 | 42.3 | 14,759 | 44.8*** | 3,923 | 28.0*** | 246 | 29.4*** | 2,414 | 41.5 |
| Primary care case management (no carve-out plan) | 20,371 | 19.8 | 9,361 | 19.1 | 6,931 | 22.1*** | 2,896 | 20.6*** | 189 | 22.6* | 994 | 17.1*** |
| More than 1 type of plan | 22,986 | 22.4 | 9,013 | 18.4 | 6,732 | 20.4*** | 5,691 | 40.6*** | 280 | 33.5*** | 1,270 | 21.8*** |
| Medicaid eligibility category | ||||||||||||
| Blind or disabled | 10,811 | 10.5 | 1,749 | 3.6 | 3,527 | 10.7*** | 1,658 | 11.8*** | 76 | 9.1*** | 3,801 | 65.3*** |
| Foster care | 7,164 | 7.0 | 3,361 | 6.9 | 2,486 | 7.6*** | 1,103 | 7.9*** | 55 | 6.6 | 159 | 2.7*** |
| Otherc | 84,694 | 82.5 | 43,936 | 89.6 | 26,916 | 81.7*** | 11,274 | 80.3*** | 706 | 84.4*** | 1,862 | 32.0*** |
| General medical comorbidity | ||||||||||||
| Asthma | 18,013 | 17.5 | 7,404 | 15.1 | 6,580 | 20.0*** | 2,604 | 18.6*** | 158 | 18.9** | 1,267 | 21.8*** |
| Any other chronic conditiond | 4,079 | 4.0 | 1,785 | 3.6 | 999 | 3.0*** | 670 | 4.8*** | 41 | 4.9 | 584 | 10.0*** |
| Mental comorbidity | ||||||||||||
| Depressive disorder | 7,802 | 7.6 | 3,492 | 7.1 | 2,584 | 7.9*** | 1,328 | 9.5*** | 54 | 6.5 | 344 | 5.9*** |
| Conduct disorder or oppositional defiant disorder | 23,448 | 22.8 | 9,815 | 20.0 | 8,965 | 27.2*** | 3,060 | 21.8*** | 267 | 31.9*** | 1,341 | 23.0*** |
| Anxiety disorder | 6,610 | 6.4 | 3,600 | 7.3 | 1,660 | 5.0*** | 925 | 6.6** | 39 | 4.7*** | 386 | 6.6* |
| Bipolar disorder | 9,347 | 9.1 | 4,351 | 8.9 | 2,870 | 8.7 | 1,362 | 9.7** | 66 | 7.9 | 698 | 12.0*** |
| Schizophrenia or other psychotic disorder | 1,029 | 1.0 | 341 | .7 | 442 | 1.3*** | 148 | 1.1*** | 8 | 1.0 | 90 | 1.6*** |
| Other | 35,430 | 34.5 | 16,823 | 34.3 | 10,247 | 31.1*** | 5,406 | 38.5*** | 344 | 41.1*** | 2,610 | 44.8*** |
| Received any psychotherapy service in treatment period | 34,016 | 33.1 | 15,206 | 31.0 | 10,866 | 33.0*** | 5,521 | 39.3*** | 233 | 27.8* | 2,190 | 37.6*** |
| Received any new medication in treatment period | 27,701 | 27.0 | 14,213 | 29.0 | 8,746 | 26.6*** | 3,107 | 22.1*** | 211 | 25.2* | 1,424 | 24.5*** |
| Switched medication in treatment period | 41,722 | 40.6 | 21,709 | 44.3 | 11,836 | 35.9*** | 5,324 | 37.9*** | 314 | 37.5*** | 2,539 | 43.6 |
| County level characteristic (M±SD) | ||||||||||||
| % living in urban area | 65.6±29.8 | 54.4±29.3 | 73.7±27.3*** | 85.2±18.8*** | 59.6±29.7*** | 68.2±29.0*** | ||||||
| % living in poverty | 17.0±6.0 | 16.0±5.1 | 17.0±5.2*** | 20.8±8.4*** | 19.0±7.9*** | 16.8±5.7*** | ||||||
| Outpatient mental health facilities per 100,000 residents | 1.3±2.0 | 1.6±2.2 | 1.2±1.8*** | .6±1.3*** | 1.0±1.4*** | 1.3±2.0*** | ||||||
| Community health centers per 100,000 residentse | 3.7±6.6 | 4.4±7.6 | 3.0±5.4*** | 3.2±5.8*** | 5.2±6.0*** | 3.1±5.7*** | ||||||
| Primary care physicians per 100,000 residents | 60.0±27.7 | 56.2±28.6 | 67.6±28.0*** | 54.5±19.6*** | 58.2±28.3* | 61.7±26.9*** | ||||||
| Psychologists per 100,000 residents | 15.8±17.0 | 13.5±15.9 | 20.3±18.8*** | 12.5±12.6*** | 14.5±20.4 | 17.9±17.7*** | ||||||
