Evidence-Based Use of Second-Generation Antipsychotics in a State Medicaid Pediatric Population, 2001–2005
Use of antipsychotic medications in office-based clinical practice has increased significantly in adult populations since the 1990s, and numerous studies have reported evidence of increasing use of these medications among children and adolescents ( 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 ). The increase has been most dramatic for newer, second-generation antipsychotics compared with first-generation agents.
For adults, the U.S. Food and Drug Administration (FDA) has approved use of second-generation antipsychotics for treatment of schizophrenia, bipolar and mood disorders, and selected other conditions ( 10 , 11 , 12 , 13 ). The upward trend in use of second-generation antipsychotics among children and adolescents has alarmed some authorities, partly because the FDA has approved limited use of only two of these agents in pediatric populations. In October 2006 risperidone was first approved for limited pediatric use—for irritability in autistic disorder in populations aged five to 16 years. Approval was given the following year for its use in the treatment of adolescents with schizophrenia and bipolar disorders. In 2007 aripiprazole was approved for treatment of schizophrenia among adolescents, and approval was expanded to include use for children with bipolar I disorder on the basis of a four-week trial involving patients aged ten to 17 ( 14 ). As of December 2009, several other second-generation antipsychotic medications were approved by the FDA for short-term use in certain pediatric groups and for serious mental health conditions.
Although most use of second-generation antipsychotics in pediatric populations has not been reviewed by the FDA, there is body of clinical trial evidence about use of these medications by children. These published trials have shortcomings in terms of sample size and study duration, which underlines the necessity for further study in this area. Nevertheless, clinicians may view favorable results of such clinical trials as evidence supporting use of second-generation antipsychotics by their pediatric patients. Use of a medication to treat a patient with a diagnosis for which some clinical trial evidence exists may be considered "evidence based." Conversely, use of a medication to treat a condition or a type of patient when there has been no effort to systematically study safety and effectiveness can be considered "not evidence based."
The purpose of this study was to describe use of second-generation antipsychotics in a pediatric population. Specifically, the study had three objectives. First, the study identified new users under age 18 who received second-generation antipsychotics in a state Medicaid population from 2001 through 2005. Second, for each child treated, the study classified use of the agent as either evidence based or not depending on the child's diagnoses. This approach followed a concept used in a study of anticonvulsants in the Georgia Medicaid population ( 15 ). Third, the study identified factors associated with the likelihood of receipt of drug treatment categorized as evidence based.
Methods
Medicaid data
This project was reviewed and approved by the University of Arkansas for Medical Sciences Institutional Review Board. The study involved a retrospective analysis of data from a state Medicaid administrative claims database. The database allowed examination of all medical claims and diagnoses and of all outpatient medication claims. The study population consisted of children who were new users of second-generation antipsychotics. A new user was operationally defined as receiving his or her first prescription for one of these agents—the index prescription—between January 1, 2001, and December 31, 2005, with no antipsychotic use during the 12 months before the date of service associated with the index prescription. The participant was required to be under age 18 on the index date and to have been continuously eligible for Medicaid one year before and one year after the index date.
Relevant information from pharmacy, inpatient, outpatient, and nursing home claims and from enrollment records was collected from January 2000 to December 2006. Information from before the study period was used to ensure that patients were excluded if they had been treated with an antipsychotic in the year before the index date. Because a time lag may occur between initial treatment and establishment of a definitive psychiatric diagnosis, medical claims data were obtained for the 12 months after the study to ensure the maximum likelihood of capturing all relevant diagnoses for children who started treatment late in the study period.
All prescription claims indicating that clozapine, olanzapine, risperidone, quetiapine, ziprasidone, or aripiprazole was dispensed for a child were extracted from the pharmacy claims database on the basis of specific generic codes for the medications. Outpatient and inpatient medical claims for 365 days before and after the index prescription date were obtained. All diagnosis codes from a participant's medical claims were documented. By use of a scheme similar to those found in other studies ( 16 , 17 , 18 , 19 , 20 ), participants were classified on the basis of their diagnoses into different disease groups for which antipsychotics may be used. We used a list of ICD-9-CM codes for identifying different disease groups in the sample. The codes were first identified from the literature and then validated by using the sixth edition of ICD-9-CM .
