Defining and Measuring Clinical Effectiveness in the Treatment of Schizophrenia
Abstract
OBJECTIVES: Expectations in treating schizophrenia are expanding beyond just controlling psychotic symptoms to include functional recovery. This report describes an approach to define and measure the clinical effectiveness of treatment in achieving these objectives. METHODS: A comprehensive literature review established that there is limited information about the meaning of the term "clinical effectiveness." To address this gap a consensus conference of schizophrenia researchers was held to consider the components of clinical effectiveness in real-world community practice and how these components can best be measured. RESULTS: The consensus of the researchers was that effective clinical treatment is characterized by four outcome domains: symptoms of disease, treatment burden, disease burden, and health and wellness. A clinical instrument to measure these four domains was constructed: Global Outcome Assessment of Life in Schizophrenia (GOALS). In using GOALS, clinicians rate each of the four domains on a scale of 1, very much improved, to 7, very much worse. Field-testing of this instrument is planned. CONCLUSIONS: Effective treatment interventions that combine optimal pharmacotherapy and targeted psychosocial treatments are raising expectations about the prospects of functional recovery among patients with schizophrenia. GOALS is proposed as one tool that can provide busy clinicians with a simple, objective measure of the effectiveness and outcomes of the clinical treatment they provide to patients with schizophrenia.
Advances in pharmacologic and psychosocial treatments have ushered in an era of broader stabilization, better reintegration, and more favorable outcomes for persons who have schizophrenia. The management of schizophrenia has moved beyond the mere control of psychotic symptoms to include functional recovery and social and vocational reintegration (1,2,3,4). These heightened expectations may require a change in perspective by some clinicians who may limit their treatment planning to short-term goals and management of the acute symptoms of illness.
Most clinicians agree that the primary treatment goal for patients with schizophrenia is to maximize the clinical effectiveness of the interventions employed to achieve the best possible outcome. A dearth of information exists in the literature about the meaning of the term "clinical effectiveness" as it applies to patients in real-world treatment settings. Furthermore, although standardized measures of efficacy in treating symptoms are available, there are not any generally accepted operational criteria that clinicians can use to measure the clinical effectiveness of the treatments they provide. To address this gap in knowledge, a roundtable entitled Towards Identifying Criteria for Clinical Effectiveness was set up so that a group of experts on schizophrenia who met in June 2002 could consider the following questions about the measurement of clinical effectiveness in real-world clinical practice: What do we mean by clinical effectiveness, and how does it differ from efficacy? What target outcome domains should be included in an assessment of clinical effectiveness? What tools can clinicians use to measure clinical effectiveness in those target areas? How can clinicians incorporate the concept of clinical effectiveness in their treatment planning?
The purpose of this article is to propose a definition of clinical effectiveness, suggest critical outcome domains and tools to measure clinical effectiveness, and review factors that may affect these outcomes. It is hoped that the formulation presented here will help clinicians integrate these outcome domains into a new and progressive model of treatment planning that is more progressive.
Defining clinical effectiveness
Both efficacy and effectiveness studies aim to assess the impact of an intervention on outcomes, but the criteria used for these evaluations can differ markedly (4,5,6). Efficacy trials seek to limit variables that can influence outcomes by stipulating strict inclusion and exclusion criteria for study entry (7). Effectiveness studies, on the other hand, approximate real-world conditions by allowing multiple variables that can affect adherence with the treatment regimen and patient outcomes (8). The box on this page summarizes the differential outcomes that are often considered in efficacy and effectiveness trials.
Outcomes considered in efficacy and effectiveness trials of schizophrenia
Efficacy trials
Positive symptoms
Negative symptoms
Acute safety
Depression and anxiety
Cognition
Tolerability
Treatment adherence
Hospital discharge
Effectiveness trials
Continuing symptom relief
Daily functioning
Ambulatory status
Social relations
Family relations
Occupational or academic functioning
Independent living and autonomy
Goal attainment, satisfaction, and possession of common objects
On the basis of this distinction, we propose the following operational definition of clinical effectiveness. A clinically effective treatment is characterized by sustained adherence by the patient to the prescribed treatment regimen; long-term reduction in symptoms of disease, treatment burden (side effects), and impact of the disease on the patient and members of his or her social circle; and long-term increase in healthy behaviors and restoration of wellness.
Clinical effectiveness can best be measured by examining several interrelated outcome domains that yield a broader clinical perspective on the progress of treatment.
Outcome domains for measuring clinical effectiveness
Liberman and colleagues (9) proposed operational criteria for recovery from schizophrenia that included symptom remission, improved vocational functioning, independent living, and improved peer relationships (Table 1). Although this model goes beyond symptom control as the only desirable outcome, it does not consider the side effects of treatment or the large burden that schizophrenia places on families, caregivers, and society. Consequently, we propose adding these elements to our definition of effective treatment, which includes four distinct, but interrelated, outcome domains: symptoms of disease, treatment burden, disease burden, and health and wellness. An overview of each of the four outcome domains is presented in the following sections. However, before reviewing the domains in detail it is important to briefly discuss two areas that cut across all four outcome domains: the perspective of the evaluator and adherence to treatment.
Treatment goals and expectations vary depending on the perspective of various individuals involved in the treatment (10,11,12). Whereas patients may be concerned about their ability to stay out of the hospital, family members may be more concerned about the patient's ability to live independently, and health care providers may be focused on improving symptoms and reducing the overall cost of care. The selected outcome domains reflect both the patient's perspective and the perspectives of other concerned parties—for example, treatment could focus on alleviating families' social and financial burden and society's economic burden. Clearly, a clinician cannot manage all these problems alone and will need to look for additional resources to maximize the clinical effectiveness of the intervention.
As noted in the definition we proposed for clinically effective treatment, sustained patient adherence to treatment is essential for achieving optimal outcomes in all domains. For example, a treatment cannot produce symptom improvement if it is not taken appropriately, and the side effects associated with a particular medication will affect patients' willingness to take it. Improved cognition as a result of effective treatment may increase patients' understanding of the need for medication and improve adherence. Finally, if patients improve their quality of life and associate these gains with the treatment they are receiving, they are likely to be more willing to continue with that treatment.
