Diffusion of Antipsychotics in the U.S. and French Markets, 1998–2008
Abstract
Objective
Second-generation antipsychotics captured most of the U.S. antipsychotic market shortly after their introduction. Little is known about how second-generation antipsychotics have diffused in other countries with different health systems. The study objective was to describe trends in antipsychotic use in the United States and France from 1998 to 2008.
Methods
Pharmaceutical policies in France and the United States are briefly described, followed by descriptive data on quarterly prescriptions for oral antipsychotics dispensed between January 1998 and September 2008. Data are from Xponent for the United States and the GERS database for France. Trends in the use of first- versus second-generation antipsychotics and in ingredient formulations of second-generation antipsychotics used are reported.
Results
Between 1998 and 2008, total antipsychotic use in the United States increased by 78%. Total use in France was consistently higher despite a 9% decrease during the period. By 2008, second-generation antipsychotics represented 86% of the antipsychotics sold in the U.S. market, versus only 40% of the French market. However, average annual growth rates in use of second-generation antipsychotics were similar in the two countries. In France, use of all but one second-generation antipsychotic steadily increased, whereas in the United States trends in the use of newer drugs varied substantially by drug. For example, use of olanzapine decreased after 2003, but use of quetiapine increased.
Conclusions
These results highlight markedly divergent trends in the diffusion of new antipsychotics in France and the United States. Some differences may be explained by differences in health systems; others may reflect physicians’ preferences and norms of practice.
The antipsychotic market in the United States is dominated by second-generation antipsychotics. Seven new drugs introduced between 1989 and 2006 quickly took nearly 90% of the market for antipsychotics and expanded the total number of users of antipsychotic medication, with a substantial portion of expanded use owing to off-label indications (1–3). The rapid diffusion of second-generation antipsychotics has been controversial in light of evidence of increased metabolic risks (4,5) and their significantly higher costs compared with first-generation antipsychotics (6–8).
Other countries have also seen significant increases in use of more costly second-generation antipsychotics (9–13); however, few studies have compared trends in the use of antipsychotics in the United States and other countries. We compared trends in use of antipsychotics in the United States with those of France for two reasons. First, according to the World Health Organization, France has the highest-performing health system (14). Second, physicians in the United States and France had access to a somewhat similar list of approved second-generation antipsychotics but differed with respect to reimbursement policy, use of cost sharing, and other functions.
The study reported here examined trends between 1998 and 2008. We present trends in use overall, by class (first- versus second-generation antipsychotics), and by product. To provide some context for our findings, we briefly present background on the two countries’ health system features that may influence medication utilization. Although a full examination of the impact of these features on antipsychotic diffusion is beyond the scope of this article, we offer some hypotheses in our discussion section as to how antipsychotic use may be shaped by health system and other factors. We also provide information on the drugs available (specifically, drugs approved by regulators) for use in both countries.
Background on the French and U.S. health systems
Health systems support the development and appropriate diffusion of technology through their regulatory approval process, reimbursement policy, postmarketing surveillance of drug safety, and provision of safety information to clinicians and consumers (Table 1). More detailed overviews of the French system have been published elsewhere (15,16). Both pharmaceutical systems have similar drug approval processes and pharmacovigilance systems, and both countries offer coverage for low-income and elderly populations. Conversely, the universality and comprehensiveness of health insurance benefits, the policies regarding drug pricing, and drug promotion regulations differ dramatically between the two countries.
