Early Intervention to Preempt Major Depression Among Older Black and White Adults
Abstract
Objective
The study objective was to assess the efficacy of problem-solving therapy for primary care (PST-PC) for preventing episodes of major depression and mitigating depressive symptoms of older black and white adults. The comparison group received dietary coaching.
Methods
A total of 247 participants (90 blacks, 154 whites, and three Asians) with subsyndromal depressive symptoms were recruited into a randomized depression prevention trial that compared effects of individually delivered PST-PC and dietary coaching on time to major depressive episode and level of depressive symptoms (Beck Depression Inventory) over two years. Cumulative intervention time averaged 5.5−6.0 hours in each study arm.
Results
The two groups did not differ significantly in time to major depressive episodes, and incidence of such episodes was low (blacks, N=8, 9%; whites, N=13, 8%), compared with published rates of 20%–25% over one year among persons with subsyndromal symptoms and receiving care as usual. Participants also showed a mean decrease of 4 points in depressive symptoms, sustained over two years. Despite greater burden of depression risk factors among blacks, no significant differences from whites were found in the primary outcome.
Conclusions
Both PST-PC and dietary coaching are potentially effective in protecting older black and white adults with subsyndromal depressive symptoms from developing episodes of major depression over two years. Absent a control for concurrent usual care, this conclusion is preliminary. If confirmed, both interventions hold promise as scalable, safe, nonstigmatizing interventions for delaying or preventing episodes of major depression in the nation’s increasingly diverse older population.
Major depressive disorder is prevalent, with adequate treatment difficult to access and only partially successful in averting years lived with disability (1). In later life, particularly, major depressive disorder has public health importance because of its prevalence and associated disability, morbidity, health care costs, and mortality, especially among primary care outpatients and people from racial-ethnic minority groups (2). Major depressive disorder is also a risk factor for dementia (3). The limitations of treatment underscore the need to develop public health–relevant approaches to prevent depression and its downstream consequences for high-risk older adults.
Elderly adults who are from racial-ethnic minority groups show particular vulnerability to common mental illnesses. Older blacks, for example, endorse significantly greater depressive symptoms than whites (4) and bear a higher burden of risk of depression rooted in social and medical disadvantages (5): more disability, greater health risks (including obesity, smoking, and substance use disorders), lower education attainment, and lower likelihood of marriage (6). Blacks also have a higher incidence of dementia (7), and preventing depression may delay or prevent dementia (8). In addition, inequalities in the utilization of mental health services and the treatment rate for depression continue to grow (9) and are compounded by barriers of trust, stigma, and shortages of providers who have the same race-ethnicity as their patients (10).
Mildly symptomatic individuals are at highest risk of developing episodes of major depression (11–13). Bereavement, social isolation, sleep disturbance, disability, previous depression, and female gender are important risk factors for depression among older community residents (14). Per the Institute of Medicine, focusing depression prevention for mildly symptomatic persons (“indicated” prevention) may have the greatest efficiency from a public health perspective, with a lower number needed to treat to prevent one incident case (14,15).
The dearth of randomized controlled prevention trials with older adults, however, raises the question of which interventions to use. Older patients, especially blacks, prefer psychosocial interventions to antidepressant medication for treatment of depression (16). Moreover, antidepressant medications, while effective in severe depression, appear to show minimal benefit relative to placebo in mild depression (17), although the notion that mild depression does not respond to antidepressant medication is not settled (18).
Problem-solving therapy for primary care (PST-PC) is a brief intervention with antidepressant treatment efficacy that is deliverable by non–mental health clinicians in primary care (19,20). It delays or prevents depression of older adults with macular degeneration (21) and after stroke (22). The antidepressant and depression-preventing effects of PST-PC may be mediated by a seven-step approach to better problem solving (including behavioral activation), leading to improved self-efficacy and resilience, together with reduction in learned helplessness (23).
In designing this trial, we sought a culturally acceptable, active comparison intervention to control for nonspecific effects of time and attention inherent in PST-PC. The choice of coaching in healthy dietary practices grew out of field data collected from 1,244 black participants in the Healthy Black Family Project at the University of Pittsburgh’s Graduate School of Public Health, in which many of the respondents with high levels of stress were either overweight (45%) or obese (50%). Our Community Research Advisory Board endorsed the choice of dietary coaching as an active control arm and as a culturally acceptable strategy consistent with clinical equipoise and one that would facilitate recruitment of black participants (many of whom were not receiving primary care services) more easily than treatment as usual or a no-intervention control.