TABLE 1. Characteristics of Medicaid-insured youths ages six to 12 who initiated ADHD medication, by race-ethnicitya
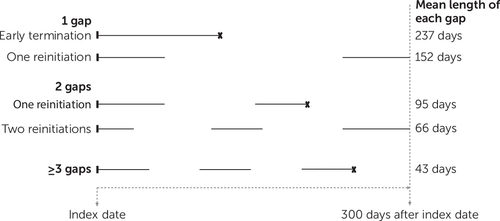
FIGURE 1. Treatment patterns among 62,540 Medicaid-insured children who had discontinuous medication treatment for ADHDa
aSolid lines indicate receipt of ADHD medication in the 300 days after the index prescription. An X at the end of a line indicates termination of treatment. Consistent with HEDIS guidelines, children who had discontinuous treatment did not fill a sufficient number of ADHD medication prescriptions to provide continuous treatment for at least 210 days of the 300-day treatment period. A medication gap was a period in which medication was not filled for at least 14 days. Among children who had three or more gaps, the number of medication gaps ranged from three to six.
Racial-Ethnic Differences in Medication Patterns
In bivariate comparisons (Table 2), children from minority groups were more likely than whites to experience early termination, two gaps, and three or more gaps; the largest absolute black-white difference (marginal effect=12.2%, p<.001) and Hispanic-white difference (marginal effect=8.2%, p<.001) occurred for those with three or more gaps.
| Variable | Continuous treatment | Early termination | 1 gap with reinitiation | 2 gaps | ≥3 gaps | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MEb | SE | MEb | SE | MEb | SE | MEb | SE | MEb | SE | |
| Non-Hispanic black | –24.1*** | .6 | 4.1*** | .3 | .4* | .2 | 7.4*** | .2 | 12.2*** | .3 |
| Hispanic | –19.2*** | 1.3 | 4.1*** | .7 | .3 | .2 | 6.6*** | .6 | 8.2*** | .4 |
| Non-Hispanic other | –16.3*** | 3.0 | 5.0** | 1.7 | .9 | .6 | 5.5*** | 1.0 | 5.0*** | 1.4 |
| Unknown | –11.0*** | .9 | 1.2* | .5 | .5 | .3 | 3.7*** | .5 | 5.7*** | .6 |
| Interceptc | 49.6 | 11.6 | 4.3 | 15.6 | 18.9 | |||||
TABLE 2. Unadjusted multinomial logistic model of racial-ethnic differences in discontinuous medication treatment among Medicaid-insured children who initiated ADHD medicationa
In multivariate analyses (Table 3), the marginal effects indicated that the model-adjusted percentages of black children with early termination, two gaps, and three or more gaps were 3.0, 6.6, and 10.8 percentage-points higher, respectively, compared with whites (p<.001). For example, the model-adjusted percentage of those with three or more gaps was 19.4% for whites (intercept=19.4%) and 30.2% (19.4+10.8) for black youths. The differences are depicted graphically in Figure 2. These absolute black-white differences represent a 25% (3.0/12.2) relative increase in the likelihood of early termination, a 41% (6.6/16.2) relative increase in the likelihood of two gaps, and a 56% (10.8/19.4) relative increase in the likelihood of three or more gaps (p<.001). Compared with white children, Hispanic children had a 21% relative increase in the likelihood of terminating medication, a 29% relative increase in the likelihood of having two gaps, and a 40% relative increase in the likelihood of experiencing three or more gaps (p<.001).