Unfortunately, studies have found that problems with adherence are a major concern among patients with schizophrenia. McCombs and colleagues (13) reported that only 11.6 percent of patients with schizophrenia in the California Medicaid program continued to purchase antipsychotic medications consistently for one year. In a similar study Lyu and colleagues (14) estimated the one-year adherence rate to be 20 percent by using Medicaid data for four additional states. Both studies found evidence that many patients are not continuing antipsychotic drug therapies and suggest that clinicians need to do a better job in monitoring treatment adherence.
Symptoms of disease
Positive and negative symptoms. Traditionally, the positive and negative symptoms of schizophrenia have been the focus of clinical drug trials (15), and extensive empirical and research data support the clinical efficacy of pharmacotherapy for controlling these symptoms (7).
Beyond positive and negative symptoms, there is increasing awareness that cognitive function and mood and anxiety symptoms have significant effects on the outcome of long-term treatment as well. Several studies have suggested that functional outcomes associated with schizophrenia may be strongly associated with these other aspects of the illness (16,17,18,19,20,21).
Cognitive dysfunction. Evidence is accumulating that impairment in cognitive functioning is a core part of the pathology of schizophrenia (22). Cognitive difficulties may precede the onset of positive symptoms, and problems with attention, learning, verbal memory, and executive function may persist even when psychotic symptoms are in remission (16). Furthermore, cognitive deficits may cause more social and vocational disability than positive or negative symptoms and may play a role in relapse and rehospitalization (4,16,18,19,23). Alternatively, improvement in verbal memory, attention, and executive functioning has been associated with better functional and vocational outcomes (23,24,25).
Mood and anxiety symptoms. High rates of depressive symptoms have been reported for 22 to 75 percent of patients who experience a first episode of schizophrenia (26) and for up to 50 percent of patients who are experiencing an acute psychotic episode (27,28). Depression may persist among a significant number of patients with schizophrenia (28) and has been associated with an increased risk of relapse, poor social adjustment, and suicide (29,30). In fact, one study found that high ratings for depression and anxiety were more strongly correlated with low ratings of subjective quality of life than any other symptoms (21).
Treatment burden
The second outcome domain, treatment burden, focuses on efforts to reduce or eliminate the adverse effects of antipsychotic treatment, such as extrapyramidal side effects, other side effects, and health risks—for example, obesity and cardiac effects (31,32,33).
Extrapyramidal side effects of medication. First-generation antipsychotics can cause extremely uncomfortable and disfiguring extrapyramidal side effects that often lead to poor adherence, frequent relapses, chronicity, and impaired functioning (29,31,34,35). These side effects can also increase patients' social isolation and contribute to vocational disability. Although the incidence of extrapyramidal side effects varies among the different second-generation antipsychotics, at commonly prescribed doses all are associated with a significantly lower incidence of extrapyramidal side effects than first-generation antipsychotics, which promotes better adherence and hence leads to fewer relapses (31,36).
Other adverse effects of medication. Antipsychotics can cause other types of adverse effects that can be difficult for patients to tolerate and may lead to nonadherence and poorer outcomes. Some of these side effects include weight gain, sexual dysfunction, breast engorgement, anticholinergic side effects (blurry vision, dry mouth, and constipation), drooling, sedation, and sleep disturbances (31,33). These side effects have an adverse impact on the quality of life of individuals who receive treatment. Consequently, an assessment of clinical effectiveness must include consideration of a drug's side-effect profile.
Health risks related to drug treatments. Some antipsychotic medications can cause adverse effects related to weight gain, metabolic disturbances, and cardiac effects that increase medical health risks.
Although many antipsychotics are associated with weight gain, the average gain varies from one medication to another (37). In the short term, excessive weight may cause fatigue, shortness of breath, sweating, sleep disturbances, and back pain. Patients who become obese are also at increased risk of arthritis, gall bladder disease, stress incontinence, sleep apnea, major depression, hyperglycemia, hyperlipidemia, hypertension, type 2 diabetes, ischemic heart disease, stroke, and cancer (breast, endometrial, colon, and prostate) (4,33).
The rate of diabetes has been found to be two to four times greater among patients with schizophrenia than in the general population (38). This greater rate of diabetes is also found among patients with bipolar disorder (39). Furthermore, some antipsychotics affect endocrine-metabolic function, including glucose utilization, insulin resistance, and lipid levels (40,41,42,43).
Elevated prolactin levels, which are associated with some antipsychotics, may cause amenorrhea, galactorrhea, gynecomastia, decreased libido, and erectile dysfunction (44).
Cardiac function—for example, QTc interval—can be affected by some antipsychotic medications. Older patients and patients who have a history of chronic heart disease, have low calcium or magnesium (electrolyte imbalance), have a family history of a long QTc, or are taking multiple drugs are at particular risk of cardiac complications (45).
Disease burden
The third domain considers the burden of the disease on the patient; on his or her family, caregivers, and friends; on health care systems that provide treatment; and on society as a whole. This domain extends the concept of clinical effectiveness beyond the model of Liberman and colleagues (9) to consider the broader impact of illness on everyone. The wide range of burden of disease includes family stress and resulting interpersonal problems, financial pressures (treatment expenses and lost income from missed work), response to the illness by the extended social network (stigma, social relations, and work), and the impact on health care use (cost of treatment and resource allocation).
The stigma attached to schizophrenia can be widespread and can affect patients and their family. Stigma complicates patients' efforts to reintegrate socially and occupationally, even when their symptoms are in remission. Motor side effects (dyskinesias and dystonias) can add to the stigma.
This domain also encompasses costs to health care systems and to society. Westermeyer and Harrow (46) reported that 40 to 50 percent of patients with schizophrenia are rehospitalized within the first year after discharge and that overall up to 85 percent are eventually rehospitalized. Rice (47) reported that the total economic burden of schizophrenia in 1990 in the United States was $32.5 billion, of which $17.3 billion was attributed to direct medical costs.
Studies have shown that effective treatment with antipsychotics can substantially improve overall disease management for patients with schizophrenia and more than offset the higher cost of the second-generation antipsychotics because of reduced hospital bed-days, which are the largest component of direct costs (48,49,50,51,52).
Health and wellness
The ultimate objective of any treatment intervention is to improve the functional capacity and the quality of life of the individual. This fourth domain encompasses a wide range of outcomes: physical health, independent living (ability to handle the requirements for autonomy), vocational or educational function, social integration (family and peer relationships), instrumental competence, and quality of life.