Levels of evidence for antipsychotic use
During the study period, none of the second-generation antipsychotics had been reviewed and approved by the FDA to treat children with any condition, and thus all use was considered off label. Off-label use—that is, use for conditions other than the approved indications—is not prohibited. It may be supported by a robust data set of clinical trial information or may have no support in peer-reviewed literature or clinical practice experience. Therefore, characterizing a particular use of a medication as off label is not especially informative. Although the appropriateness of various off-label uses may be debated, there is a line of thinking that justifies such use when it is based on rational scientific theory or evidence from controlled clinical trials ( 21 ). Thus it has been suggested that compared with off-label use for which no scientific data are available, off-label use for which evidence exists is justifiable to some extent.
We used the approach of assigning hierarchical levels of evidence on the basis of research design. This approach is based on guidelines developed by the Agency for Healthcare Research and Quality (AHRQ) and by two of the AHRQ Evidence-based Practice Centers ( 1 , 22 ). Use of a specific second-generation antipsychotic for a particular condition was classified as "strong evidence-based use" if it was supported for that condition by at least one published randomized clinical trial with a sample size of more than 30 individuals in a population under 18 years of age. Use was classified as "plausible evidence-based use" if it was supported for the specific condition by at least one published nonrandomized trial with a comparison group and a sample size of more than 30 individuals in a population under 18 years of age. Finally, use was classified as "weak evidence-based use" if use for the condition was based on published reports of studies involving individuals less than 18 years of age, including open-label studies, retrospective studies with a sample size of less than 30, or nonrandomized trials with a sample size of more than 30 but without a comparison group. Any other specific use of a medication that did not fall into one of these categories was classified as "non-evidence-based use."
A MEDLINE literature search identified clinical trials of second-generation antipsychotics in pediatric populations. All trials published before December 31, 2005, were considered. However, the date of publication was not considered in assigning the medication uses to the evidence categories, which allowed assignment in the most liberal fashion as well as consistent assignment of specific medication uses for the entire period of analysis. This approach acknowledges that certain findings may be disseminated among clinicians before studies are published. Studies were not considered if any of the participants were aged 18 years or older, if the study had three or fewer total participants, and if the results were not favorable for the antipsychotic.
The MEDLINE search identified 86 unique clinical studies. Most were open-label studies involving risperidone. All identified studies were reviewed to compile a comprehensive list of medications and uses ( ICD-9-CM diagnosis codes) along with the level of evidence to support each use (strong, plausible, or weak evidence).
Because patient charts were not examined, it was difficult to establish a direct link between a specific diagnosis and the decision to initiate treatment with a second-generation antipsychotic. Thus we elected an approach that increased the likelihood of including a diagnosis for which use of a second-generation antipsychotic would be classified as evidence based. As noted, all diagnosis codes assigned to a study participant in the year before and after the study period were available. If a recipient was assigned at least one diagnosis that was associated with some level of evidence support, the medication use for that recipient was considered evidence based. Use of a medication was assigned to the no-evidence category when there was no published support for use of the medication with any of the diagnoses assigned to a patient during the observation period.
A sensitivity analysis was conducted that included diagnoses given to the children only in the six months before and after their prescription index date. A second sensitivity analysis included 24 additional studies identified in the MEDLINE search that were published after the cutoff date of December 31, 2005; these included nine for risperidone and six for quetiapine.
Data analysis
To determine the trend of new users of second-generation antipsychotics in the state Medicaid population under age 18 from 2001 through 2005, we calculated the number of new users in each year. Trend graphs and ordinary least-squares regression were used to determine whether the trend was significant. For each second-generation antipsychotic, the number and percentage of new users in each disease category were determined over the five-year study period.