Our primary study hypothesis was that PST-PC would reduce incident episodes of major depression by 50% over two years compared with dietary coaching. Our second hypothesis was that participants in PST-PC would report more and better sustained decline in depressive symptoms compared with dietary coaching.
Methods
Informed consent, screening, assessment, and enrollment
The protocol was overseen by a data safety monitoring board and reviewed and approved annually by the University of Pittsburgh’s Institutional Review Board.
Beginning in September 2006, and extending over a period of 42 months, we enrolled a sample of 247 participants: 154 (62%) whites, 90 (36%) blacks, and three (1%) Asians. To recruit participants with subsyndromal depressive symptoms, we screened individuals who were 50 or older, using the Center for Epidemiological Studies Depression Scale (CES-D) (24) and requiring a score of 11 or greater and an absence of a major depressive episode during the previous year. We administered the Structured Clinical Interview for DSM-IV Disorders (SCID) (25) to rule out current major depressive disorder. Participants were also required to have a Mini-Mental State score of 24 or higher, to exclude probable dementia (26). An episode of alcohol or other substance use disorder within the past 12 months and a history of bipolar disorder, other psychotic disorder, or neurodegenerative disorder also were conditions for study exclusion. Recruitment pathways to study participation differed for blacks and whites, largely reflecting the different settings in which help-seeking takes place. For example, the major source for recruiting white participants was referrals from primary care practices, whereas for black participants the major source was community-based agencies, including black churches.
Randomization
A project statistician randomly assigned participants to either the PST-PC or dietary coaching condition, using permuted-block randomization stratified by the presence or absence of a history of major depression (because a past history is a strong risk factor for future episodes) and by site of recruitment—primary care, community agencies, or specialty mental health care. Randomization accounted for the different sociodemographic characteristics of participants (including race), recruitment site, and the possibility that recruitment site could influence rates of occurrence of major depressive episodes. Random assignment was communicated by the statistician to the project co-coordinator but concealed from independent evaluators. There were no instances of breaking the blind.
Interventions
Both interventions—PST-PC and dietary coaching—had similar numbers of sessions (six to eight sessions) and semiannual boosters (lasting 30–45 minutes at three, nine, and 15 months). Both interventions were provided by interventionists in individual sessions. Interventionists were trained in our National Institute of Mental Health–sponsored center for depression prevention and treatment of older adults. Both interventions included homework assignments and monitoring of adherence, and they focused on concerns identified by each participant.
The experimental group received manualized PST-PC. To teach the model to participants, the first session lasted an hour, and the subsequent sessions lasted 30 minutes each (total time 4.55±1.46 hours of PST-PC and 3.92±2.19 hours of dietary coaching).
Participants in the control condition received coaching in healthy eating practices. Using a manualized educational intervention, interventionists reviewed general nutrition guidelines, including the U.S. Department of Agriculture food pyramid; helped with preparing weekly menus and grocery lists; saved coupons for food items; and reviewed food intake since last visit. Topics discussed included access to healthy food, cost of food, meal preparation, culturally specific and acceptable foods, and specific topics raised by participants.
Interventionists were six white social workers and mental health nurses. The same interventionists delivered both PST-PC and dietary coaching, to avoid confounding intervention with clinician effects. To ensure fidelity of intervention delivery, we randomly selected 20% of audiotapes of intervention sessions for evaluation, and we supervised groups and gave one-on-one feedback to the interventionists. PST-PC adherence ratings assessing quality were completed by the intervention supervisor, who used two sessions for each case, an early session (sessions 1–3) and a later session (sessions 4–8). Once ratings were completed, corrective feedback was provided. Most sessions (N=41 of 56, 73%) of both study conditions were rated as adherent. A treatment fidelity scale was also developed to document the absence of intervention contamination effects. With this scale, ratings were completed on seven consecutive minutes of the session, starting five minutes into the session. Two raters independently rated the sessions for the presence of PST-PC and dietary coaching elements. Using blind ratings, we found the two interventions to be highly discriminable (κ=.91), even though they were delivered by the same interventionists. Interventions were delivered primarily face to face in settings the participants chose: primary care offices, community agencies, and participants’ homes. About 9% (N=173 of 1,884) of sessions were delivered over the telephone.