| Variable | Continuous treatment | Early termination | 1 gap with reinitiation | 2 gaps | ≥3 gaps | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MEb | SE | MEb | SE | MEb | SE | MEb | SE | MEb | SE | |
| Race-ethnicity (reference: non-Hispanic white) | ||||||||||
| Non-Hispanic black | –20.9*** | .5 | 3.0*** | .3 | .4* | .2 | 6.6*** | .2 | 10.8*** | .3 |
| Hispanic | –15.4*** | .7 | 2.6*** | .5 | .1 | .2 | 4.7*** | .4 | 7.8*** | .5 |
| Non-Hispanic other | –13.1*** | 2.1 | 3.4** | 1.2 | 1.2 | .6 | 4.7*** | 1.1 | 3.8** | 1.3 |
| Unknown | –10.1*** | .9 | .7 | .5 | .2 | .3 | 3.1*** | .5 | 6.1*** | .7 |
| Age | –2.7*** | .1 | .5*** | .1 | .03 | .03 | 1.0*** | .1 | 1.1*** | .1 |
| Female (reference: male) | –.6 | .3 | .9*** | .2 | .01 | .1 | .003 | .2 | –.4 | .2 |
| Plan type (reference: fee-for-service only) | ||||||||||
| Any behavioral health care carve-out plan | .5 | 1.7 | .3 | .9 | –.4 | .5 | –.5 | .9 | .05 | 1.1 |
| Comprehensive managed care plan (no carve-out plan) | .7 | 1.1 | –.4 | .6 | .05 | .3 | –.6 | .6 | .3 | .8 |
| Primary care case management (no carve-out plan) | .02 | 1.2 | .3 | .6 | .4 | .4 | –.2 | .7 | –.5 | .7 |
| More than one type of plan | –4.4*** | 1.1 | 1.0* | .5 | .8* | .3 | 1.7** | .6 | .9 | .7 |
| Medicaid eligibility category (reference: blind or disabled) | ||||||||||
| Foster care | 19.1*** | .8 | –3.2*** | .5 | –.8* | .3 | –7.4*** | .6 | –7.8*** | .7 |
| Other | –2.0** | .7 | –.03 | .4 | .1 | .3 | .5 | .4 | 1.5** | .5 |
| General medical comorbidity (reference: no indicated condition) | ||||||||||
| Asthma | .2 | .4 | –.5 | .3 | .4** | .2 | –.5 | .3 | .3 | .3 |
| Any other chronic condition | 1.8* | .9 | –.3 | .5 | .3 | .3 | .1 | .6 | –2.0** | .6 |
| Mental comorbidity (reference: no indicated disorder) | ||||||||||
| Depressive disorder | .9 | .7 | 1.3*** | .4 | –.02 | .2 | –.02 | .5 | –2.1*** | .5 |
| Conduct or oppositional defiant disorder | 1.3** | .4 | 1.2*** | .3 | –.2 | .2 | –.3 | .3 | –2.0*** | .3 |
| Anxiety disorder | .9 | .7 | 1.1** | .4 | .4 | .3 | –.7 | .4 | –1.7** | .5 |
| Bipolar disorder | 2.5*** | .6 | 1.6*** | .4 | .6** | .2 | –.5 | .4 | –4.1*** | .4 |
| Schizophrenia or other psychotic disorder | 2.5 | 1.6 | .1 | 1.0 | .9 | .5 | –1.5 | 1.2 | –2.1 | 1.2 |
| Other | 1.5*** | .4 | 1.1*** | .2 | –.2 | .1 | –.8** | .3 | –1.6*** | .3 |
| Received any psychotherapy service in treatment period (reference: no) | 6.3*** | .4 | –2.5*** | .3 | –.2 | .1 | –1.2*** | .3 | –2.5*** | .3 |
| Received any new medication in treatment period (reference: no) | 11.9*** | .4 | –6.9*** | .3 | –.1 | .1 | –3.3*** | .3 | –1.6*** | .3 |
| Switched medication in treatment period (reference: no) | 13.0*** | .4 | –11.8*** | .4 | 2.2*** | .1 | .4 | .2 | –3.9*** | .3 |
| County level characteristicc | ||||||||||
| % living in urban area | –.8* | .3 | .2 | .2 | .01 | .1 | .1 | .2 | .4 | .2 |
| % living in poverty | –1.9*** | .3 | .6** | .2 | .1 | .1 | .9*** | .1 | .3 | .2 |
| Outpatient mental health clinics per 100,000 residents | .03 | .2 | .04 | .1 | –.1 | .1 | .04 | .1 | –.001 | .2 |
| Community health centers per 100,000 residents | –.1 | .2 | –.01 | .1 | .02 | .1 | –.2 | .1 | .3 | .2 |
| Primary care physicians per 100,000 residents | –.01 | .3 | –.2 | .2 | .02 | .1 | –.3 | .2 | .4 | .2 |
| Psychologists per 100,000 residents | –.2 | .3 | –.1 | .2 | –.1 | .1 | .3* | .2 | .1 | .2 |
| Interceptd | 47.9 | 12.2 | 4.3 | 16.2 | 19.4 | |||||
TABLE 3. Multivariate multinomial logistic model of racial-ethnic differences in discontinuous medication treatment among Medicaid-insured children who initiated ADHD medicationa
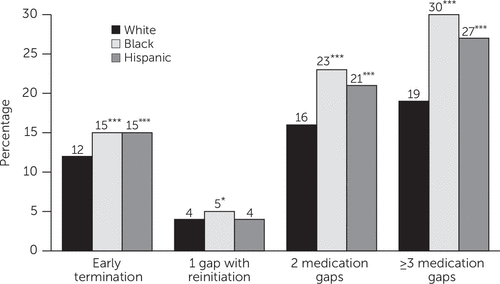
FIGURE 2. Model-adjusted patterns of discontinuous medication treatment among 102,669 Medicaid-insured children who initiated ADHD medication, by race-ethnicitya
