Characteristics and Use Patterns of Patients Taking First-Generation Depot Antipsychotics or Oral Antipsychotics for Schizophrenia
Patients with schizophrenia often improve with long-term antipsychotic treatment but are prone to relapse when they do not follow antipsychotic medication regimens. In a literature review Cramer and Rosenheck ( 1 ) reported that the average rate of nonadherence among patients with schizophrenia is 58% and ranges from 24% to 90%, depending on the definition and the method of adherence measurement. Prior research has shown that nonadherence to antipsychotics is associated with psychotic relapse and that clinicians have difficulties identifying nonadherent patients and predicting who will be nonadherent in the future ( 2 ).
Depot antipsychotics may help patients adhere to treatment because depot agents are administered by injection every two to four weeks, eliminating the need for daily dosing ( 3 , 4 ). The delivery system itself does not prevent nonadherence—that is, some patients do not return for their scheduled injections. However, depot delivery gives health care providers an opportunity to readily identify nonadherence and thereby to provide early and effective follow-up efforts ( 5 ). As a result, treatment guidelines for schizophrenia recommend that clinicians strongly consider depot medication for patients who may be nonadherent to antipsychotic treatment regimens ( 6 , 7 ).
Compared with oral antipsychotics, particularly second-generation antipsychotics, depot first-generation antipsychotics are more often associated with increased side effects of extrapyramidal symptoms ( 8 ), fear of injections, pain after injection, and stigma attached to the medication delivery method. Only sparse information is available on the clinical and functional characteristics of patients treated with first-generation depot antipsychotics, partly because they are a relatively underutilized option in usual practice settings ( 9 ).
To help address this information gap and expand the existing information, we used data from a three-year, multisite study of patients treated for schizophrenia at various systems of care across the United States. We aimed to compare the characteristics and antipsychotic use patterns between individuals treated in usual care with depot first-generation antipsychotics or oral antipsychotics.
Methods
Study design
This study used data from the U.S. Schizophrenia Care and Assessment Program (US-SCAP), a large, nonrandomized, naturalistic, three-year, prospective, multisite study of individuals treated for schizophrenia in the United States ( 10 , 11 , 12 ). The objectives of US-SCAP, inclusion and exclusion criteria, and sample characteristics are described elsewhere ( 11 , 12 ). In brief, 2,327 patients participated in the study, which was conducted between July 1997 and September 2003. Approximately 400 patients were enrolled at each of the six study sites. These sites were located in six states (California, Colorado, Connecticut, Florida, Maryland, and North Carolina) and represented treatment in diverse systems of care, including community mental health centers, university health care systems, the Department of Veterans Affairs Health Services (VA), and community and state hospitals. Institutional review board approval was received at each regional site, and informed consent was received from all participants. They were diagnosed as having schizophrenia, schizoaffective, or schizophreniform disorders on the basis of DSM-IV criteria and were at least 18 years old. Patients were excluded if they were unable to provide informed consent or had participated in a clinical trial within 30 days before enrollment.
Enrolled patients were observed and naturalistically monitored for up to three years. Patients could continue with the treatment they were receiving before enrollment for as long as deemed necessary by their treating physicians. All treatment decisions, including medication initiations and discontinuations, if any, were made by treating physicians as they occurred in usual care. Of 2,327 enrollees, most participated through year 1 (78%), and fewer completed year 2 (70%) or year 3 (65%).
Sample
The study included US-SCAP participants for whom clinical and functional assessments were conducted at enrollment and for whom medication information was available for the year after enrollment (2,186 of 2,327 enrollees, or 94%). These patients were classified into two cohorts: the depot cohort, in which patients were treated with at least one injection of first-generation depot antipsychotic at any time during the three-year study, and the oral cohort, in which patients were treated with only oral antipsychotics during the three-year study (first-generation or second-generation antipsychotics or both). Therefore, there was a possible gap of up to three years between a patient's study enrollment and the first injection of first-generation antipsychotic.