Outcomes
The primary outcome was incident episodes of major depression, per the SCID section for mood disorders (25), administered by independent evaluators blind to randomized intervention assignment at baseline (time 1), at the end of intervention, and every three months subsequently until 24 months, for a total of nine time points. Also assessed at the same time points were levels of depressive symptoms (Beck Depression Inventory [BDI]) (27) and health-related quality of life (Medical Outcomes Study 12-Item Short Form) (28). Other domains of assessment encompassed coexisting general medical illness per total score on the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) (29), problem-solving skills (Social Problem Solving Inventory [SPSI], a self-report measure of problem-solving style) (30), and anxiety (Brief Symptom Inventory [BSI]) (31). (Outcomes other than depression will be reported elsewhere.)
Data analysis
Outcomes analyses were conducted by study statisticians operating independently of the investigators and blind to study arm. All analyses were performed on the basis of the intent-to-treat principle so that comparisons were made according to the assigned intervention groups. All data were examined for normality before the analyses; transformations were used where necessary. Baseline demographic and clinical differences between participants by random assignment and by race were tested with t tests for continuous variables and chi square tests with continuity correction for categorical variables. Kaplan-Meier curves were used to illustrate the effects of PST-PC and dietary coaching on incidence of major depressive episodes. Formal inferences between groups were made with log-rank tests if five or more events were expected in both arms or with Fisher’s exact tests otherwise. Multivariate Cox proportional hazard models were used to explore the strongest predictors of major depressive disorder.
To compare depression levels (BDI), we first tested whether baseline differences were present between intervention groups. In cases where no differences were apparent, we then used a mixed-models approach to compare the trajectories of the variables over time between the groups. If there was a significant baseline difference between groups, we used the baseline value as a covariate in the fitted models. To characterize and compare the trajectories between PST-PC and dietary coaching, we used mixed models examining intervention, time, time squared, and the potential interactions among intervention and the time variables. In analyses involving race, we included race and the interactions among race and other variables. We documented reasons for missing data and handled missing data using mixed-model analyses. Formal tests were conducted to determine whether the missingness of data was random.
To examine effects of problem solving on depressive symptoms, we conducted exploratory analyses and included SPSI score as a time-varying covariate in the whole-group longitudinal model. To examine the possibility of bidirectional relationship of SPSI score and depressive symptoms, we also examined SPSI scores as outcome, using the same model of treatment, time, and treatment × time effects but including BDI scores as a time-varying covariate.
Results
The groups did not differ in sociodemographic, general health, cognitive, mental health, and recruitment pathways (Table 1). Primary care referrals provided the main source of enrollment, followed by recruitment in community-based agencies and by self-referral in response to print and on-air advertisements.
| PST-PC (N=125) | Dietary coaching (N=122) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Total N | N | % | N | % | Test statistic | df | p |
| Sociodemographic | ||||||||
| Age (M±SD years) | 247 | 65.8±10.9 | 65.4±11.0 | t=.28 | 245 | >.778 | ||
| Female | 247 | 86 | 69 | 90 | 74 | χ2=.52 | 1 | >.470 |
| Race | 247 | |||||||
| Asian/Pacific Islander | 2 | 2 | 1 | 1 | >.541a | |||