aPredicted percentages represent model-adjusted likelihoods of having a specific pattern of discontinuous medication treatment among white children, with other covariates held at their observed values, estimated with a multinomial logistic regression model. The model also controlled for state indicators, the county-level measures of sociodemographic characteristics and mental health care resources, and the individual-level measures of sociodemographic and need-related characteristics, medication switching, and use of a new medication.
*p<.05, ***p<.001
These results were similar to those from the intermediate model specification that did not include measures of newer medication use, medication switches, and county-level characteristics [see table in online supplement]. When the intermediate and final model specifications for race-ethnicity were compared, supplemental analyses (not shown) indicated that the inclusion of county-level covariates accounted for most of the change in the estimates associated with race-ethnicity across these two models.
Other Factors Associated With Medication Patterns
Medicaid eligibility through foster care (versus disability) and the presence of comorbid mental and general medical conditions were associated with a higher likelihood of continuous medication and a lower rate of three or more gaps (p<.05). (Table 3) Moreover, use of newer medication, medication switches, and the receipt of psychotherapy were associated with an increased likelihood of continuous medication, along with decreased likelihoods of early termination and three or more gaps (p<.001).
Supplemental Analyses
We estimated a multinomial probit model, which relaxes the assumption of the independence from irrelevant alternatives in the multinomial logistic model (52). In addition, we bootstrapped standard errors as a robustness check (53). Our findings remained similar (not shown). Finally, we conducted descriptive analyses to examine whether there were racial-ethnic differences in the likelihood of having a medication gap during the summer months, a proxy for a drug holiday. No racial-ethnic differences were observed in the rate of having any medication gap during the summer; however, youths from minority groups who stopped medication in the summer were more likely than whites to have a longer medication break [see table in online supplement].
Discussion
This study examined medication treatment patterns among Medicaid-insured children initiating pharmacotherapy for ADHD and differences in these patterns across racial-ethnic groups. Approximately three-fifths of our sample did not have continuous medication treatment according to HEDIS guidelines; of these, only one-fifth terminated medication entirely without reinitiating at a later date. Most youths without continuous medication experienced multiple medication gaps. Youths from minority groups were more likely than white youths to terminate medication without reinitiating and to experience multiple medication gaps. Our findings build on prior analyses that used aggregated adherence measures or examined the initial medication discontinuation (5,14,54) by highlighting how most youths who temporarily stopped medication reinitiated at a later date but experienced multiple medication gaps.
Consistent with evidence of the increased risk of early discontinuation among youths from racial-ethnic minority groups (5,14,54), we found that black and Hispanic children were more likely than white children to terminate medication entirely. However, most of the racial-ethnic differences in discontinuous medication were driven by the fact that youths from minority groups were more likely to experience multiple medication gaps with subsequent reinitiation, rather than to terminate medication entirely. Importantly, reinitiation after medication gaps would suggest that clinicians have additional opportunities to interact with families at subsequent visits to enhance treatment continuity.