| Measure | United States | France |
|---|---|---|
| Drug approval | ||
| Agency | Food and Drug Administration | French drug agency (ANSMa), European Medicine Agency, or other European drug agency |
| Criteria | Efficacy, safety, and product quality | Efficacy, safety, and product quality |
| Timing of market launch postapproval | Immediate | Delay (on average 1 year) to allow reimbursement and price decisions |
| Coverage | ||
| Formulary | Varies by payer and plan | National decision by French Ministry for Health |
| Level of coverage (share of cost covered by payer) | Varies by plan, and within plan by drug | National decision by French Ministry for Health according to the severity of disease and value of the drug |
| Price paid to manufacturer | Varies by drug and plan | Nationally set and varies by drug |
| Out-of-pocket cost | Varies by payer and drug; 100% of the drug cost for the uninsured (17% of the U.S. population in 2010) | Copayment level varies by therapeutic class (0%, 35%, 65%, or 85% of the drug cost). Patients registered with a chronic condition or with supplemental insurance (94%) typically have no out-of-pocket costs for drugs but have a deductible of €.50 per drug package (up to €50 per year) |
| Pharmacovigilance | ||
| Adverse drug reactions collection | Voluntary reporting system | Voluntary reporting. Noxious and unintended adverse drug reactions must be reported to ANSM |
| Safety warnings | Public health advisories, safety alerts, and “Dear health care provider” letters | Various public communications (mise au point, point d’information, and “Dear health care provider” letters) |
| Label change | Black box warning | Inserted into monograph sections |
| Labeling changes specific to antipsychotic | ||
| Metabolic risk of second-generation antipsychotics | September 2003 | None |
| Second-generation antipsychotics in dementia | April 2005 | March 2004 |
| First-generation antipsychotics in dementia | June 2008 | December 2008 |
| Promotion of prescription drugs | ||
| Direct to consumer | Permitted | Banned |
| Drug samples | Permitted | Tightly restricted |
| To physicians | Regulated | Regulated |
| Physicians per pharmaceutical representative in 2006b | 7.4 | 8.9 |
| Off-label use status | Allowed but may be restricted by some payers (such as prior authorization for antipsychotic use by children) | Allowed if evidence based and if there is no other alternative |
Drug approval and marketing
Approval of new drugs is based on the same criteria in both countries: drug quality, efficacy, and safety. However, whereas drugs are typically marketed by manufacturers shortly after approval by the Food and Drug Administration (FDA) in the United States, commercialization in France does not occur until separate agencies make price-setting and reimbursement decisions.
Coverage, payment, and pricing
The U.S. system lacks universal coverage; approximately 49.9 million individuals were uninsured in 2010 (17). Insured individuals obtain their coverage through commercial insurers (often employer sponsored) or through public sources such as Medicare or Medicaid. Adults with severe mental disorders are more likely than those without such disorders to be uninsured (21.0% versus 16.5%); if they are insured, they are more likely to receive coverage through Medicare or Medicaid than through commercial insurers (18). In contrast, France mandates enrollment of all persons in its health system, which includes prescription drug benefits.
Systems for determining reimbursement prices for prescription drugs also differ greatly between the two countries. In the French ambulatory care setting, drug prices are set nationally through negotiation between the health authorities and pharmaceutical firms and depend on the drug’s innovation (19,20). Prices paid for pharmaceutical drugs in the United States vary by payer.
In the United States, each payer takes a different approach to coverage of medications, including antipsychotics. For example, Medicare requires the plans with which it contracts to cover “all or substantially all” antipsychotics (21). Similarly, most Medicaid programs do not restrict coverage of antipsychotic medications, although some states impose prior-authorization requirements on some (22). Other third-party payers may limit coverage of or impose higher cost sharing for some antipsychotics.
In France, all approved antipsychotics are reimbursed. Under a general rule, patients must pay 35% of the drug cost. However, in practice, few patients pay this share because pharmacy copayments are generally covered by the patient’s supplemental insurance (and 94% had such insurance in 2008) (23). In addition, patients with chronic psychiatric conditions are offered full coverage for health care costs related to their chronic disease.
In the United States, patients’ out-of-pocket costs vary by payer and drug. Patients enrolled in Medicaid and Medicare beneficiaries who are eligible for low-income subsidies face little to no out-of-pocket cost (24). For other Medicare beneficiaries, costs vary widely (22). In 2010, commercially insured patients spent out of pocket an average of $46 monthly for a brand-name second-generation antipsychotic and $12 monthly for a generic form (25). Uninsured patients are responsible for the full cost of drugs, and large cost differences exist between first-generation antipsychotics and most second-generation antipsychotics (7).