This outcome domain incorporates three of the four categories for recovery from schizophrenia proposed by Liberman and colleagues (9): vocational functioning, independent living, and peer relationships. Unlike the previous domains, the focus of this domain is on improving outcome rather than eliminating or reducing symptoms and burden. This domain raises expectations and anticipates that the newer second-generation antipsychotics and cognitive-behavioral and psychoeducational treatments can accomplish more for the quality of life of patients with schizophrenia than previously expected.
The first step in the pursuit of health and wellness is to identify specific areas for growth, such as school, work, family, and intimate relationships. Goal setting is often difficult for patients with schizophrenia because of core negative symptoms and cognitive deficits. Furthermore, an assessment of both real and perceived barriers—that is, the individual's level of disability—that may interfere with the achievement of defined goals is also needed. Ultimately, the clinical effectiveness of a treatment intervention will be reflected in the successful achievement of these predefined social, educational, vocational, and interpersonal goals.
Social, educational, and vocational function. Employment is a primary goal for a majority of unemployed patients with schizophrenia (53,54). Sustained employment has been associated with reduced health care use and costs and increased levels of self-esteem and satisfaction (55,56,57). As noted earlier, improvement in positive symptoms appears to be less of a predictor of community functioning (independent living skills, vocational status, and quality of life), social skills, and skill acquisition (18,20) than improvement in negative symptoms and neurocognitive abilities (18,20,58,59,60).
Physical health. Compared with the general population, patients with schizophrenia are at an increased risk of suicide, physical illness, and early mortality (61,62). The high mortality rates are associated with unnatural death as a result of homicide and accidents, as well as cardiovascular illness and pulmonary infections (4,63,64). Smoking, poor nutrition, lack of exercise, obesity and its resultant health complications (cardiac disease and diabetes), and lack of routine primary health care are common problems in this population and are partly responsible for the high rates of cardiovascular and pulmonary illness found among patients with schizophrenia and their high rate of morbidity and mortality in comparison with the general population (63,64). Unfortunately, preventive health care for patients with serious mental illness is often inadequate (64,65,66). Also, as noted above, some antipsychotic medications may increase long-term health risks of problems such as obesity, metabolic problems, and cardiac disease (37,43,67,68,69). Patients with schizophrenia have also been found to have high rates of behaviors that put them at increased risk of hepatitis C and AIDS (70,71).
Measuring clinical effectiveness
Clinical effectiveness in the treatment of schizophrenia is best reflected by the progression of change in the four outcome domains from the baseline assessment. Treatment interventions have a direct impact on clinical symptoms—and thereby on disease burden—and on treatment burden. In turn, disease and treatment burden interact with other factors to influence health and wellness (Figure 1). Continuing assessment of outcomes involves short-term and long-term follow-up to track progress through the longitudinal course of illness.
The time frame for evaluating outcomes may vary with the domain that is being considered. For example, symptoms generally remit before improvements are seen in social or work functioning (72). During the first few months of treatment, clinicians focus primarily on reducing symptoms of disease while causing as few side effects as possible. Three to 12 months after initiating a treatment intervention, the focus will shift toward relapse prevention; treatment adherence; minimizing potential medical health risks; social, educational, and vocational reintegration; and patient and family satisfaction with treatment gains. Beyond a year the focus extends to longer-term goals, such as promoting full recovery, independent living, and physical health and optimizing the quality of life. Thus the first two outcome domains take a leading role early in treatment, and the third and fourth domains become increasingly important as treatment progresses.
Numerous tools can evaluate the effectiveness of schizophrenia treatment. In a real-world treatment setting clinicians need tools that are relatively quick and simple to administer, sensitive to change, and easily interpreted. Global ratings of clinical effectiveness may be the fastest and most practical type of tool for day-to-day clinical use. Beyond that, some clinicians may find it useful to employ instruments that examine specific outcome areas.
Global ratings of clinical effectiveness
The Global Assessment of Functioning (GAF) scale produces a single, composite score of psychological, social, and occupational functioning on a hypothetical continuum from mental health to severe illness (15). The GAF is a widely used reliable and valid measure of psychiatric disturbance among patients with severe mental illness (73). A modified version includes more detailed criteria and a more structured scoring system and may be particularly appropriate for use by raters with varying skills and backgrounds (74).
Alternatively, the Clinical Global Impressions (CGI) scale can be used to assess overall clinical changes in symptoms, behavior, and function (75). The subscales of the CGI include a measure of current severity and overall global change from a specific reference point (usually the beginning of treatment).
The GAF and CGI scales do not examine the progression of independent domains. For instance, symptoms of disease may progress differently from the ability to live independently. Therefore, a slightly expanded CGI tool that measures progress in each of the four domains by using a 7-point scale may offer a better view of how a specific patient is progressing. The panel of schizophrenia researchers proposed an expanded global assessment grid, called the Global Outcome Assessment of Life in Schizophrenia (GOALS) scale, which broadens the evaluation to consider each of the proposed four outcome domains. In using GOALS, clinicians rate each of the four domains on a scale of 1, very much improved, to 7, very much worse. (The instrument is available on request from the first author.) Clinicians can use specific rating instruments, as described below, in conjunction with clinical interviews of the patient and involved family members or caregivers to score the grid and use it in the treatment planning process.
Rating instruments for specific outcome domains
Table 2 suggests domain-specific measurement tools that may be appropriate for assessing symptoms of disease (first domain), treatment burden (second domain), disease burden (third domain), and health, quality of life, and health and wellness (fourth domain). Although some quantifiable methods can be used to assess the third outcome domain (for example, assessments of cost of treatment or resource use and caregiver burden scales), interviews with the patient and family may be more revealing. In fact, rating scales cannot supplant the importance or value of clinical interviews with the patient and information gathered from family members.
Symptoms of disease. Two scales are often used to measure severity and change in positive and negative symptoms. The Brief Psychiatric Rating Scale (BPRS) was developed to assess change in severity of psychopathology among patients with psychotic illness, such as schizophrenia and psychotic major depression (76). The Positive and Negative Syndrome Scale (PANSS) was developed to assess psychopathology among patients with schizophrenia, with an emphasis on positive and negative symptoms but without neglecting other general psychopathology features (77). In the recovery model of Liberman and colleagues (9) symptom remission is defined as a score of 4 or less (moderate symptoms) for two consecutive years on each of the positive and negative symptom items on the PANSS.