Outcome measures
Data were collected at baseline and at six-month and one-year intervals. At six-month intervals, participants gave self-reports on a health questionnaire (SCAP-HQ) ( 13 ) and medical records were systematically extracted for medications and other psychiatric resources used in the prior six-month interval. At one-year intervals, clinicians administered and rated standard psychiatric scales.
The SCAP-HQ has been validated to assess patient-reported health domains in the treatment of schizophrenia. In this study the SCAP-HQ was used to assess substance use, suicidal thoughts, and suicide attempts in the four weeks before enrollment as well as arrests in the six months before enrollment.
The clinician-rated measures included the Positive and Negative Syndrome Scale (PANSS) and the PANSS factor subscales ( 14 ), the Montgomery-Åsberg Depression Rating Scale ( 15 ), and the Global Assessment of Functioning scale (GAF) ( 16 ).
Systematic semistructured interviews with patients at enrollment provided information about sociodemographic characteristics, including age, gender, ethnicity, education level, marital status, veteran status, and health insurance coverage. Patients' medical records provided information on psychiatric diagnosis and psychiatric hospitalizations in the year before enrollment. Medical records also provided information about prescribed psychiatric medications. Trained and annually certified examiners extracted medical record information for the six months before enrollment and for each six-month interval thereafter. In addition, patients were queried about their use of medications and other mental health resources outside those received at their regular treatment site. When patients reported such use, systematic efforts were made to extract offsite medical records.
In addition, patients' patterns of depot antipsychotic use were assessed to identify a subgroup of patients who received injections in the year after enrollment. The outcomes of depot use patterns included an annual medication possession ratio (MPR), which is the ratio of the cumulative number of days covered with any first-generation depot (14 days and 28 days for each injection of fluphenazine depot or haloperidol depot, respectively) over 365 days, the proportion of patients concurrently treated with a first-generation depot and any oral antipsychotic, the mean duration of this concurrent antipsychotic use pattern, and the mean modal dosages and frequencies of treatment with each depot first-generation antipsychotic.
Statistical analysis
The depot and oral antipsychotic treatment groups were compared on patients' characteristics at and before enrollment with chi square tests for categorical variables and t tests for continuous variables. Descriptive statistics were used for analyses of treatment patterns during the year after enrollment. For variables with skewed distributions, the estimated median is also presented. To confirm findings from univariate analysis, we also conducted a multivariate logistic regression analysis with the dependent variable of having received a depot injection at any time during the three-year study. For this logistic regression, explanatory variables included sociodemographic, illness, and utilization characteristics at study enrollment that showed statistical significance in the univariate analyses. The final model was determined by a stepwise selection of explanatory variables. All statistical analyses were completed with SAS version 8.2. Significance level was set at α =.05.
Results
About one-fourth of the study participants (569 of 2,186, or 26%,) were treated with depot first-generation antipsychotics at least once during the three-year study, whereas 74% (1,617 of 2,186) were treated with only oral agents and all of this group received first-generation antipsychotic drugs. However, 30 of 569 (5%) depot-treated patients received only one injection. Table 1 presents patient sociodemographic characteristics at enrollment for the depot and oral treatment groups. Compared with the oral treatment group, patients in the depot treatment group were significantly more likely to be younger and male and to have never married, and they were less likely to have more than a high school education or veteran status. The depot and oral treatment groups also significantly differed in ethnic background and health insurance coverage. About 51% of the depot group were African American, compared with 32% of the oral group. The depot group also differed in Medicaid or Medicare health insurance or dual eligibility.