In France, there is usually no out-of-pocket cost associated with antipsychotic medications apart from a €.50 deductible per package of drugs purchased (up to a limit of €50 per year). Thus patients do not spend more for branded or nonpreferred drugs versus generics, as they typically do in the United States.
Pharmacovigilance
After drugs have been approved, both the FDA and French Agence Nationale de Sécurité du Médicament may issue safety warnings as evidence emerges on a drug’s risk profile. The FDA may require labeling changes to be printed in black box warnings or added to the drug’s monograph. In France the new safety information is added to the drug’s monograph and patient information leaflet found in every drug package.
Information concerning the risks of antipsychotics has been handled differently by the two countries’ regulatory agencies. In September 2003, the FDA issued a warning about the metabolic risks of second-generation antipsychotics. No such warning has been issued in France. Both countries’ regulatory agencies issued warnings about increased mortality risk with second-generation antipsychotics used by older adults with dementia (in March 2004 in France and April 2005 in the United States); these warnings were later expanded in 2008 in both countries to include all antipsychotics.
Promotion
Promotion of pharmaceuticals to health care professionals is regulated in both countries. Promotional materials should be consistent with drug labels and, consistent with FDA and ANSM language, “not be false or misleading”; in particular, promoting off-label use is forbidden. Drug samples and direct-to-consumer promotion of prescription drugs are permitted in the United States but are tightly restricted in France.
Methods
Data sources
For the United States, the data were collected from prescriptions dispensed for oral antipsychotics from IMS Health’s Xponent database. Xponent directly captures over 70% of all U.S. prescriptions filled in retail pharmacies and uses a patented, proprietary projection methodology to represent 100% of prescriptions filled in these outlets. We obtained monthly data on all oral antipsychotic prescriptions that were filled from January 1, 1998, through September 30, 2008, by patients of a 10% random sample of U.S. physicians; these patients filled at least one prescription for an antipsychotic over the period. To extrapolate to all U.S. physicians, we multiplied the prescriptions observed in our data set by 10.
French prescription fills for oral antipsychotics from 1998 to 2008 were extracted from the GERS (Groupement pour l’élaboration et la réalisation de statistiques) database, which collects data on sales to community pharmacies for all pharmaceutical products in France.
Drugs
Antipsychotics were identified by an ATC (anatomical, therapeutic, and chemical) classification code starting with N05A (excluding lithium N05AN). In some analyses, we report product-specific use among the second-generation antipsychotics available in one or both countries by the end of the study period.
Use of injectable antipsychotics was not included because this form is mainly administered in hospitals or physicians’ offices and thus is not recorded in the databases we used.
Analysis
We combined all drug formulations at the ingredient level and report quarterly market shares or rates of use. For both data sources, we converted drug quantities into a monthly unit of treatment (the quantity needed for 30 days of treatment regardless of strength). For the United States, we assumed that a prescription equaled a 30-day supply. Although this assumption might underestimate the total number of prescriptions if a substantial number were filled for a 90-day supply by mail order pharmacies, we did not expect mail order use to vary by class or product. Furthermore, antipsychotics were highly likely to be filled for a 30-day supply because of dispensing limits imposed by most state Medicaid programs (24), which finance a majority of antipsychotics (21).
We converted the French data from number of packages sold each quarter into monthly supplies. For second-generation antipsychotics, packages of 30 or 60 tablets of any strength corresponded to a monthly supply (assuming daily intake and that a dispensed package corresponded to a monthly prescription). However, because first-generation antipsychotics’ packages are frequently dispensed for quantities other than a month’s supply (with 20 or 50 tablets), we used 2006 IMS estimates of mean tablets used per day for commonly used first-generation antipsychotics to calculate average monthly supplies (26).
To determine population-based rates of antipsychotic use, we calculated the number of monthly treatments per 1,000 inhabitants using yearly estimates of population size from the U.S. Census Bureau and its French equivalent (the Institut National de la Statistique et des Études Économiques).