To develop strategies to reduce racial-ethnic disparities in medication discontinuities, it is important to identify the mechanisms that explain these disparities. In our study, we specifically examined two medication-related mechanisms—medication switching and use of newer medication formulations on the market. Adjusting for these measures, however, did little to explain the observed racial-ethnic differences. Other potential explanations for the differential treatment patterns may be related to the health care system, particularly unequal geographic access to care, language barriers, and access to different types of prescribers (55,56). To capture differences in geographic access to care, we controlled for measures of geographic accessibility to health care resources; however, the racial-ethnic differences in medication gaps remained similar. Language barriers may be also problematic in treatment continuity for mental disorders for some minority groups, given that changes in emotions associated with these disorders are largely identified by patients’ ability to verbalize those feelings (55). Furthermore, research suggests that white children are more likely to receive mental health care from specialist physicians, whereas those from minority groups are more likely to see pediatricians (56). However, we could not measure language or types of prescribing provider (for example, psychiatrist versus pediatrician) with the available data. Future research should further explore these unmeasured system-related factors to illuminate the mechanisms for addressing the differential patterns of ADHD medication discontinuities among youths from diverse racial-ethnic backgrounds.
Recent studies have highlighted the role of parents’ preferences in choices of treatment for their child’s ADHD, which can largely affect treatment continuity (57–60). Research also indicates that parents from racial-ethnic minority groups may value the risks and benefits of medication differently than white parents (14,61,62). Specifically, parents from minority groups may be more concerned than white parents that these medications have harmful side effects (61). Accordingly, nonwhite parents are more likely than white patients to be dissatisfied with drug therapy overall (14,61), and some parents prefer living with their child’s symptoms off medication to living with the medication’s side effects (63). Furthermore, many nonwhite parents believe that taking ADHD medication can lead to drug addiction (61,62). Thus the culturally influenced preferences for and perceptions of pharmacotherapy may be another explanation for the differential medication patterns.
Notably, medication discontinuities do not necessarily reflect undesirable care quality. A gap period may represent a drug holiday (17), which may occur during school breaks (14). Indeed, our supplementary analyses showed that for most children who did not have continuous medication according to HEDIS, an extended gap occurred between May and August. Although no racial-ethnic difference was observed in the rate of any medication gap during the summer, youths from minority groups who stopped medication in the summer were more likely than white youths to have a longer medication break [see online supplement]. These extended medication breaks may nevertheless raise concerns, given prior research indicating that most Medicaid-enrolled youths who discontinue ADHD pharmacotherapy do not receive any psychotherapy and disengage from treatment entirely (8).
Several study limitations should be noted. As with all claims-based analyses, we could not distinguish clinically appropriate discontinuation from discontinuation without clinical guidance. In addition, there may have been unmeasured differences in clinical severity or patients’ preferences for treatment across racial-ethnic groups. Moreover, the prescription claims do not provide information on whether children actually took the medication. Similarly, no data were available on services reimbursed by other payers, and it is unknown whether these findings would be generalizable to privately insured youths. Nevertheless, the MAX data remain our best source of information on patterns of care continuity among diverse, low-income youths.
Furthermore, the results were generated from nine states and may not generalize to other states. Nevertheless, Medicaid-enrolled youths in these states are an important research population; more than one-third of black youths and over one-fourth of Hispanic youths in the United States reside in these states (64–66), which have less generous Medicaid benefits and lower mental health expenditures than the national average (67,68). Finally, our data are relatively dated. Nonetheless, there is no reason to believe that the relationship between race-ethnicity and medication treatment patterns has changed since our study period.
Conclusions
This study raises important questions regarding discontinuity of ADHD pharmacotherapy and multiple medication gaps among Medicaid-enrolled youths. Children from racial-ethnic minority groups were much less likely than white children to be classified as having continuous medication treatment according to HEDIS. Most of these differences in discontinuous medication were accounted for by an increased likelihood that black and Hispanic (versus white) youths experienced multiple medication gaps, rather than an increased likelihood of terminating medication entirely. Future research is needed to identify the most salient factors that contribute to these racial-ethnic differences in medication gaps and discontinuities and to develop intervention strategies to improve treatment continuity among all youths with ADHD.