We used a logistic regression model to determine which groups were more or less likely to have an evidence-based use of a second-generation antipsychotic. This model used age as a continuous independent variable; categorical independent variables were gender, race, geographic location, metropolitan county status, receipt of psychotherapy, psychiatric hospitalization, and long-term care. For the model, evidence-based use was defined as meeting the standard for strong evidence-based use, and plausible and weak evidence-based use were considered to be non-evidence-based use. The significance level was set at .05. Log of odds (logit) as a linear function of the independent variables was employed, and the maximum likelihood estimation procedure was used to obtain the parameter estimates. SAS, version 9.1, was used for all data manipulations and statistical analyses.
Results
Of the 52,521 Medicaid-covered children with at least one prescription claim for a second-generation antipsychotic from 2001 to 2005, a total of 11,700 met the inclusion criteria. Table 1 presents data on demographic characteristics of the sample. Compared with the Arkansas Medicaid pediatric population, the sample was much older and had a larger proportion of females.
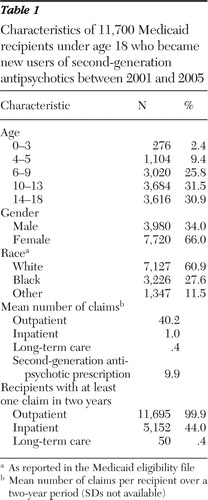 |
Table 2 shows the number of children who initiated therapy over five years with each of the second-generation antipsychotics. The denominator used to calculate the percentages was the number of children continuously enrolled in Medicaid each year, which was fairly constant across the five years. None of the children received initial treatment with clozapine. For most children, therapy was initiated with risperidone (51.2%). The agent least used for initial treatment was ziprasidone (2.5%). Of interest, .5% of the sample received more than one second-generation antipsychotic prescription on their index date. Results of the regression analysis indicated that, on average, 431 children each year initiated treatment with a second-generation antipsychotic (p<.001).
 |
The disease categories to which diagnoses obtained from the children's medical claims were assigned are shown in Table 3 , along with the ICD-9-CM codes that define the categories. It should be noted that a child with two or more diagnoses could be counted in more than one category. The most common condition identified was attention-deficit hyperactivity disorder, followed by depression, conduct disorder, oppositional defiant disorder, and adjustment reactions.
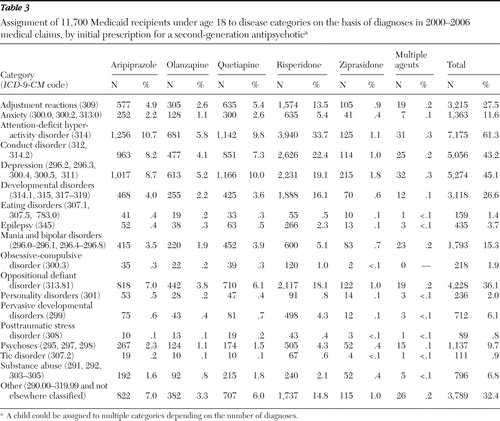 |
At the time of the study there were four conditions for which treatment with risperidone was well supported on the basis of the reports published before December 31, 2005: conduct disorder, developmental disorders, pervasive developmental disorders, and psychoses. Plausible evidence for risperidone use was found for the treatment of mania and bipolar disorders. Weak evidence supported use of risperidone for three disease categories—epilepsy, obsessive-compulsive disorder, and tic disorder. For olanzapine, strong evidence was found for use in treating psychoses and plausible evidence was found for mania and bipolar disorders. For quetiapine, strong evidence was found for use in treating mania and bipolar disorders. No strong or plausible evidence was found for use of aripiprazole and ziprasidone to treat any condition.
Table 4 shows the proportion of use of the five medications in each of the evidence categories. Among users of risperidone, 64.1% of use was in the category of strong evidence-based use—the largest percentage for any of the five medications. Among users of aripiprazole, 77.1% of use was not supported by any published evidence.