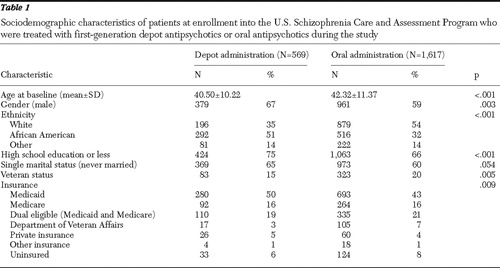 |
Table 2 presents the clinical and functional characteristics of the depot and oral treatment groups at enrollment. The two groups significantly differed on diagnostic mix, such that the depot group was more likely to be diagnosed as having schizophrenia, paranoid type, and less likely to have a diagnosis of schizoaffective disorder. Compared with the oral group, the depot group exhibited significantly higher levels of psychopathology, as measured by the PANSS total score and the PANSS subscale scores on the positive symptoms, hostility and excitability, and a higher level of disorganized thinking. The depot group was functioning at a significantly poorer global level (as assessed with the GAF) but tended to report significantly fewer suicidal thoughts or attempts. In addition, the depot group was significantly more likely to be hospitalized for psychiatric reasons in the year before enrollment, to use alcohol or illicit drugs, and to have been arrested.
 |
Table 3 presents the odds ratios in point estimates and 95% confidence intervals for all predictors of the use of first-generation depot antipsychotic agents during the US-SCAP study. Ethnicity was found to be a significant predictor. Compared with other ethnic groups, white patients were 33% less likely to use first-generation depot injections, whereas African Americans were 53% more likely to use them. Patients who received care through VA Health Systems were approximately 60% less likely than those with other coverage or the uninsured to receive a first-generation depot. Compared with non-substance-using patients, patients using alcohol or illicit drugs during the four weeks before enrollment were twice as likely to be treated with first-generation depot antipsychotics at some point during the study. Patients who had been arrested during the six months before enrollment were 83% more likely to be treated with first-generation depot than patients without such prior arrests. Utilization of inpatient psychiatric care during the year before study enrollment was associated with 41% greater subsequent use of first-generation depot antipsychotic agents, compared with patients without such prior inpatient hospitalizations. However, the differences in psychopathology scores (depression, anxiety, and disorganized thinking) at enrollment produced only very small effects on predicting the use of depot regimens.
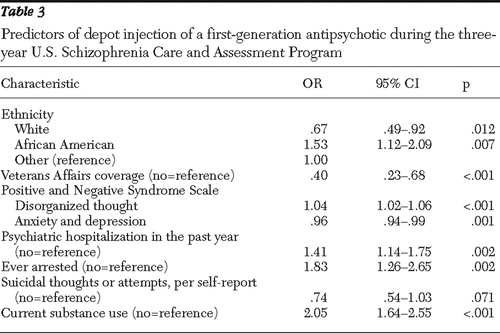 |
The use patterns of first-generation depot antipsychotics in the full year after enrollment were assessed for a subgroup of 505 patients who received at least one depot injection of first-generation medication during this period (260 patients received depot fluphenazine; 226 patients, depot haloperidol; 19 patients, fluphenazine depot and haloperidol depot in separate treatment episodes). Through most of the year the depot group received first-generation depot (mean±SD=331±74 days; median of 365 days), and the average MPR was 91%. Medication for about two-thirds of the depot group was augmented with oral antipsychotics (68%) for approximately six months (mean=164±153 days; median=144 days). Furthermore, patients treated with any haloperidol depot significantly differed from patients treated with any fluphenazine depot on treatment duration (mean=316±93 versus 332±75 days, respectively; p=.029) and took concurrent oral antipsychotic agents for 172 days, compared with 150 days for patients treated with fluphenazine depot (p=.092).
The doses and frequencies of the two depot first-generation antipsychotics were mostly consistent with the product package insert. For patients who received only one of the depot drugs, the modal dose and frequency of fluphenazine depot was 25 mg biweekly, based on 662 injections given to 273 patients. A majority (78%) of the injections were given biweekly (N=521), 11% (N=70) every three weekss, 6% (N=39) monthly, and 1% (N=8) weekly. Only 24 injections (4%) were given in other patterns (such as a single injection in an isolated episode) during the study period. The modal dose and frequency of haloperidol depot was 100 mg monthly, based on 626 injections given to 234 patients. A total of 345 injections (55%) were given monthly, 158 (25%) were given biweekly, 70 (11%) every three weeks, and six (1%) weekly. Approximately 47 injections (8%) administered haloperidol depot in other patterns.