| Black | 42 | 34 | 48 | 39 | ||||
| White | 81 | 65 | 73 | 60 | ||||
| Education (M±SD years) | 247 | 14.4±2.8 | 14.7±2.7 | t=–.78 | 245 | >.436 | ||
| Marital status | ||||||||
| Cohabiting or married | 247 | 58 | 46 | 56 | 46 | χ2=1.68 | 3 | >.640 |
| Divorced or separated | 21 | 17 | 27 | 22 | ||||
| Never married | 17 | 14 | 12 | 10 | ||||
| Widowed | 29 | 23 | 27 | 22 | ||||
| Employed | 247 | 52 | 42 | 47 | 39 | χ2=.13 | 1 | >.716 |
| Median household income (M±SD $) | 243 | 50,511±25,787 | 45,545±21,599 | t=1.62 | 241 | >.105 | ||
| General health | ||||||||
| Cumulative Illness Rating Scale (M±SD score) | ||||||||
| Totalb | 245 | 7.7±3.6 | 8.0±4.2 | t=–.60 | 243 | >.550 | ||
| Countc | 246 | 4.9±2.1 | 5.0±2.4 | t=–.48 | 244 | >.628 | ||
| Heart and vasculard | 246 | 2.0±1.5 | 1.9±1.5 | t=.60 | 244 | >.549 | ||
| Body mass index | ||||||||
| Total (M±SD) | 245 | 30.5±6.6 | 30.6±7.3 | t=–.07 | 243 | >.942 | ||
| ≥30 points (obesity indicator) | 245 | 57 | 46 | 62 | 52 | χ2=.68 | 1 | >.411 |
| Rand Health Status Inventory (M±SD)e | ||||||||
| Physical health component | 207 | 41.3±11.8 | 42.9±11.8 | t=–.95 | 205 | >.342 | ||
| Mental health component | 207 | 42.4±9.8 | 43.7±9.0 | t=–1.04 | 205 | >.297 | ||
| Cognition: Mini-Mental Status Examination (M±SD)f | 246 | 28.1±1.7 | 28.4±1.5 | t=–1.38 | 244 | >.170 | ||
| Mental health | ||||||||
| Hamilton Rating Scale for Depression (M±SD)g | 246 | 11.6±4.0 | 10.8±3.5 | t=1.68 | 244 | >.094 | ||
| Center for Epidemiologic Studies Depression Scale (M±SD)h | 246 | 21.9±8.3 | 20.4±7.5 | t=1.48 | 244 | >.141 | ||
| Beck Depression Inventory (M±SD)i | 233 | 11.1±5.9 | 9.9±5.5 | t=1.60 | 231 | >.110 | ||
| Brief Symptom Inventory anxiety (M±SD)j | 235 | .5±.5 | .5±.5 | t=–.16 | 233 | >.875 | ||
| History of major depressive disorder | 41 | 33 | 42 | 34 | χ2=.02 | 1 | >.892 | |
| History of anxiety disorder | 27 | 22 | 25 | 20 | χ2=.00 | 1 | >.954 | |
| Current anxiety disorder | 27 | 22 | 33 | 27 | χ2=.72 | 1 | >.395 | |
| Social Problem Solving Inventory (M±SD)k | ||||||||
| Total | 214 | 99.8±13.7 | 103.1±13.0 | t=–1.82 | 212 | >.070 | ||
| Positive problem orientation | 229 | 95.8±16.4 | 99.8±14.7 | t=–1.92 | 227 | >.056 | ||
| Referral source | ||||||||
| Community outreachl | 22 | 18 | 24 | 20 | χ2=3.54 | 5 | >.618 | |
| Mental health specialist | 5 | 4 | 5 | 4 | ||||
| Primary care | 53 | 43 | 57 | 47 | ||||
| Research (research program or registry) | 13 | 11 | 12 | 10 | ||||
| Self-referred (media, brochure, presentation, peer educator) | 23 | 19 | 13 | 11 | ||||
| Word of mouth | 7 | 6 | 10 | 8 |
Participant descriptive data
Black participants differed significantly from whites in having fewer years of formal education, greater likelihood of not living with a spouse or partner, less likelihood of being employed, lower household income, greater rate of obesity, lower general health–related quality of life, lower scores on cognitive screening measures, and lower rate of current anxiety disorder (Table 2). Despite the greater burden of social and medical disadvantages, black participants did not differ from whites on preintervention measures of emotional distress (CES-D), depression (BDI), or anxiety (BSI) and proportion with a history of major depressive disorder. Participants were similar on SPSI scores (30) regardless of race, with the exception that higher positive problem orientation (a measure of active coping and resilience) was evident among black participants. More whites than blacks had a current anxiety disorder, despite lower social and medical burden among whites.
| Whites (N=154) | Blacks (N=90) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Total N | N | % | N | % | Test statistic | df | pa |
| Sociodemographic | ||||||||
| Age (M±SD years) | 244 | 65.5±11.7 | 65.8±9.7 | t=.24 | 242 | >.813 | ||
| Female | 244 | 104 | 68 | 70 | 78 | χ2=2.44 | 1 | >.118 |
| Education (M±SD years) | 244 | 15.2±2.8 | 13.3±2.2 | t=–5.56 | 242 | <.001 | ||