No consensus exists about an appropriate office-based assessment of cognitive function. Full-scale cognitive test batteries are beyond the scope of ordinary office-based practices, and it is not clear whether abbreviated tests are satisfactory (78). Clinicians may want to refer some patients for specialized neurocognitive assessments. Nevertheless, it is important to evaluate the impact of treatment interventions on cognitive function as well as the impact of cognition on other outcomes. The following queries might help in this assessment. To what extent have critical cognitive functions (for example, executive function and working and verbal memory) improved since the initiation of treatment? Have functional skills altered as a result of changes in cognitive capacity? To what extent has adherence to treatment benefited from better insight? To what extent have medications (for example, anticholinergics) adversely affected cognitive function?
Mood symptoms can be measured with the Calgary Depression Scale for Schizophrenia, a reliable and validated instrument developed specifically for assessing mood among patients with schizophrenia (79,80). Questions to assess mood symptoms might include the following: To what extent do depressive symptoms or persistent anxiety impede social, vocational, or occupational function? Are negative symptoms related to underlying depression?
Treatment burden. One clear measure of the consequences of treatment burden is adherence to drug treatment, because patients are more likely to continue taking their medication if the treatment burden is tolerable. Discontinuation of treatment also contributes to increased burden on others.
Movement disorders or extrapyramidal side effects are one of the most well recognized components of treatment burden. A number of scales are available to measure emergent or persistent extrapyramidal side effects. These include the Barnes Akathisia Rating Scale (81), the Simpson-Angus Extrapyramidal Side Effects Scale (82), and the Abnormal Involuntary Movement Scale (83).
Subjective distress related to extrapyramidal side effects may be a better predictor of treatment adherence than the commonly used extrapyramidal side effects scales (84,85,86). A new scale to measure side effects was recently developed by the Approaches to Schizophrenia Communication (ASC) steering group to address these shortcomings (87). The ASC-SR is a self-report checklist to be completed by the patient, and the ASC-C is a clinician-administered version to be completed by the physician and the patient together as part of a semistructured interview (87,88,89). The short list of items in these questionnaires (see box on this page) are designed to help the patient better verbalize concerns about side effects and to help the treatment team gain a better understanding of what side effects are most bothersome to the patient.
Disease burden. Schizophrenia can have a tremendous impact on the patient and his or her family. Effective treatment may help alleviate some of the negative repercussions of schizophrenia on the patient's social network. An assessment of the burden of illness requires interviews with the patient and family to assess changes since the onset of the illness. The following questions are examples of how to assess this domain: Has there been a change in the level of interpersonal or family function? Has there been a change in the cost of treatment (for families or the health care system)? Has there been a change in vocational or educational function (productivity) of family members?
Health and wellness. This broad category may require several different types of assessment. Instruments to measure health and wellness among patients with severe mental illness include the Multnomah Community Ability Scale (90), the Quality of Life Interview (91), the Quality of Life Scale (92), the Wisconsin Quality of Life Index (93), and the Schizophrenia Outcomes Module (94). Although such detailed rating scales may not be suitable for use in day-to-day practice, clinicians should be alert for changes in the key areas assessed by these scales. Because this domain assesses successes rather than burdens, assessment questions need to include the following: Is there a change in the level of satisfaction with life, with work, with school? Is the patient more capable of independent living (self-management)? Is there a change in social relationships and intimacy with others? Is there a change in self-care (instrumental living function)? Is there a change in motivation and sense of purpose? Is there a change in health care practices—for example, smoking, substance abuse, and exercise?
The operational definitions of recovery proposed by Liberman and colleagues (9) may be helpful in assessing vocational functioning, independent living, and peer relationships (Table 1). However, the overall GOALS grid score for the domain of health and wellness also anticipates consideration of the impact of treatment on quality of life.
Factors affecting the assessment of clinical effectiveness
A variety of intervening factors can affect treatment outcomes for patients with schizophrenia and should be considered in the evaluation of clinical effectiveness. These factors can be grouped by whether they apply to the patient, the family, the treating physician, the health care system, or society at large (4). Certain factors can directly affect treatment outcomes (for example, substance abuse), influence expectations about outcomes (for example, age), or affect both treatment outcome and expectations (for example, educational level and available resources); they may also influence choice of treatment (for example, age or education can affect whether vocational rehabilitation is chosen).
Patient factors
Several factors may alter treatment outcomes in real-world settings: age (for example, adolescents and elderly patients), medical status and comorbid conditions, gender, substance use or abuse, cognitive capacity, treatment adherence, cultural issues (for example, race or ethnicity, educational levels, cultural expectations, belief systems, and religious background), employment status, marital status, lifestyle (for example, diet, exercise, involvement with others, and leisure pursuits), insight into illness and coping skills, and attitude toward physicians and health care systems.
Physician factors
A number of factors related to the physician have a significant influence on treatment selection and clinical outcomes and effectiveness (4): commitment to educating the patient and family about the illness, expertise with new treatments, familiarity with rehabilitation resources, ability to make appropriate referrals for rehabilitation services, and understanding that the ultimate goal of treatment extends beyond simply controlling symptoms and avoiding rehospitalization to encompass wellness and restoration of functioning.
ASC-C side effect checklist
1. Loss of energy or drive
2. Feeling unmotivated or numb
3. Daytime sedation or drowsiness
4. Sleeping too much
5. Muscles being too tense or stiff
6. Muscles trembling or shaking
7. Feeling restless or jittery
8. Need to move around and pace; inability to sit still
9. Trouble getting to sleep or staying asleep (insomnia)
10. Blurry vision
11. Dry mouth
12. Drooling
13. Memory and concentration problems
14. Constipation
15. Weight changes
16. Changes in sexual functioning
17. Menstrual or breast problem
The ASC scales can be obtained at www.seroquel.com/prof_asp/tools/asc_c_tool/asc-c_scales_writable.pdf
Institutional and societal factors
Institutional factors often influence treatment effectiveness. The clinical setting (for example, a busy clinic or a private office), consumer friendliness, convenience of location, and formulary limitations on choice of available medications (4) may facilitate or impede effective treatment. The financial and personnel resources of the health care providers and the system of communication between providers will have an impact on clinical effectiveness.