1 : Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics 125:75–81, 2010Crossref, Medline, Google Scholar
2 : Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry 49:980–989, 2010Crossref, Medline, Google Scholar
3 : Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Annals of Pharmacotherapy 42:24–31, 2008Crossref, Medline, Google Scholar
4 : The impact of long-acting medications on attention-deficit/hyperactivity disorder treatment disparities. Journal of Child and Adolescent Psychopharmacology 23:401–409, 2013Crossref, Medline, Google Scholar
5 : Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Archives of Pediatrics and Adolescent Medicine 159:572–578, 2005Crossref, Medline, Google Scholar
6 : Treatment of attention-deficit hyperactivity disorder: patterns of evolving care during the first treatment episode. Psychiatric Services 63:122–129, 2012Link, Google Scholar
7 FY 2014 Unduplicated Number of Children Ever Enrolled in Medicaid and CHIP. Baltimore, Centers for Medicare and Medicaid Services, 2014. https://www.medicaid.gov/chip/downloads/fy-2014-childrens-enrollment-report.pdf. Accessed May 22, 2017Google Scholar
8 : Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics 139:e20162444, 2017Crossref, Medline, Google Scholar
9 : National trends in the treatment of attention deficit hyperactivity disorder. American Journal of Psychiatry 160:1071–1077, 2003Link, Google Scholar
10 : Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics 108:1033–1044, 2001Crossref, Medline, Google Scholar
11 : National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics 113:754–761, 2004Crossref, Medline, Google Scholar
12 : Stimulant treatment over five years: adherence, effectiveness, and adverse effects. Journal of the American Academy of Child and Adolescent Psychiatry 43:559–567, 2004Crossref, Medline, Google Scholar
13 : Medication adherence in the MTA: saliva methylphenidate samples versus parent report and mediating effect of concomitant behavioral treatment. Journal of the American Academy of Child and Adolescent Psychiatry 48:501–510, 2009Crossref, Medline, Google Scholar
14 : Risk factors for discontinuing drug therapy among children with ADHD. Health Services and Outcomes Research Methodology 8:134–158, 2008Crossref, Google Scholar
15 : Patient characteristics associated with medication adherence. Clinical Medicine and Research 11:54–65, 2013Crossref, Medline, Google Scholar
16 : Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clinical Therapeutics 34:944–956.e4, 2012Crossref, Medline, Google Scholar
17 : Drug holidays from ADHD medication: international experience over the past four decades. Journal of Attention Disorders 19:551–568, 2015Crossref, Medline, Google Scholar
18 : Potential adverse effects of discontinuing psychotropic drugs: part 4. benzodiazepine, glutamate, opioid, and stimulant drugs. Journal of Psychosocial Nursing and Mental Health Services 48:11–14, 2010Crossref, Google Scholar
19 : Review of medication adherence in children and adults with ADHD. Postgraduate Medicine 122:184–191, 2010Crossref, Medline, Google Scholar
20 : Attention-deficit/hyperactivity disorder: an update on medication adherence and persistence in children, adolescents and adults. Expert Review of Pharmacoeconomics and Outcomes Research 13:791–815, 2013Crossref, Medline, Google Scholar
21 : Stimulant withdrawal. Addiction 89:1477–1481, 1994Crossref, Medline, Google Scholar
22 Evaluating Encounter Data Completeness for Researchers Using the Centers for Medicare and Medicaid Services Chronic Condition Data Warehouse (CCW). Falls Church, VA, LewinGroup, May 2012. https://www.ccwdata.org/web/guest/white-papers-presentations. Accessed Aug 22, 2017Google Scholar
23 Medicaid Analytic eXtract (MAX) General Information. Baltimore, Centers for Medicare and Medicaid Services. https://www.medicaid.gov/medicaid/data-and-systems/collection-systems/max/index.html. Accessed May 1, 2017Google Scholar
24 Byrd VLH, Dodd AH, Malsberger R, et al: Assessing the Usability of MAX 2008 Encounter Data for Enrollees in Comprehensive Managed Care. Princeton, NJ, Mathematica Policy Research, July 2012. https://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/Downloads/MAX_IB7_EncounterData_071312.pdf. Accessed May 20, 2017Google Scholar