Discussion
In the US-SCAP study, approximately one-fourth of the participants received depot first-generation antipsychotics at least once during the three-year period. Depot antipsychotic medications appeared to be relegated to a relatively small subgroup of patients who could potentially benefit from them, considering the twofold difference between the rate of depot use during the first year of the US-SCAP study (26%) and the reported annual rate (59%) of treatment-noncompliant patients with schizophrenia ( 17 ). The guidelines for the treatment of schizophrenia have specified the need to treat medication-nonadherent patients with antipsychotics in depot formulation and to potentially expand current uses of depot antipsychotic agents ( 6 , 18 ).
According to univariate comparisons, the depot treatment group appeared to significantly differ from the oral treatment group on several baseline characteristics, suggesting that patients treated with depot antipsychotics have a clinical, functional, and sociodemographic profile distinctively different from patients treated with only oral antipsychotics. Approximately half of the patients treated with first-generation depot antipsychotics were treated at inpatient psychiatric facilities during the year before enrollment. These patients also tended to be substance users and to have had prior arrests. These characteristics have been found to be significant predictors of nonadherence to antipsychotic medications ( 19 , 20 ), which suggests that clinicians may have prescribed depot first-generation agents to help address prior nonadherence to medication regimens. Although the US-SCAP study did not assess the reasons for medication initiation or discontinuation, this possible explanation can be inferred only from other studies. Recently, a large, prospective, observational study of schizophrenia patients treated in ten European countries, the European Schizophrenia Outpatient Health Outcomes (EU-SOHO), reported that more than 50% of patients who were initiated on first-generation depot antipsychotics or were switched to them were treated with depot formulations to help address problems of nonadherence, rather than for lack of efficacy or other reasons ( 21 ).
The use of first-generation depot antipsychotics is less frequent in the United States than in Western Europe and Australia. In Australia, for example, during the latter half of 2001 depot medications accounted for 16.7% of total prescriptions for antipsychotic medications dispensed through community pharmacies ( 22 ). According to a study from the Schizophrenia Patients Outcomes Research Team survey of patterns of usual care for schizophrenia, only 11.8% of the VA outpatients were considered to receive first-generation depot agents ( 23 ). Our findings of an annual rate of 26% (although 5% of them received only one injection) and the information on dose frequency would suggest that this treatment option is being implemented in the United States as part of usual care and that first-generation depot antipsychotics are being used at doses and frequencies consistent with the drugs' package inserts.
The association between first-generation depot use and African-American race was originally reported in a study of fluphenazine decanoate in 1985 ( 24 ). The finding was further replicated in more recent studies after the introduction of second-generation antipsychotics ( 25 , 26 , 27 ). The reasons for this ethnic disparity (that is, that African Americans are more likely than Caucasians to receive first-generation depot medication) may include more severe psychotic symptoms with hostility or suspiciousness at symptom presentation ( 28 ), greater acceptability of injections and tolerance of medication side effects ( 24 ), and clinicians' perceptions of poorer adherence to antipsychotic regimen ( 29 ). It is also likely that clinicians (mostly white) tend to misdiagnose bipolar disorder as schizophrenia among African-American patients ( 30 ).
The use of first-generation agents in depot formulations may differ, however, across systems of care in the United States. Our findings suggest that participants covered by VA are less likely than uninsured participants or those covered by other types of health insurance to be treated with depot first-generation agents. Although we are unclear about the specific factors that may contribute to the system-level differences in depot utilization, the findings are consistent with a facility-level study of inpatient facilities of the New York Office of Mental Health, which found significant differences in depot utilization across facilities ( 31 ).