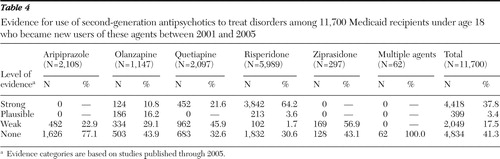 |
Almost half of the children in the sample (41.3%) appeared to have received a second-generation antipsychotic without having a diagnosis associated with any supporting evidence ( Table 4 ). On the basis of this finding, we further investigated the diagnoses of these 4,834 participants. Over half of them (N=2,790, 58%) had a diagnosis of hyperkinetic syndrome of childhood with hyperactivity. Other frequently observed diagnoses among these 4,834 children were oppositional defiant disorder (N=1,617, 34%), depressive disorder (N=1,354, 28%), hyperkinetic syndrome of childhood without mention of hyperactivity (N=955, 20%), and unspecified disturbance of conduct (N=692, 14%).
Table 5 presents the results of the logistic regression model that focused on factors associated with strong evidence-based uses of the medications. All the variables used in the model were significantly associated with strong evidence. Participants who had received psychotherapy were nearly twice as likely as those who had not to have an index prescription that was evidence based (odds ratio [OR]=1.92). Having a previous psychiatric hospitalization significantly increased the odds of receipt of an evidence-based prescription (OR=1.92).
 |
We conducted a sensitivity analysis that included diagnoses given to children in only the six months before and after their index prescription date. In this analysis the percentage of children given prescriptions that were not based on any evidence increased from 41.3% to 55.1%. Another sensitivity analysis included evidence from clinical studies that were published after the cutoff date of December 31, 2005. In this analysis the overall percentage of uses supported by weak evidence increased from 17.5% to 33.5%; the proportion of strong evidence-based use was not affected.
Discussion
These results add to the evidence that treatment of children with second-generation antipsychotic medications increased dramatically in the early years of this new century. Although prevalence is not a focus of this report, the number of children who received an initial prescription for a second-generation antipsychotic doubled between 2001 and 2005 in the Medicaid population that we examined. Because Medicaid enrollment grew more slowly than the number of patients initiating such treatment, we conclude that use of these medications in this population is increasing. Further studies are needed to determine whether this trend is evident in other pediatric populations, especially among children not enrolled in Medicaid.
It was not surprising that for every year examined most new users of second-generation antipsychotics were given an initial prescription for risperidone. Risperidone had been commercially available for several years before the study period. In addition, of the five second-generation medications examined, at the time of the study risperidone was supported by the largest base of evidence for use among children and adolescents. Risperidone accounted for the highest number of new users each year, and the proportion of new users who were prescribed risperidone as an initial treatment was consistent throughout the study period.
The growth in aripiprazole use is of particular interest. We identified five studies of its use in pediatric populations, and all of them offered only weak evidence; thus it is not surprising that our results revealed that 77% of its use was not evidence based. Nevertheless, the number of new pediatric users of aripiprazole, which was introduced in 2002, increased dramatically over the study period. New users increased by 338% between 2003 and 2004 and by 368% between 2003 and 2005. It is notable that in 2005 the proportion of children who received aripiprazole as an initial therapy (8.2%) was almost equal to the proportion who received initial therapy with risperidone (9.8%).
It is apparent that the increased acceptance of second-generation antipsychotics in the treatment of children during the study period was independent of a comparably robust expansion of the medical literature supporting their use. There continue to be only a limited number of specific conditions among children for which use of second-generation antipsychotics is supported by any evidence. The conditions for which there is current FDA approval for pediatric use—for aggressive or self-injurious behavior among autistic children, for schizophrenia, and for bipolar I disorder—are rare for children. In our sample of children treated with second-generation antipsychotics between 2001 and 2005, these conditions were apparently present in only a small minority. Nevertheless, clinicians seem to be finding an increasing role for these agents in clinical settings.
Little information has been published about the clinical indications for which clinicians prescribe second-generation antipsychotics for children. This study identified diagnoses given to children who received these agents. In addition, we attempted to determine the proportion of use of these medications that could be described as evidence based. Our literature review of articles published through March 2007 identified 110 clinical studies of second-generation antipsychotic use in pediatric populations. Only 19 of the 110 studies were randomized placebo-controlled clinical trials, of which 12 studied risperidone. On the basis of our criteria, only ten clinical studies provided strong evidence for use of a second-generation antipsychotic in a pediatric population and four provided plausible evidence. This finding emphatically underlines the scarcity of well-designed, high-quality clinical studies to inform clinicians about the safety and effectiveness of these medications for conditions affecting children.