Results
Antipsychotic drugs available in each country
More first-generation antipsychotics were approved and marketed in France than in the United States (15 versus 11, respectively) during the study period, and the specific drugs available differed, with only four first-generation drugs (chlorpromazine, haloperidol, pimozide, and loxapine) available with oral forms in both countries. By 2008, more second-generation antipsychotics were available in the United States than in France (seven versus five) (Table 2). Four drugs were available in both countries: clozapine, risperidone, olanzapine, and aripiprazole. Amisulpride, the first second-generation antipsychotic introduced in France, is not commercialized in the United States, whereas quetiapine, available since 1997 in the United States, was not approved until 2010 in France. On average, second-generation antipsychotics were launched 2.1 years (range 18–32 months) earlier in the United States than in France.
| United States | France | ||||
|---|---|---|---|---|---|
| Drug | Approval date | Indication | Approval date | Marketing date | Indication |
| Paliperidone | Dec. 2006 | Schizophrenia | June 2007 | — | |
| Aripiprazole | Nov. 2002 | Schizophrenia | June 2004 | June 2004 | Schizophrenia in adults and adolescents age ≥15 |
| Sept. 2003 | Maintenance treatment in schizophrenia | ||||
| Sept. 2004 | Acute treatment of manic or mixedb episodes in bipolar disorder type I | March 2008 | Acute treatment of manic episodes in bipolar disorder | ||
| March 2005 | Preventive treatment in bipolar disorder type I | March 2008 | Maintenance treatment in bipolar disorder | ||
| Nov. 2007 | Major depressive disorder (adjunct)c | ||||
| Oct. 2007 | Schizophrenia in children ages 13–17c | ||||
| Feb. 2008 | Acute manic or mixed episodes in bipolar disorder in children ages 10–17c | ||||
| Nov. 2009 | Irritability in autistic disorder in children ages 6–17c | ||||
| Ziprasidone | Feb. 2001 | Schizophrenia | — | — | Special authorization (autorisation temporaire d’utilisation) since 2007 |
| June 2002 | Acute agitation in schizophrenic patients | ||||
| Aug. 2004 | Acute treatment of manic or mixed episodes in bipolar disorder type I | ||||
| Nov. 2009 | Maintenance treatment in bipolar disorder type I (adjunct) | ||||
| Quetiapine | Sept. 1997 | Schizophrenia | Nov. 2010 | Oct. 2011 | Schizophrenia |
| Jan. 2004 | Acute treatment of manic episodes in bipolar disorder type I | Nov. 2010 | Acute treatment of manic episodes in bipolar disorder | ||
| Jan. 2004 | Acute treatment of depressive episodes in bipolar disorder | Nov. 2010 | Acute treatment of depressive episodes in bipolar disorder | ||
| Jan. 2004 | Maintenance treatment in bipolar disorder type I | Nov. 2010 | Maintenance treatment in bipolar disorder | ||
| Nov. 2010 | Major depressive disorder (adjunct)c | ||||
| Olanzapine | Sept. 1996 | Schizophrenia | Sept. 1996 | June 1999 | Schizophrenia |
| March 2000 | Acute treatment of manic or mixedb episodes in bipolar disorder type I (monotherapy or in combinationd July 2003) | June 2002 | Acute treatment of manic episodes in bipolar disorder | ||
| Jan. 2004 | Maintenance treatment in bipolar disorder type I | Oct. 2003 | Maintenance treatment in bipolar disorder type I | ||
| Dec. 2003 | Depressive episodes associated with bipolar I disorder with fluoxetinec | ||||
| March 2009 | Severe depression (with fluoxetine)c | ||||
| Dec. 2009 | Schizophrenia or acute manic or mixed episodes in bipolar disorder in children ages 13–17c | ||||
| Risperidone | Dec. 1993 | Schizophrenia | May 1995 | Feb. 1996 | Schizophrenia |
| March 2002 | Maintenance treatment in schizophrenia | ||||
| Dec. 2003 | Acute treatment of manic or mixedb episodes in bipolar disorder type I | Aug. 2006 | Acute treatment of manic episodes in bipolar disorder | ||
| Aug. 2007 | Schizophrenia and bipolar disorder in children ages 13–17c | Aug. 2006 | Aggressiveness in children ≥5 years with mental retardation | ||
| Oct. 2006 | Irritability associated with autistic disorder in children | July 2008 | Aggressiveness in patients with dementiac | ||
| Clozapine | Sept. 1989 | Treatment-resistant schizophrenia | June 1991 | Nov. 1991 | Treatment-resistant schizophrenia; psychotic disorders in Parkinson’s diseasec |
| Amisulpride | — | — | Jan. 1986 | March 1991 | Schizophrenia |
The extension of second-generation antipsychotics’ labels to cover additional indications also occurred sooner in the United States than in France, and drugs generally had a greater number of indications in the United States (Table 2).