25 Byrd VLH, Dodd AH: Assessing the Usability of Encounter Data for Enrollees in Comprehensive Managed Care Across MAX 2007–2009. Princeton, NJ, Mathematica Policy Research, Dec 2012. https://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/Downloads/MAX_IB_15_AssessingUsability.pdf. Accessed May 20, 2017Google Scholar
26 Area Health Resource Files, 2011–2012. Rockville, MD, US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Professions, 2012Google Scholar
27 National Survey of Mental Health Treatment Facilities. Rockville, MD, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, 2008Google Scholar
28 : Medication use and spending trends among children with ADHD in Florida’s Medicaid program, 1996–2005. Psychiatric Services 63:115–121, 2012Link, Google Scholar
29 : Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 19:187–195, 2009Crossref, Medline, Google Scholar
30 HEDIS Archives: HEDIS2014. Washington, DC, National Committee on Quality Assurance, 2014. http://www.ncqa.org/hedis-quality-measurement/hedis-measures/hedis-archives. Accessed May 2, 2017Google Scholar
31 HEDIS 2014 Final NDC Lists. Washington, DC, National Committee on Quality Assurance, 2014. http://www.ncqa.org/hedis-quality-measurement/hedis-measures/hedis-2014/hedis-2014-final-ndc-lists. Accessed Aug 1, 2017Google Scholar
32 : Effect of methylphenidate formulation for attention deficit hyperactivity disorder on patterns and outcomes of treatment. Journal of Child and Adolescent Psychopharmacology 14:575–581, 2004Crossref, Medline, Google Scholar
33 : Maintenance treatment with long-acting injectable risperidone in first-episode schizophrenia: a randomized effectiveness study. Journal of Clinical Psychiatry 73:1224–1233, 2012Crossref, Medline, Google Scholar
34 : Revisiting the behavioral model and access to medical care: does it matter? Journal of Health and Social Behavior 36:1–10, 1995Crossref, Medline, Google Scholar
35 Bagchi AD, Verdier J, Esposito D: Chartbook: Medicaid Pharmacy Benefit Use and Reimbursement in 2007. Princeton, NJ, Mathematica Policy Research, May 2011. https://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/Downloads/Pharmacy_Rx_Chartbook_2007.pdf. Accessed May 13, 2017Google Scholar
36 General Dynamics Information Technology: Considerations for Researchers Using MAX Data for Researchers Using the Centers for Medicare and Medicaid Services’ Chronic Condition Data Warehouse (CCW). Falls Church, VA, LewinGroup, Sept 2012. Accessed May 13, 2017Google Scholar
37 General Dynamics Information Technology: Evaluating Medicaid Long-Term Services and Supports Utilization for Researchers Using the Centers for Medicare and Medicaid Services’ Chronic Condition Data Warehouse (CCW). Falls Church, VA, LewinGroup, Sept 2012. https://www.ccwdata.org/documents/10280/19002254/evaluating-medicaid-long-term-services-and-supports-utilization.pdf. Accessed Aug 22, 2017Google Scholar
38 : Ten-year trends in treatment services for children with attention-deficit hyperactivity disorder enrolled in Medicaid. Health Affairs 35:1266–1270, 2016Crossref, Medline, Google Scholar
39 : ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011Crossref, Medline, Google Scholar
40 : Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. Clinical Psychology Review 26:486–502, 2006Crossref, Medline, Google Scholar
41 : Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics 100:662–666, 1997Crossref, Medline, Google Scholar
42 : Racial differences in medication switching and concomitant prescriptions in the treatment of bipolar disorder. Psychiatric Services 57:666–672, 2006Link, Google Scholar
43 National Drug Code Directory. Silver Spring, MD, US Department of Health and Human Services, US Food and Drug Administration, 2017. https://www.fda.gov/drugs/informationondrugs/ucm142438.htm. Accessed May 1, 2017Google Scholar
44 : Availability of youth services in US mental health treatment facilities. Administration and Policy in Mental Health and Mental Health Services Research 43:717–727, 2016Crossref, Medline, Google Scholar
45 : Contextual socioeconomic status and mental health counseling use among US adolescents with depression. Journal of Youth and Adolescence 43:1151–1162, 2014Crossref, Medline, Google Scholar
46 : Geography and the Medicaid mental health care infrastructure: implications for health care reform. JAMA Psychiatry 70:1084–1090, 2013Crossref, Medline, Google Scholar