On the one hand, despite the relatively thin base of evidence, we found that 38% of the children in the sample had a diagnosis for which use of a second-generation antipsychotic was supported by strong evidence. Most was attributable to use of risperidone to treat children with diagnoses of conduct disorder and oppositional defiant disorder. However, use of other agents to treat children in this sample lacked substantial evidence.
On the other hand, 62% of children who started treatment with a second-generation antipsychotic during the study period did not have a diagnosis for which such treatment was supported by strong evidence. Examination of the diagnoses given to children in this sample reveals important information about the clinical applications of these medications. Clearly, behavioral problems, including oppositional and conduct disorders and hyperkinetic-hyperactivity symptoms, were frequently seen among the children treated with second-generation antipsychotics. If the primary off-label uses for these medications are for children with behavioral problems, there is an obvious and urgent need to compare the safety and effectiveness of these agents and of other treatment approaches that are effective in modifying children's behaviors.
Other conditions that appear to be frequently diagnosed among children receiving second-generation antipsychotics include depression and adjustment disorder. It is not clear in our sample whether these conditions were primary diagnoses or comorbid conditions. In addition, because of the design of this analysis, these diagnoses may have been given after initiation of treatment with second-generation antipsychotics. Therefore, the possibility that treatment of maladaptive behaviors with second-generation antipsychotics may lead to high rates of depression cannot be discounted and should prompt urgent assessment.
As with all studies, a number of limitations bear consideration. A significant limitation is that the design could not establish a link between initiation of second-generation antipsychotic treatment and a specific triggering diagnosis. Administrative claims data can help to establish a temporal relationship between the start of a treatment and a new or changed diagnosis, but this relationship may or may not reflect the clinical reasons for a change in therapy. Obviously, this analysis was dependent on the diagnosis data that were recorded, and we did not have access to the working diagnoses that a clinician might have been considering with a trial of a medication. The validity of diagnoses is also a concern, particularly because—for younger children especially—certain psychiatric conditions may be difficult if not impossible to establish. Another concern is that we may have overestimated the number of evidence-based users because we grouped specific diagnoses into broad disease categories and classified evidence-based used on the basis of the broad categories.
Clearly, further research is needed to understand prescribing of second-generation antipsychotics in pediatric populations. The questions to answer are numerous, and the answers should be informed by an awareness, which is highlighted in this report, that much of the use of these medications is unrelated to the current FDA-approved indications. Further, pediatric use of second-generation antipsychotics may be only marginally related to the specific conditions that have previously been studied. Important considerations include specific sequelae of such treatment in terms of patients' mental and social well-being as well as their overall physical health. Certainly, the most useful studies would compare these powerful new agents with other medications that have established uses and with behavioral and psychosocial interventions.
Conclusions
The results of this study indicate that use of second-generation antipsychotics in the treatment of children and adolescents increased considerably from 2001 to 2005 in a state Medicaid population. Much of the growth was attributable to rapid adoption of aripiprazole as an initial treatment, despite a paucity of evidence from clinical trials supporting use among children at the time of the study. Fewer than half of the children initiating treatment were given a diagnosis—either before or after treatment initiation—for which strong clinical evidence supported use of the second-generation agent. Children who were treated with psychotherapy and those who had been admitted to psychiatric hospitals before the index date of their initial treatment with a second-generation medication were more likely to have a diagnosis for which medication use was supported by strong clinical evidence. Further research is needed to understand the prescribing of second-generation antipsychotics in the pediatric population.
Acknowledgments and disclosures
The authors thank Jill Johnson, Pharm.D., for guidance and advice in developing evidence-based criteria and conducting the literature search and Gary Moore for assistance in preparing the data extracts from the original claims. The Arkansas Department of Human Services provided access to the data at no charge.
The authors report no competing interests.