Trends in total antipsychotic use
From 1998 to 2008, the two countries saw different trends in total use (Figure 1). In the United States, total antipsychotic use per 1,000 inhabitants increased by 78%, while in France, it declined slightly, by 9%. Yet the overall level of antipsychotic use was consistently higher in France than in the United States during the study period, although the gap narrowed; use in France was more than threefold higher in 1998 but was less than twice as high by 2008.
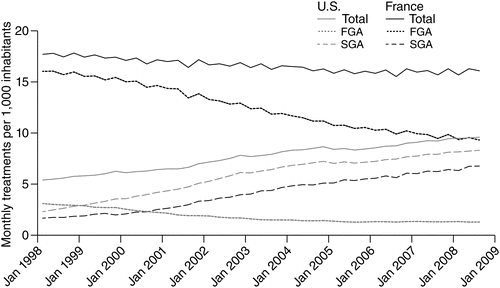
aSources: United States, IMS Xponent, 1996–2008, IMS Health, Inc. France, GERS database 1998–2008. FGA, first-generation antipsychotics; SGA, second-generation antipsychotics
First-generation versus second-generation antipsychotics
In 2008, first-generation antipsychotics represented 59% of the antipsychotic market in France compared with only 14% in the United States (Figure 2). Indeed, second-generation antipsychotics had captured a majority of the market for antipsychotics in the United States by 1999. However, the mean±SE annual growth rate in second-generation antipsychotic use did not significantly differ between the two countries: 12.7%±2.8% in the United States versus 13.9%±2.5% in France.
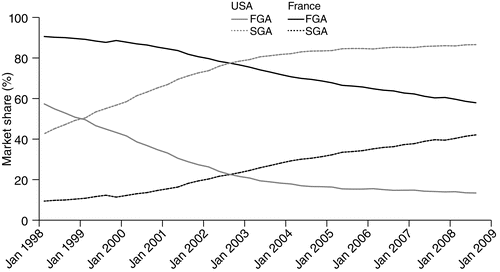
aSources: United States, IMS Xponent, 1996–2008, IMS Health, Inc.; France, GERS database 1998–2008. FGA, first-generation antipsychotics; SGA, second-generation antipsychotics
Trends in use of second-generation antipsychotics
Trends in product-level use of second-generation antipsychotics also followed different patterns (Figure 3). In France, there was a steady increase in use of all newer drugs but amisulpride. Trends in use of the second-generation antipsychotics varied substantially by drug in the United States, where use of clozapine and olanzapine decreased and use of risperidone leveled off. Even several years after their commercialization and adoption by physicians, quetiapine and aripiprazole in the United States have seen changes in use: a recent slowing down for quetiapine after a rapid increase in use and a sharp increase for aripiprazole (for example, 5% and 25% increases, respectively, between 2007 and 2008).