Although participants in this study were treated with first-generation depot antipsychotics for most of the year after enrollment, their treatment regimens were likely to include prolonged antipsychotic polypharmacy, with concurrent use of oral and depot antipsychotics. This adjunctive use suggests that clinicians may use depot first-generation antipsychotics to extend antipsychotic coverage and supplement oral antipsychotics for patients who are anticipated to have difficulties with medication adherence. Despite this prevalent clinical practice, there is little scientific evidence to support the use of antipsychotic polypharmacy ( 32 ) regardless of drug formulation.
Our study appears to be the first to report the MPR of depot antipsychotic agents in usual care settings. Calculations of this adherence proxy measure were feasible because of systematic extraction of medical records of each patient's medication prescriptions throughout the three-year study. For depot drugs, there is a methodological issue of using computerized prescription claims databases in the measurement of drug exposure, because the number of days supplied in paid claims for depot prescriptions was found to be recorded in an inconsistent or inaccurate manner compared with the days of supply calculated from dosing instructions in the medical record ( 33 ).
Study findings need to be interpreted in the context of study limitations. First, this was a cross-sectional analysis of a three-year time window during the long-term treatment of patients with schizophrenia, and as such, it attributed enrollment characteristics to patients who were treated by first-generation depot at any time during those three years. Therefore, except for the few time-irrelevant variables, such as gender and ethnicity, the clinical and functional variables were not assessed at the time the patient initiated the first-generation depot antipsychotic and thus may not accurately reflect the patient's status at that time.
Second, medication regimens were based on prescription information in patients' medical records and may not necessarily reflect the actual filling of the prescription and use of the antipsychotic. However, previous research has demonstrated a very high concordance rate between the presence of a prescription for antipsychotics and the filling of the prescription in a patient population that resembles US-SCAP participants (patients with severe mental illness, diagnosed primarily as having schizophrenia, covered by Medicaid) ( 34 ). In this study, patients were queried about the use of medications and other mental health resources outside those received at their regular treatment site. When patients reported such use, systematic efforts were made to extract offsite medical records. However, if the patient did not report use of other medications, there was no review of offsite records, thus creating a potential source of information bias. Last, results may not generalize to patients treated in the private sector because public payers covered almost all US-SCAP participants.
Conclusions
Results from this large, prospective, naturalistic study of patients treated for schizophrenia in various systems of care in the United States suggest that patients treated with depot first-generation antipsychotics were distinctively different from those receiving only oral medication in socioeconomic status and social support structures and were high consumers of health care resources. Although long-term use of first-generation depot antipsychotics might address some unmet needs of a unique subgroup of patients with schizophrenia, the use of these depot medications in usual care is typically augmented with oral antipsychotics for extended periods.
Acknowledgments and disclosures
The US-SCAP study was funded by Eli Lilly and Company and administered by the Medstat Group. The authors thank the site investigators and others who collaborated in the research. Maryland: AnthonyF. Lehman, M.D., M.S.P.H., University of Maryland School of Medicine, and Gerard Gallucci, M.D., M.H.S., Johns Hopkins Bayview Medical Center. Colorado: Courtenay Harding, Ph.D., University of Colorado (formerly). Florida: David Shern, Ph.D., Florida Mental Health Institute, University of South Florida, and Terri Saunders, M.S. (formerly Florida Mental Health Institute). North Carolina: Jeffrey Swanson, Ph.D., Lawrence A. Dunn, M.D., and Marvin Swartz, M.D., Duke University Medical School. California: RichardL. Hough, Ph.D., and Concepcion Barrio, Ph.D., Child and Adolescent Services Research Center and San Diego State University. Connecticut: Robert A. Rosenheck, M.D., and Rani Desai, Ph.D., Department of Veterans Affairs Connecticut Health Care System. Medstat group: Pat Russo, Ph.D., M.S.W. (formerly), Liisa Palmer, Ph.D., Lito Torres, M.B.A., and Brian Cuffel, Ph.D. (formerly). Eli Lilly and Company: Don Buesching, Ph.D., Brian M. Johnstone, Ph.D., and Thomas Croghan, M.D. (formerly). Consultants: David Salkever, Ph.D., Johns Hopkins University, Eric P. Slade, Ph.D. (formerly of Johns Hopkins University), WilliamHargreaves, Ph.D., and Martha Shumway, Ph.D., University of California, San Francisco.