A patient's ability to achieve improved social and vocational functioning can ultimately be affected by the attitude of the society in which he or she lives (4). Stigmatization of mental illness, job discrimination, misunderstanding about psychiatric illness and care, publicity by antipsychiatry groups, shortage of appropriate community resources, confusing and contradictory public-sector policies, and red tape can have a serious impact on clinical effectiveness, even when the illness has remitted and the patient is poised for social and vocational reintegration.
Special issues related to adolescents and older patients
Age is an important patient variable in treatment selection and outcome expectations and warrants further examination. A discussion of treatment issues related to these particular patient populations will also illustrate how all the variables described above—patient, physician, institutional, and societal—interact to affect treatment outcomes.
Adolescents. Education, social relations, and substance abuse are critical issues during the developmental phase of adolescence and may affect treatment outcomes among adolescents who become psychotic. Studies have found that early intervention among patients with schizophrenia is likely to lead to better long-term outcomes (95,96). For this reason assertive treatment intervention with adolescents is recommended to improve the long-term prognosis (95). Several studies are currently under way to investigate the effectiveness of early intervention programs for adolescents with psychosis (95,97,98).
Adolescents appear to be less tolerant than adults of the physical side effects of antipsychotic medications (for example, movement disorders); hence, second-generation antipsychotics may be especially indicated in this population (95). In selecting and evaluating the effectiveness of specific treatment approaches among adolescent patients, clinicians need to focus on the following points: developmental issues related to antipsychotic treatment, impact of treatment on educational and social functioning, comorbid substance abuse as a modifier of the effectiveness of treatment, drug tolerability and treatment adherence, and stigma encountered by young people with psychosis.
Elderly patients. Critical issues affecting elderly patients with psychosis differ markedly from those affecting adolescent patients (99). Current patterns of antipsychotic use among elderly patients fall into two distinct and nonoverlapping domains: management of psychosis among patients with chronic conditions (for example, schizophrenia) and short-term, acute symptomatic relief for specific conditions (for example, agitation in Alzheimer's disease). The settings in which care is delivered to elderly patients include the full range of ambulatory and inpatient public and private facilities, facilities in the Department of Veterans Affairs, the long-term-care sector (skilled and intermediate care), and the rapidly growing assisted-living sector, including both proprietary and not-for-profit general and disease-specific (Alzheimer's) facilities.
The elderly population is large, growing, and increasingly sophisticated. In selecting and evaluating the effectiveness of specific treatment approaches among elderly patients, clinicians need to focus on the following points: shifts in levels of care and associated cost of treatment, polypharmacy, expectations about treatment outcome, existing levels of function and disability that can affect outcome expectations, and caregiver and family burden.
Conclusions
The recent introduction of a wide range of new treatment interventions, both pharmacologic and psychosocial, is raising expectations about long-term outcomes for patients with schizophrenia. Consequently, there is more optimism about what patients with schizophrenia can achieve after remission. In this article we have proposed a working definition, given practical suggestions for measuring clinical effectiveness in a real-world setting, and offered tools that clinicians may find helpful in measuring progress in practice. GOALS allows a clinician or treatment team to evaluate the progress of treatment and to consider new strategies to maximize each of the four outcome domains. It is hoped that this formulation will help clinicians improve their ability to formulate an effective treatment process and to evaluate the impact of intervention on patients' short- and long-term outcomes.
Acknowledgments
The authors thank Peter Weiden, M.D., for contributing significantly to the ideas presented in this paper. The participants in the Towards Identifying Criteria for Clinical Effectiveness roundtable were John P. Docherty, M.D., Philip P. Harvey, Ph.D., John Kane, M.D., Jeffrey S. McCombs, Ph.D., Robin M. Murray M.D., D.Sc., Henry A. Nasrallah, M.D., S. Charles Schulz, M.D., Rajiv Tandon, M.D., Steven D. Targum, M.D., and Peter J. Weiden, M.D. The authors also thank AstraZeneca Pharmaceuticals LP for providing support for the roundtable.
Dr. Nasrallah is affiliated with the department of psychiatry, neurology, and neuroscience at the University of Cincinnati Medical Center, 231 Albert Sabin Way, P.O. Box 670559, Cincinnati, Ohio 45267-0559 (e-mail, [email protected]). Dr. Targum is with Pharmastar in Swarthmore, Pennsylvania. Dr. Tandon is with the department of psychiatry at the University of Michigan in Ann Arbor. Dr. McCombs is with the department of pharmaceutical economics and policy at the University of Southern California in Los Angeles. Ms. Ross is with Ross Editorial in Independence, Virginia.