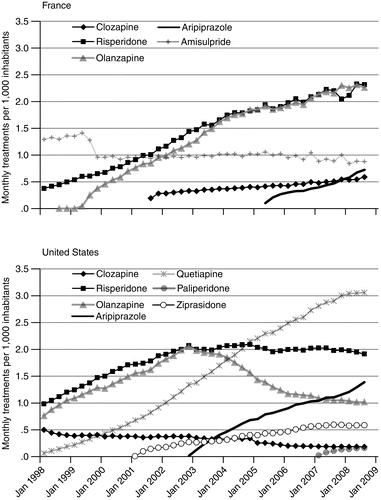
aSources: United States, IMS Xponent, 1996–2008, IMS Health, Inc.; France, GERS database 1998–2008
From 1998 to 2003, France and the United States had nearly equivalent levels and upward trends in use of risperidone and olanzapine. After 2003, this increasing trend continued in France, while in the United States there was a steep decrease in olanzapine use and stabilization in risperidone use. In 2008, olanzapine accounted for only 12% of the U.S. second-generation antipsychotic market, down from 33% in 1998. Opposite trends in clozapine use were observed in the United States (decreasing use) and in France (increasing use), although the level of use was low in both countries, with shares of 8.7% of the second-generation antipsychotic market in France and 2.2% in the United States.
In 2008, the U.S. market was slightly less concentrated in the top two drugs. U.S. market leaders were quetiapine with 37% of prescriptions and risperidone with 23% of use. In contrast, in France, olanzapine and risperidone shared equally approximately 70% of the second-generation antipsychotic market.
Discussion
Our study offers three main findings. First, the two countries had divergent trends in antipsychotic use overall, with use per population increasing in the United States and declining in France, where the initial level of use was higher. Second, we found large differences between the two countries in the market shares of first and second generations of antipsychotics. By 2008, first-generation antipsychotics accounted for only 14% of antipsychotic use in the United States, while they still made up a majority of use in France. Finally, the product-level market shares for second-generation antipsychotics showed different patterns, with the most notable difference in olanzapine’s share of use in the two countries.
The difference in absolute rates of overall antipsychotic use between the two countries is notable. However, because the data available to us were drawn with different sampling strategies, we were unable to determine whether these rates were truly different, perhaps due to differences in medication access and affordability or whether they were an artifact of the way we measured use. As a result, we focus our discussion on the divergent trends between the two countries.
Physicians in the United States were much more rapid to shift toward use of newer over older antipsychotics than were physicians in France. Because of the huge price differences between first- and second-generation antipsychotics during the study period (differences that will narrow with the launch of generic second-generation antipsychotics), the rapid adoption of newer drugs among U.S. physicians had important economic consequences for payers, particularly Medicare and Medicaid, which finance most antipsychotic prescriptions (21).
Physicians’ prescribing of antipsychotics was likely influenced by patients’ clinical characteristics, which we were unable to measure. Prescribing was also influenced by physician preferences. The long clinical tradition in France of combining two first-generation antipsychotics (one mainly sedative and one more active on positive symptoms of schizophrenia) (27,28) may not have existed in the United States and may explain the higher use of first-generation drugs in France compared with the United States. However, the difference in the speed with which second-generation antipsychotics captured the antipsychotic market in the two countries also may have been influenced by health system factors, some of which we briefly reviewed. For example, the slower adoption of new second-generation antipsychotics in France may be explained by the 2.1-year delay in drug approval. The higher use of second- over first-generation antipsychotics in the United States may also be due in part to the availability of more second-generation antipsychotics and the number of indications for which manufacturers sought regulatory approval. Likewise, the higher use of first-generation antipsychotics in France may reflect the greater number of first-generation drugs available there.
Other findings may not have been predicted on the basis of an assessment of health system differences alone. For instance, there is substantial evidence that choice of medication class is responsive to relative out-of-pocket price (29). On this factor alone, one might have expected greater use of first-generation antipsychotics in the United States, where there is a steeper gradient in cost sharing between generic and brand-name drugs, compared with France, where most patients face no copay for antipsychotics.
Regulatory agencies and physicians in both countries appear to have responded differently to information regarding the comparative effectiveness and safety of second-generation antipsychotics (30,31). The dramatic decrease in olanzapine use in the United States beginning in 2003 coincided with an FDA warning in that year on the metabolic effects of new antipsychotics and with a consensus statement published shortly thereafter ranking olanzapine as having high metabolic risk (32). In response to these concerns, some state Medicaid programs placed restrictions on second-generation use after the warnings (22,33). In contrast, the French drug regulatory agency issued no such warning and did not place any market restrictions on drugs. In addition to differences in the actions of the regulatory agencies, it is possible that French and American physicians differed in their perception of antipsychotic risks. Perceptions of American physicians may have been shaped by the extensive publicity surrounding U.S. lawsuits against olanzapine’s manufacturer (34).