1. Cooper WO, Hickson GB, Fuchs C, et al: New users of antipsychotic medications among children enrolled in TennCare. Archives of Pediatric and Adolescent Medicine 158:753–759, 2004Google Scholar
2. Martin A, Leslie D: Trends in psychotropic medication costs for children and adolescents, 1997–2000. Archives of Pediatric and Adolescent Medicine 157:997–1004, 2003Google Scholar
3. Pappadopulos E, Jensen PS, Schur SB, et al: "Real world" atypical antipsychotic prescribing practices in public child and adolescent inpatient settings. Schizophrenia Bulletin 28:111–121, 2002Google Scholar
4. Patel NC, Crismon ML, Hoagwood K, et al: Trends in the use of typical and atypical antipsychotics in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 44:548–556, 2005Google Scholar
5. Zito JM, Safer DJ, DosReis S, et al: Psychotropic practice patterns for youth: a 10-year perspective. Archives of Pediatric and Adolescent Medicine 157:17–25, 2003Google Scholar
6. Campbell M, Rapoport JL, Simpson GM: Antipsychotics in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 38:537–545, 1999Google Scholar
7. Curtis LH, Masselink LE, Ostbye T, et al: Prevalence of atypical antipsychotic drug use among commercially insured youths in the United States. Archives of Pediatric and Adolescent Medicine 159:362–366, 2005Google Scholar
8. Cooper WO, Arbogast PG, Ding H, et al: Trends in prescribing of antipsychotic medications for US children. Ambulatory Pediatrics 6:79–83, 2006Google Scholar
9. Olfson M, Blanco C, Liu L, et al: National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Archives of General Psychiatry 63:679–685, 2006Google Scholar
10. Cortese L, Pourcher-Bouchard E, Williams R: Assessment and management of antipsychotic-induced adverse events. Canadian Journal of Psychiatry 43(suppl 1):15S–20S, 1998Google Scholar
11. Essock SM, Hargreaves WA, Dohm FA, et al: Clozapine eligibility among state hospital patients. Schizophrenia Bulletin 22:15–25, 1996Google Scholar
12. Kelly DL, Love RC: Ziprasidone and the QTc interval: pharmacokinetic and pharmacodynamic considerations. Psychopharmacology Bulletin 35:66–79, 2001Google Scholar
13. Leucht S, Pitschel-Walz G, Abraham D, et al: Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized controlled trials. Schizophrenia Research 35:51–68, 1999Google Scholar
14. Data on file, study 31-03-240. Rockville, Md, Otsuka Maryland Research Institute, 2005–2007Google Scholar
15. Chen H, Deshpande AD, Jiang R, et al: An epidemiological investigation of off-label anticonvulsant drug use in the Georgia Medicaid population. Pharmacoepidemiology Drug Safety 14:629–638, 2005Google Scholar
16. Harpaz-Rotem I, Leslie DL, Martin A, et al: Changes in child and adolescent inpatient psychiatric admission diagnoses between 1995 and 2000. Social Psychiatry and Psychiatric Epidemiology 40:642–647, 2005Google Scholar
17. Levine LJ, Schwarz DF, Argon J, et al: Discharge disposition of adolescents admitted to medical hospitals after attempting suicide. Archives of Pediatric and Adolescent Medicine 159:860–866, 2005Google Scholar
18. Harpaz-Rotem I, Leslie D, Rosenheck RA: Treatment retention among children entering a new episode of mental health care. Psychiatric Services 55:1022–1028, 2004Google Scholar
19. Guevara J, Lozano P, Wickizer T, et al: Utilization and cost of health care services for children with attention-deficit/hyperactivity disorder. Pediatrics 108:71–78, 2001Google Scholar
20. Guevara J, Lozano P, Wickizer T, et al: Psychotropic medication use in a population of children who have attention-deficit/hyperactivity disorder. Pediatrics 109:733–739, 2002Google Scholar
21. Blumer JL: Off-label uses of drugs in children. Pediatrics 104:598–602, 1999Google Scholar
22. Harris RP, Helfand M, Woolf SH, et al: Current methods of the US Preventive Services Task Force: a review of the process. American Journal of Preventive Medicine 20:21–35, 2001Google Scholar