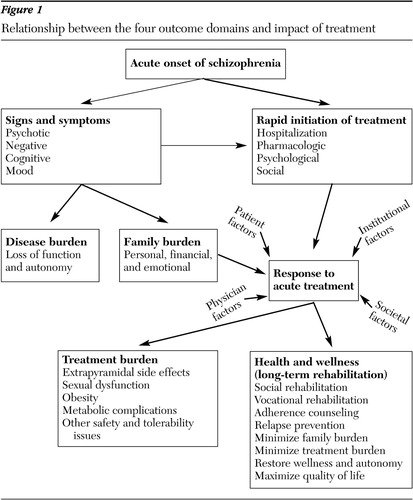
Figure 1. Relationship between the four outcome domains and impact of treatment
 |
Table 1. Operational definition of recovery from schizophreniaa
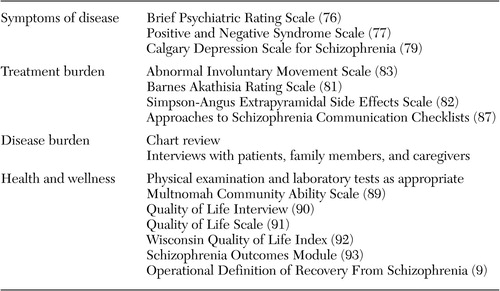 |
Table 2. Clinically useful domain-specific measurement tools in the treatment of schizophrenia
1. DeQuardo JR, Tandon R: Do atypical antipsychotic medications favorably alter the long-term course of schizophrenia? Journal of Psychiatric Research 32:229–242, 1998Google Scholar
2. Lebowitz BD, Rudorfer MV: Treatment research at the millennium: from efficacy to effectiveness. Journal of Clinical Psychopharmacology 18:1, 1998Crossref, Medline, Google Scholar
3. Kane J: Progress defined: short-term efficacy, long-term effectiveness. International Clinical Psychopharmacology 16(suppl 1):S1-S8, 2001Google Scholar
4. Nasrallah HA, Smeltzer DJ: Contemporary diagnosis and management of the patient with schizophrenia. Newtown, Penn, Handbooks in Health Care, 2002Google Scholar
5. Olfson M: Emerging methods in mental health outcomes research. Journal of Practical Psychiatry and Behavioral Health 5:20–24, 1999Crossref, Google Scholar
6. Streiner DL: The 2 "Es" of research: efficacy and effectiveness trials. Canadian Journal of Psychiatry 47:552–556, 2002Crossref, Medline, Google Scholar
7. Tandon R, Jibson MD: Efficacy of newer generation antipsychotics in the treatment of schizophrenia. Psychoneuroendocrinology 28(suppl 1):9–26, 2003Medline, Google Scholar
8. Fichtner CG, Luchins DJ, Malan RD, et al: Real-world pharmacotherapy with novel antipsychotics. Journal of Practical Psychiatry and Behavioral Health 5:37–43, 1999Crossref, Google Scholar
9. Liberman RP, Kopelowicz A, Ventura J, et al: Operational criteria and factors related to recovery from schizophrenia. International Review of Psychiatry 14:256–272, 2002Crossref, Google Scholar
10. Fischer EP, Shumway M, Owen RR: Priorities of consumers, providers, and family members in the treatment of schizophrenia. Psychiatric Services 53:724–729, 2002Link, Google Scholar
11. Lee TT, Ziegler JK, Sommi R, et al: Comparison of preferences for health outcomes in schizophrenia among stakeholder groups. Journal of Psychiatric Research 34:201–210, 2000Crossref, Medline, Google Scholar
12. Crane-Ross D, Roth D, Lamber BG: Consumers' and case managers' perceptions of mental health and community support service needs. Community Mental Health Journal 36:161–178, 2000Crossref, Medline, Google Scholar
13. McCombs JS, Nichol MB, Stimmel GL, et al: Use patterns for antipsychotic medications in Medicaid patients with schizophrenia. Journal of Clinical Psychiatry 60(suppl 19):5–11, 1999Medline, Google Scholar
14. Lyu RR, McCombs JS, Johnstone BM, et al: Use of conventional antipsychotics and the cost of treating schizophrenia. Health Care Financing Review 23:83–99, 2001Medline, Google Scholar
15. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision. Washington, DC, American Psychiatric Association, 2000Google Scholar
16. Gold JM, Harvey PD: Cognitive deficits in schizophrenia. Psychiatric Clinics of North America 16:295–312, 1993Crossref, Medline, Google Scholar
17. Goldman RS, Axelrod BN, Tandon R, et al: Neuropsychological prediction of treatment efficacy and one-year outcome in schizophrenia. Psychopathology 26:122–126, 1993Crossref, Medline, Google Scholar
18. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry 153:321–330, 1996Google Scholar
19. Harvey PD, Howanitz E, Parrella M, et al: Symptoms, cognitive functioning, and adaptive skills in geriatric patients with lifelong schizophrenia: a comparison across treatment sites. American Journal of Psychiatry 155:1080–1086, 1998Link, Google Scholar
20. Green MF, Kern RS, Braff DL, et al: Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophrenia Bulletin 26:119–136, 2000Google Scholar
21. Huppert JD, Weiss KA, Lim R, et al: Quality of life in schizophrenia: contributions of anxiety and depression. Schizophrenia Research 51:171–180, 2001Crossref, Medline, Google Scholar
22. Goldberg TE, Ragland JD, Torrey EF, et al: Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Archives of General Psychiatry 47:1066–1072, 1990Crossref, Medline, Google Scholar
23. Bellack AS, Gold JM, Buchanan RW: Cognitive rehabilitation for schizophrenia: problems, prospects, and strategies. Schizophrenia Bulletin 25:257–274, 1999Crossref, Medline, Google Scholar
24. Bryson G, Bell MD, Kaplan E, et al: The functional consequences of memory impairments on initial work performance in people with schizophrenia. Journal of Nervous and Mental Disease 186:610–615, 1999Crossref, Google Scholar
25. Meltzer HY, McGurk SR: The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophrenia Bulletin 25:233–255, 1999Crossref, Medline, Google Scholar
26. Koreen AR, Siris SG, Chakos M, et al: Depression in first-episode schizophrenia. American Journal of Psychiatry 150:1643–1648, 1993Link, Google Scholar
27. Mauri MC, Bravin S, Fabiano L, et al: Depressive symptoms and schizophrenia: a psychopharmacological approach. L'Encephale 21:555–558, 1995Medline, Google Scholar
28. Lancon C, Auquier P, Reine G, et al: Relationships between depression and psychotic symptoms of schizophrenia during an acute episode and stable period. Schizophrenia Research 47:135–140, 2001Crossref, Medline, Google Scholar
29. Herz MI, Melville C: Relapse in schizophrenia. American Journal of Psychiatry 137:801–805, 1980Link, Google Scholar
30. Roy A: Depression, attempted suicide, and suicide in patients with chronic schizophrenia. Psychiatric Clinics of North America 9:193–206, 1986Crossref, Medline, Google Scholar
31. Nasrallah HA, Mulvihill T: Iatrogenic disorders associated with conventional vs atypical antipsychotics. Annals of Clinical Psychiatry 13:215–227, 2001Medline, Google Scholar
32. Tandon R: Safety and tolerability: how do newer generation "atypical" antipsychotics compare? Psychiatric Quarterly 73:297–311, 2002Google Scholar