Our study had some limitations. First, this descriptive study could not generate causal estimates of the relationships between features of the health system and trends in antipsychotic use. Second, the data for the two countries came from different sources: prescriptions (written and filled) from a random sample of physicians in the United States and sales in the ambulatory care market in France. To compare use between the two countries, we used the most comparable unit available from both sources: monthly treatments. However, to convert the number of sold packages in France to the number of monthly treatments, we had to make assumptions about the average daily dose for first-generation antipsychotics because packages are not standardized for monthly treatments. However, although this may have led to some error in estimating differences between countries in first-generation antipsychotic use, we do not expect this measurement error to change over time, and therefore it cannot explain differences in trend. In addition, unlike U.S. data, French sales data capture prescriptions for outpatients and most of the consumption by patients living in nursing homes. Last, we did not record the use of long-acting or other injectable antipsychotics. Hence we were not able to explore differences in the use of the entire class of antipsychotics. The use of long-acting antipsychotics in the early 2000s for patients with schizophrenia has been reported to be similar in the United States (26%) (35) or France (21%) (36) and thus should not affect the comparisons.
Conclusions
The diffusion of second-generation antipsychotics clearly followed different patterns in the United States and France both in terms of their share of the total antipsychotic market as well as individual product market share among second-generation drugs. Some of these differences appear to be consistent with health system differences between the two countries, such as the timing of drug approval and the issuance of safety warnings, although these influences need to be further explored. Other differences between the two countries may reflect physicians’ reaching different conclusions about the comparative effectiveness and safety of antipsychotics.
1 : Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiology and Drug Safety 20:177–184, 2011Crossref, Medline, Google Scholar
2 : Off-label use of antipsychotic medications in the Department of Veterans Affairs health care system. Psychiatric Services 60:1175–1181, 2009Link, Google Scholar
3 : Off-label use of antipsychotic medications in Medicaid. American Journal of Managed Care 18:e109–e117, 2012Medline, Google Scholar
4 : Impact of FDA black box advisory on antipsychotic medication use. Archives of Internal Medicine 170:96–103, 2010Crossref, Medline, Google Scholar
5 : Prevalence, utilization patterns, and predictors of antipsychotic polypharmacy: experience in a multistate Medicaid population, 1998–2003. Clinical Therapeutics 29:183–195, 2007Crossref, Medline, Google Scholar
6 : Policy implications of CATIE [Letter]. Psychiatric Services 59:695, 2008Link, Google Scholar
7 : Second-generation antipsychotics: cost-effectiveness, policy options, and political decision making. Psychiatric Services 59:515–520, 2008Link, Google Scholar
8 : Are older antipsychotic drugs obsolete? British Medical Journal 332:1346–1347, 2006Crossref, Medline, Google Scholar
9 : Antipsychotic prescribing trends: a review of pharmaco-epidemiological studies. Acta Psychiatrica Scandinavica 121:4–10, 2010Crossref, Medline, Google Scholar
10 : Increased use of second generation antipsychotic drugs in primary care: potential relevance for hospitalizations in schizophrenia patients. European Journal of Clinical Pharmacology 64:73–76, 2008Crossref, Medline, Google Scholar
11 : Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995–2009. PLoS ONE 6:e28725, 2011Crossref, Medline, Google Scholar
12 : National changes in oral antipsychotic treatment for people with schizophrenia in primary care between 1998 and 2007 in the United Kingdom. Pharmacoepidemiology and Drug Safety 21:161–169, 2012Crossref, Medline, Google Scholar