Dr. Shi has received grants from Eli Lilly and Company. Dr. Ascher-Svanum, Dr. Zhu, Dr. Faries, and Mr. Montgomery are employees of Eli Lilly and Company, and Dr. Faries is also a stockholder. Dr. Marder has received grant support from Eli Lilly and Company and Janssen Pharmaceuticals and is a consultant for Bristol-Myers Squibb, Otsuka, Solvay, and Pfizer.
1. Cramer JA, Rosenheck R: Compliance with medication regimens for mental and physical disorders. Psychiatric Services 49:196–201, 1998Google Scholar
2. Diaz E, Levine HB, Sullivan MC, et al: Use of the Medication Event Monitoring System to estimate medication compliance in patients with schizophrenia. Journal of Psychiatry and Neuroscience 26:325–329, 2001Google Scholar
3. Kane JM: Strategies for improving compliance in treatment of schizophrenia by using a long-acting formulation of an antipsychotic: clinical studies. Journal of Clinical Psychiatry 64(suppl 16):34–40, 2003Google Scholar
4. Marder SR: Overview of partial compliance. Journal of Clinical Psychiatry 64(suppl 16):3–9, 2003Google Scholar
5. Love RC: Strategies for increasing treatment compliance: the role of long-acting antipsychotics. American Journal of Health System Pharmacy 59(22 suppl 8):S10–S15, 2002Google Scholar
6. Kane JM, Leucht S, Carpenter D, et al: Expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders: introduction: methods, commentary, and summary. Journal of Clinical Psychiatry 64(suppl 12):5–19, 2003Google Scholar
7. Kane JM, Aguglia E, Altamura AC, et al: Guidelines for depot antipsychotic treatment in schizophrenia. European Neuropsychopharmacology Consensus Conference in Siena, Italy. Journal of the European College of Neuropsychopharmacology 8:55–66, 1998Google Scholar
8. Barnes TR, Curson DA: Long-term depot antipsychotics: a risk-benefit assessment. Drug Safety 10:464–479, 1994Google Scholar
9. Glazer WM, Kane JM: Depot neuroleptic therapy: an underutilized treatment option. Journal of Clinical Psychiatry 53:426–433, 1992Google Scholar
10. Faries D, Asher-Svanum H, Zhu B, et al: Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry 5(1):26, 2005Google Scholar
11. Ascher-Svanum H, Zhu B, Faries D, et al: A comparison of olanzapine and risperidone on the risk of psychiatric hospitalization in the naturalistic treatment of patients with schizophrenia. Annals of General Hospital Psychiatry 3:11, 2004Google Scholar
12. Ascher-Svanum H, Faries D, Zhu B, et al: Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. Journal of Clinical Psychiatry 67:453–460, 2006Google Scholar
13. Lehman AF, Fischer EP, Postrado L: The Schizophrenia Care and Assessment Program Health Questionnaire (SCAP-HQ): an instrument to assess outcomes of schizophrenia care. Schizophrenia Bulletin 29:247–256, 2003Google Scholar
14. Davis JM, Chen N: The effects of olanzapine on the 5 dimensions of schizophrenia derived by factor analysis: combined results of the North American and international trials. Journal of Clinical Psychiatry 62:757–771, 2001Google Scholar
15. Montgomery SA, Åsberg M: A new depression scale designed to be sensitive to change. British Journal of Psychiatry 134:382–389, 1979Google Scholar
16. Endicott J, Spitzer RL, Fleiss JL, et al: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 33:766–771, 1976Google Scholar