47 : Geographic access to specialty mental health care across high- and low-income US communities. JAMA Psychiatry 74:476–484, 2017Crossref, Medline, Google Scholar
48 Census 2000 Geographic Terms and Concepts. Washington, DC, US Census Bureau, 2000. https://www2.census.gov/geo/pdfs/reference/glossry2.pdf. Accessed May 20, 2017Google Scholar
49 : Race, insurance status, and nulliparous, term, singleton, vertex cesarean indication: a case study of a New England tertiary hospital. Women’s Health Issues 26:329–335, 2016Crossref, Medline, Google Scholar
50 : Correlates of mental health service use and type among Asian Americans. Administration and Policy in Mental Health and Mental Health Services Research 41:543–551, 2014Crossref, Medline, Google Scholar
51 Stata Statistical Software, Version 13.1. College Station, TX, Stata Corp, 2013Google Scholar
52 : Estimating a multinomial probit model of brand choice using the method of simulated moments. Marketing Science 11:386–407, 1992Crossref, Google Scholar
53 : Confidence intervals for predicted outcomes in regression models for categorical outcomes. Stata Journal 5:537–559, 2005Crossref, Google Scholar
54 : Persistence of stimulants in children and adolescents with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 22:139–148, 2012Crossref, Medline, Google Scholar
55 : Racial-ethnic differences in the use of psychotropic medication in high-risk children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 42:1433–1442, 2003Crossref, Medline, Google Scholar
56 : Ethnic differences in children’s entry into public mental health care via emergency mental health services. Journal of Child and Family Studies 18:512–519, 2009Crossref, Medline, Google Scholar
57 : Assessment of parents’ preferences for the treatment of school-age children with ADHD: a discrete choice experiment. Expert Review of Pharmacoeconomics and Outcomes Research 11:245–252, 2011Crossref, Medline, Google Scholar
58 : Parent preferences regarding stimulant therapies for ADHD: a comparison across six European countries. European Child and Adolescent Psychiatry 23:1189–1200, 2014Crossref, Medline, Google Scholar
59 : Preferences for treatment of attention-deficit hyperactivity disorder (ADHD): a discrete choice experiment. BMC Health Services Research 9:149, 2009Crossref, Medline, Google Scholar
60 : Systematic review of patients’ and parents’ preferences for ADHD treatment options and processes of care. The Patient 8:483–497, 2015Crossref, Medline, Google Scholar
61 : Parental perceptions and satisfaction with stimulant medication for attention-deficit hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics 24:155–162, 2003Crossref, Medline, Google Scholar
62 : Latina mothers’ stances on stimulant medication: complexity, conflict, and compromise. Journal of Developmental and Behavioral Pediatrics 25:311–317, 2004Crossref, Medline, Google Scholar
63 : Caught in a balancing act: parents’ dilemmas regarding their ADHD child’s treatment with stimulant medication. Qualitative Health Research 16:1267–1285, 2006Crossref, Medline, Google Scholar
64 Annual State Resident Population Estimates for 6 Race Groups by Age, Sex, and Hispanic Origin: April 1, 2010, to July 1, 2012. Washington, DC, US Census Bureau, 2012Google Scholar
65 Annual Estimates of the Resident Population by Sex, Age, Race, and Hispanic Origin for the United States and States: April 1, 2010, to July 1, 2012. Washington, DC, US Census Bureau, 2013Google Scholar
66 The State of America’s Children. Washington, DC, Children’s Defense Fund, 2014. http://www.childrensdefense.org/library/state-of-americas-children/2014-soac.pdf?utm_source=2014-SOAC-PDF&utm_medium=link&utm_campaign=2014-SOAC. Accessed May 1, 2017Google Scholar
67 Where Are States Today? Medicaid and CHIP Eligibility Levels for Children, Pregnant Women, and Adults. Menlo Park, CA, Kaiser Family Foundation, March 2017. http://files.kff.org/attachment/Fact-Sheet-Where-are-States-Today-Medicaid-and-CHIP-Eligibility-Levels-for-Children-Pregnant-Women-and-Adults. Accessed May 5, 2017Google Scholar
68 SMHA Mental Health Actual Dollar and Per Capita Expenditures by State (FY2004–FY2013). Falls Church, VA, National Association of State Mental Health Program Directors Research Institute, 2017Google Scholar