13 : Trends in treatment of newly treated schizophrenia-spectrum disorder patients in Taiwan from 1999 to 2006. Pharmacoepidemiology and Drug Safety 21:989–996, 2012Crossref, Medline, Google Scholar
14 World Health Report 2000: Health Systems: Improving Performance. Geneva, World Health Organization, 2000. Available at www.who.int/whr/2000/en/whr00_en.pdfGoogle Scholar
15 : Patient access to pharmaceuticals: an international comparison. European Journal of Health Economics 8:253–266, 2007Crossref, Medline, Google Scholar
16 : The health care system under French national health insurance: lessons for health reform in the United States. American Journal of Public Health 93:31–37, 2003Crossref, Medline, Google Scholar
17 DeNavas Walt C, Proctor BD, Smith JC: Income, Poverty, and Health Insurance Coverage in the United States: 2010. Washington, DC, US Census Bureau, Sept 2011. Available at www.census.gov/prod/2011pubs/p60-239.pdfGoogle Scholar
18 : The impact of national health care reform on adults with severe mental disorders. American Journal of Psychiatry 168:486–494, 2011Link, Google Scholar
19 Grandfils N: Drug Price Setting and Regulation in France. DT no 16. Paris, Institut de recherché et documentation en économie de la santé, Sept 2008. Available at www.irdes.fr/EspaceAnglais/Publications/WorkingPapers/DT16DrugPriceSettingRegulationFrance.pdfGoogle Scholar
20 : The drug budget silo mentality: the French case. Value in Health 6(suppl 1):S10–S19, 2003Crossref, Medline, Google Scholar
21 : Dual eligibles with mental disorders and Medicare part D: how are they faring? Health Affairs 28:746–759, 2009Crossref, Google Scholar
22 : Medicaid’s prior authorization program and access to atypical antipsychotic medications. Health Affairs 26:750–760, 2007Crossref, Google Scholar
23 Perronnin M, Pierre A, Rochereau T: Complementary Health Insurance in France: Wide-Scale Diffusion but Inequalities of Access Persist. Paris, Institut de recherché et documentation en économie de la santé, Jan 2011. Available at www.irdes.fr/EspaceAnglais/Publications/IrdesPublications/QES161.pdfGoogle Scholar
24 Hearne J: Prescription Drug Coverage Under Medicaid. Washington, DC, Congressional Research Service, Feb 6, 2008. Available at aging.senate.gov/crs/medicaid16.pdfGoogle Scholar
25 : Average out-of-pocket expenses across different drug categories and commercial third-party payers. Psychiatry 7:12–13, 2010Medline, Google Scholar
26 Avis sur les médicaments. Saint-Denis La Plaine, France, Haute Autorité de santé. Available at www.has-sante.fr/portail/jcms/c_5267/actes-medicaments-dispositifs-medicaux?cid=c_5267Google Scholar
27 : The French Consensus Conference on long-term therapeutic strategies for schizophrenic psychoses. Social Psychiatry and Psychiatric Epidemiology 30:49–52, 1995Crossref, Medline, Google Scholar
28 : Patterns of risperidone prescription: a utilization study in south-west France. Acta Psychiatrica Scandinavica 109:202–206, 2004Crossref, Medline, Google Scholar
29 : Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA 298:61–69, 2007Crossref, Medline, Google Scholar
30 : Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353:1209–1223, 2005Crossref, Medline, Google Scholar
31 : Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Archives of General Psychiatry 63:1079–1087, 2006Crossref, Medline, Google Scholar
32
33 : Effect of prior authorization of second-generation antipsychotic agents on pharmacy utilization and reimbursements. Psychiatric Services 59:540–546, 2008Link, Google Scholar
34 : Atypical antipsychotics: a case study in new era risk management. Journal of Psychiatric Practice 12:253–258, 2006Crossref, Medline, Google Scholar
35 : Characteristics and use patterns of patients taking first-generation depot antipsychotics or oral antipsychotics for schizophrenia. Psychiatric Services 58:482–488, 2007Link, Google Scholar
36 : Neuroleptic drug utilization among schizophrenic outpatients [in French]. Revue d'Epidemiologie et de Sante Publique 53:601–613, 2005Crossref, Medline, Google Scholar