33. Nasrallah H: A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology 28(suppl 1):83–96, 2003Medline, Google Scholar
34. Docherty JP, Van Kammen DP, Siris SG, et al: Stages of onset of schizophrenic psychosis. American Journal of Psychiatry 135:420–426, 1978Link, Google Scholar
35. Tandon R, Jibson MD: Extrapyramidal side effects of antipsychotic treatment: scope of problem and impact on outcome. Annals of Clinical Psychiatry 14:123–129, 2002Crossref, Medline, Google Scholar
36. Leucht S, Pitschel-Walz G, Abraham D, et al: Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophrenia Research 35:51–68, 1999Crossref, Medline, Google Scholar
37. Allison DB, Mentore JL, Heo M, et al: Antipsychotic-induced weight gain: a comprehensive research synthesis. American Journal of Psychiatry 156:1686–1696, 1999Abstract, Google Scholar
38. Dixon L, Weiden P, Delahanty J, et al: Prevalence and correlates of diabetes in national schizophrenia samples. Schizophrenia Bulletin 26:903–912, 2000Crossref, Medline, Google Scholar
39. Regenold WT, Thaper RK, Marano C, et al: Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. Journal of Affective Disorders 70:19–26, 2002Crossref, Medline, Google Scholar
40. Koro CE, Fedder DO, L'Italien GJ, et al: An assessment of the independent effects of olanzapine and risperidone exposure on the risk of hyperlipidemia in schizophrenic patients. Archives of General Psychiatry 59:1021–1026, 2002Crossref, Medline, Google Scholar
41. McIntyre RS, McCann SM, Kennedy SH: Antipsychotic metabolic effects: weight gain, diabetes mellitus, and lipid abnormalities. Canadian Journal of Psychiatry 46:273–281, 2001Crossref, Medline, Google Scholar
42. Henderson DC: Clinical experience with insulin resistance, diabetic ketoacidosis, and type 2 diabetes mellitus in patients treated with atypical antipsychotic agents. Journal of Clinical Psychiatry 62(suppl 27):10–14, 2001Medline, Google Scholar
43. Haupt DW, Newcomer JW: Hyperglycemia and antipsychotic medications. Journal of Clinical Psychiatry 62(suppl 27):15–26, 2001Medline, Google Scholar
44. Dickson RA, Seeman MV, Corenblum B: Hormonal side effects in women: typical versus atypical antipsychotic treatment. Journal of Clinical Psychiatry 61:10–15, 2000Medline, Google Scholar
45. Glassman AH, Bigger JT Jr: Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. American Journal of Psychiatry 158:1774–1782, 2001Link, Google Scholar
46. Westermeyer JF, Harrow M: Course and outcome in schizophrenia, in Handbook of Schizophrenia: Vol 3: Nosology, Epidemiology, and Genetics of Schizophrenia. Edited by Tsuang MT, Simpson JC. New York, Elsevier Science, 1988Google Scholar
47. Rice DP: The economic impact of schizophrenia. Journal of Clinical Psychiatry 60(suppl 1):4–6, 28–30, 1999Medline, Google Scholar
48. Fichtner CG, Hanrahan P, Luchins DJ: Pharmacoeconomic studies of atypical antipsychotics: review and perspective. Psychiatric Annals 28:381–396, 1998Crossref, Google Scholar
49. Csernansky JG, Schuchart EK: Relapse and rehospitalisation rates in patients with schizophrenia: effects of second generation antipsychotics. CNS Drugs 16:473–484, 2002Crossref, Medline, Google Scholar
50. Del Paggio D, Finley PR, Cavano JM: Clinical and economic outcomes associated with olanzapine for the treatment of psychotic symptoms in a county mental health population. Clinical Therapeutics 24:803–817, 2002Crossref, Medline, Google Scholar
51. Gianfrancesco F, Durkin MB, Mahmoud R, et al: Use of healthcare services by patients treated with risperidone versus conventional antipsychotic agents. Pharmacoeconomics 20:413–427, 2002Crossref, Medline, Google Scholar
52. Tilden D, Aristides M, Meddis D, et al: An economic assessment of quetiapine and haloperidol in patients with schizophrenia only partially responsive to conventional antipsychotics. Clinical Therapeutics 24:1648–1667, 2002Crossref, Medline, Google Scholar
53. Rogers ES, Walsh D, Masotta L, et al: Massachusetts survey of client preferences for community support services. Boston, Center for Psychiatric Services, 1991Google Scholar
54. McGurk SR: Neurocognition as a determinant of employment status in schizophrenia. Journal of Psychiatric Practice 6:190–196, 2000Crossref, Medline, Google Scholar
55. Bell MD, Lysaker PH, Milstein RM: Clinical benefits of paid work activity in schizophrenia. Schizophrenia Bulletin 22:51–67, 1996Crossref, Medline, Google Scholar
56. Mueser KT, Becker DR, Torrey WC, et al: Work and nonvocational domains of functioning in persons with severe mental illness: a longitudinal analysis. Journal of Nervous and Mental Disease 185:419–426, 1997Crossref, Medline, Google Scholar
57. Polak P, Warner R: The economic life of seriously mentally ill people in the community. Psychiatric Services 47:270–274, 1996Link, Google Scholar
58. Greden JF, Tandon R: Negative symptoms of schizophrenia: the need for conceptual clarity. Biological Psychiatry. 30:321–325, 1991Google Scholar
59. Breier A, Schreiber JL, Dyer J, et al: National Institute of Mental Health longitudinal study of chronic schizophrenia: prognosis and predictors of outcome. Archives of General Psychiatry 48:239–246, 1991Crossref, Medline, Google Scholar
60. McGurk SR, Meltzer HY: The role of cognition in vocational functioning in schizophrenia. Schizophrenia Research 45:175–184, 2000Crossref, Medline, Google Scholar
61. McGlashan TH: A selective review of recent North American long-term follow-up studies of schizophrenia. Schizophrenia Bulletin 14:515–542, 1988Crossref, Medline, Google Scholar
62. Medical Illness and Schizophrenia. Edited by Meyer JM, Nasrallah HA. Washington, DC, American Psychiatric Press; 2003Google Scholar
63. Brown S, Birtwistle J, Roe L, et al: The unhealthy lifestyle of people with schizophrenia. Psychological Medicine 29:697–701, 1999Crossref, Medline, Google Scholar
64. Dixon L, Postrado L, Delahanty J, et al: The association of medical comorbidity in schizophrenia with poor physical and mental health. Journal of Nervous and Mental Disease 187:496–502, 1999Crossref, Medline, Google Scholar