17. Gilmer TP, Dolder CR, Lacro JP, et al: Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. American Journal of Psychiatry 161:692–699, 2004Google Scholar
18. Lehman AF, Lieberman JA, Dixon LB, et al: Practice guideline for the treatment of patients with schizophrenia, 2nd ed. American Journal of Psychiatry 161(Feb suppl):1–56, 2004Google Scholar
19. Fenton WS, Blyler CR, Heinssen RK: Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophrenia Bulletin 23:637–651, 1997Google Scholar
20. Bhanji NH, Chouinard G, Margolese HC: A review of compliance, depot intramuscular antipsychotics and the new long-acting injectable atypical antipsychotic risperidone in schizophrenia. Journal of the European College of Neuropsychopharmacology 14(2):87–92, 2004Google Scholar
21. Haro JM, Edgell ET, Frewer P, et al: The European Schizophrenia Outpatient Health Outcomes Study: baseline findings across country and treatment. Acta Psychiatrica Scandinavica Supplementum 416:7–15, 2003Google Scholar
22. Mond J, Morice R, Owen C, et al: Use of antipsychotic medications in Australia between July 1995 and December 2001. Australian and New Zealand Journal of Psychiatry 37:55–61, 2003Google Scholar
23. Rosenheck RA, Desai R, Steinwachs D, et al: Benchmarking treatment of schizophrenia: a comparison of service delivery by the national government and by state and local providers. Journal of Nervous and Mental Disease 188:209–216, 2000Google Scholar
24. Price N, Glazer W, Morgenstern H: Demographic predictors of the use of injectable versus oral antipsychotic medications in outpatients. American Journal of Psychiatry 142:1491–1492, 1985Google Scholar
25. Kreyenbuhl J, Zito JM, Buchanan RW, et al: Racial disparity in the pharmacological management of schizophrenia. Schizophrenia Bulletin 29:183–193, 2003Google Scholar
26. Kuno E, Rothbard AB: Racial disparities in antipsychotic prescription patterns for patients with schizophrenia. American Journal of Psychiatry 159:567–572, 2002Google Scholar
27. Covell NH, Jackson CT, Evans AC, et al: Antipsychotic prescribing practices in Connecticut's public mental health system: rates of changing medications and prescribing styles. Schizophrenia Bulletin 28:17–29, 2002Google Scholar
28. Strakowski SM, Flaum M, Amador X, et al: Racial differences in the diagnosis of psychosis. Schizophrenia Research 21:117–124, 1996Google Scholar
29. Valenstein M, Copeland LA, Owen R, et al: Adherence assessments and the use of depot antipsychotics in patients with schizophrenia. Journal of Clinical Psychiatry 62:545–551, 2001Google Scholar
30. Blow FC, Zeber JE, McCarthy JF, et al: Ethnicity and diagnostic patterns in veterans with psychoses. Social Psychiatry and Psychiatric Epidemiology 39:841–851, 2004Google Scholar
31. Citrome L, Levine J, Allingham B: Utilization of depot neuroleptic medication in psychiatric inpatients. Psychopharmacology Bulletin 32:321–326, 1996Google Scholar
32. Patrick V, Levin E, Schleifer S: Antipsychotic polypharmacy: is there evidence for its use? Journal of Psychiatric Practice 11:248–257, 2005Google Scholar
33. Shireman TI, Svarstad BL, Sweeney JK: Validity of claims data for long-acting injectable medications. Journal of Pharmacoepidemiology 7:41–55, 1999Google Scholar
34. Svarstad BL, Shireman TI, Sweeney JK: Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatric Services 52:805–811, 2001Google Scholar