65. Koran LM, Sox HC Jr, Marton KI, et al: Medical evaluation of psychiatric patients: I. Results in a state mental health system. Archives of General Psychiatry 46:733–740, 1989Crossref, Medline, Google Scholar
66. Weiden PJ: Managing unhealthy behaviors in schizophrenia. Journal of Psychiatric Practice 6:160–168, 2000Crossref, Google Scholar
67. Wirshing DA, Spellberg BJ, Erhart SM, et al: Novel antipsychotics and new onset diabetes. Biological Psychiatry 44:778–783, 1998Crossref, Medline, Google Scholar
68. Wirshing DA, Wirshing WC, Kysar L, et al: Novel antipsychotics: comparison of weight gain liabilities. Journal of Clinical Psychiatry 60:358–363, 1999Crossref, Medline, Google Scholar
69. Goodnick PJ, Kato MM: Antipsychotic medication: Effects on regulation of glucose and lipids. Expert Opinion on Pharmacotherapy 2:1571–1582, 2001Crossref, Medline, Google Scholar
70. Cournos F, Herman R, Kaplan M, et al: AIDS prevention for people with severe mental illness. Journal of Practical Psychiatry and Behavioral Health 3:285–292, 1997Crossref, Google Scholar
71. Kelly JA: HIV risk reduction for persons with severe mental illness. Clinical Psychology Review 17:293–309, 1997Crossref, Medline, Google Scholar
72. Mintz J, Mintz LI, Arruda MJ, et al: Treatments of depression and the functional capacity to work. Archives of General Psychiatry 49:761–768, 1992Crossref, Medline, Google Scholar
73. Jones SH, Thornicroft G, Coffey M, et al: A brief mental health outcome scale: reliability and validity of the Global Assessment of Functioning (GAF). British Journal of Psychiatry 166:654–659, 1995Crossref, Medline, Google Scholar
74. Hall RC: Global assessment of functioning: a modified scale. Psychosomatics 36:267–275, 1995Crossref, Medline, Google Scholar
75. Guy W: ECDEU Assessment Manual for Psychopharmacology-Revised. DHEW pub no ADM 76–338. Rockville, Md, US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976Google Scholar
76. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacology Bulletin 24:97–99, 1988Google Scholar
77. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 13:261–276, 1987Crossref, Medline, Google Scholar
78. Harvey PD, Keefe RSE: Studies of cognitive change with novel antipsychotic treatment in schizophrenia. American Journal of Psychiatry 158:176–184, 2001Link, Google Scholar
79. Addington D, Addington J, Schissel B: A depression rating scale for schizophrenics. Schizophrenia Research 3:247–251, 1990Crossref, Medline, Google Scholar
80. Addington D, Addington J, Maticka-Tyndale E, et al: Reliability and validity of a depression rating scale for schizophrenics. Schizophrenia Research 6:201–208, 1992Crossref, Medline, Google Scholar
81. Barnes TRE: A rating scale for drug-induced akathisia. British Journal of Psychiatry 154:672–676, 1989Crossref, Medline, Google Scholar
82. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 212:11–19, 1970Crossref, Google Scholar
83. Guy W: ECDEU Assessment Manual for Psychopharmacology-Revised. DHEW pub no ADM 76–338. Rockville, Md, US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976Google Scholar
84. Adams SG Jr, Howe JT: Predicting medication compliance in a psychotic population. Journal of Nervous and Mental Disease 181:558–560, 1993Crossref, Medline, Google Scholar
85. Buchanan A: A two-year prospective study of treatment compliance in patients with schizophrenia. Psychological Medicine 22:787–797, 1992Crossref, Medline, Google Scholar
86. Weiden P, Rapkin B, Mott T, et al: Rating of medication influences (ROMI) scale in schizophrenia. Schizophrenia Bulletin 20:297–310, 1994Crossref, Medline, Google Scholar
87. Dott SG, Weiden P, Hopwood P, et al: An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication Checklists. CNS Spectrums 6:333–338, 2001Crossref, Medline, Google Scholar
88. Weiden PJ, Miller AL: Which side effects really matter? Screening for common and distressing side effects of antipsychotic medications. Journal of Practical Psychiatry 7:41–47, 2001Crossref, Medline, Google Scholar
89. Using the ASC Program: a training guide. Journal of Practical Psychiatry 7:69–72, 2001)Crossref, Google Scholar
90. Barker S, Barron N, McFarland BH, et al: Multnomah Community Ability Scale: User's Manual. Portland, Oreg, Western Mental Health Research Center, Oregon Health Sciences University, 1993Google Scholar
91. Lehman AF: A Quality of Life Interview for the chronically mentally ill. Evaluation and Program Planning 11:51–62, 1988Crossref, Google Scholar
92. Heinrichs DW, Hanlon TE, Carpenter WT: The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin 10:388–397, 1984Crossref, Medline, Google Scholar
93. Becker M, Diamond R, Sainfort F: A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Quality of Life Research 2:239–251, 1993Crossref, Medline, Google Scholar
94. Fischer EP, Cuffel BJ, Owen RR, et al: Schizophrenia Outcomes Module. Little Rock, Ark, University of Arkansas for Medical Sciences, 1996Google Scholar
95. Schulz SC, Findling RL, Friedman L, et al: Treatment and outcomes in adolescents with schizophrenia. Journal of Clinical Psychiatry 59(suppl 1):50–54, 1998Medline, Google Scholar
96. Shepherd M, Watt D, Falloon I, et al: The natural history of schizophrenia: a five-year follow-up study of outcome and prediction in a representative sample of schizophrenics. Psychological Medicine 15:1–46, 1989Google Scholar
97. McGorry PD, Phillips J, Yung AR, et al: A randomised controlled trial of interventions in the pre-psychotic phase of psychotic disorders. Schizophrenia Research 41:9, 2000Crossref, Google Scholar
98. Wyatt RJ, Henter I: Rationale for the study of early intervention. Schizophrenia Research 51:69–76, 2001Crossref, Medline, Google Scholar
99. Maixner SM, Mellow AM, Tandon R: The efficacy, safety, and tolerability of antipsychotics in the elderly. Journal of Clinical Psychiatry 60(suppl 8):29–41, 1999Medline, Google Scholar