| Marital status | 244 | χ2=21.74 | 3 | <.001 | ||||
| Cohabiting or married | 88 | 57 | 25 | 28 | ||||
| Divorced or separated | 22 | 14 | 25 | 28 | ||||
| Never married | 12 | 8 | 16 | 18 | ||||
| Widowed | 32 | 21 | 24 | 27 | ||||
| Employed | 244 | 71 | 46 | 27 | 30 | χ2=5.48 | 4 | <.019 |
| Median household income (M±SD $) | 240 | 58,273±23,210 | 31,003±13,137 | t=–10.16 | 238 | <.001 | ||
| General health | ||||||||
| Cumulative Illness Rating Scale (M±SD score) | ||||||||
| Totalb | 242 | 7.4±3.8 | 8.4±4.0 | t=1.89 | 240 | >.059 | ||
| Countc | 243 | 4.8±2.2 | 5.2±2.2 | t=1.60 | 241 | >.109 | ||
| Heart and vasculard | 243 | 1.8±1.5 | 2.1±1.5 | t=1.34 | 241 | >.181 | ||
| Body mass index | ||||||||
| Total (M±SD) | 242 | 29.1±6.4 | 33.0±7.0 | t=4.34 | 240 | <.001 | ||
| ≥30 points (obesity indicator) | 242 | 60 | 39 | 57 | 63 | χ2=11.95 | 1 | <.001 |
| Rand Health Status Inventory (M±SD)e | ||||||||
| Physical health component | 204 | 43.9±11.3 | 38.8±12.0 | t=–3.00 | 202 | <.004 | ||
| Mental health component | 204 | 42.9±9.2 | 43.3±9.9 | t=.27 | 202 | >.788 | ||
| Cognition: Mini-Mental Status Examination (M±SD)f | 243 | 28.7±1.3 | 27.4±1.8 | t=–6.61 | 241 | <.001 | ||
| Mental health | ||||||||
| Hamilton Rating Scale for Depression (M±SD)g | 243 | 10.9±3.7 | 11.7±3.8 | t=1.64 | 241 | >.101 | ||
| Center for Epidemiologic Studies Depression Scale (M±SD)h | 243 | 21.1±8.0 | 21.3±7.9 | t=.19 | 241 | >.851 | ||
| Beck Depression Inventory (M±SD)i | 230 | 10.6±5.3 | 10.4±6.4 | t=–.22 | 228 | >.829 | ||
| Brief Symptom Inventory anxiety (M±SD)j | 232 | .5±.5 | .5±.5 | t=–.41 | 230 | >.684 | ||
| History of major depressive disorder | 244 | 54 | 35 | 28 | 31 | χ2=.24 | 1 | >.623 |
| History of anxiety disorder | 244 | 30 | 19 | 22 | 24 | χ2=.56 | 1 | >.452 |
| Current anxiety disorder | 244 | 45 | 29 | 14 | 16 | χ2=5.06 | 1 | <.025 |
| Social Problem Solving Inventory (M±SD)k | ||||||||
| Total | 212 | 100.3±13.4 | 103.7±13.4 | t=1.80 | 210 | >.073 | ||
| Positive problem orientation | 227 | 95.9±15.2 | 100.9±16.1 | t=2.38 | 225 | <.018 | ||
| Referral source | 244 | χ2=89.62 | 5 | <.001 | ||||
| Community outreachl | 4 | 3 | 42 | 47 | ||||
| Mental health specialist | 7 | 5 | 3 | 3 | ||||
| Primary care | 95 | 62 | 15 | 17 | ||||
| Research (research program or registry) | 19 | 12 | 6 | 7 | ||||
| Self-referred (media, brochure, presentation, or peer educator) | 22 | 14 | 14 | 16 | ||||
| Word of mouth | 7 | 5 | 10 | 11 |
Survival analysis of time to major depressive episode
Participants in PST-PC and dietary coaching did not differ significantly in time to major depressive episodes. Moreover, we observed similar incidence between black participants (N=8 of 90, 9%; 95% confidence interval [CI]=4%–17%) and white participants (N=13 of 154, 8%; CI=5%−14%) and similar incidence as well by recruitment site (mental health specialty, N=7 of 67, 10%; CI=4%−19%; community agencies, N=5 of 62, 8%; CI=3%−18%; and primary care practices, N=9 of 111, 8%; CI=4%−15%). Multivariate Cox proportional hazard models identified the two strongest predictors of incident episodes: greater cumulative medical comorbidity (total CIRS-G score, hazard ratio [HR]=1.18, CI=1.07–1.31) and greater severity of depressive symptoms (BDI score, HR=1.17, CI=1.09–1.25). Every 1-unit increase in total CIRS-G score increased hazard of an event by 18%, and a 1-unit increase on the BDI increased hazard by 17%.
The overall dropout rate was 24% (N=59 of 247) and did not differ by study arm or race. Thus similar percentages of blacks (N=62, 69%) and whites (N=102, 66%) completed the study, experienced the onset of major depressive episodes (blacks, N=8, 9%; whites, N=13, 8%), died during the trial (blacks, N=2, 2%; whites, N=3, 2%; no suicides), or dropped out because of loss of interest or respondent burden, participant relocation, or additional diagnosis (blacks, N=18, 20%; whites, N=36, 23%). We observed no differences in age, race, or baseline severity of depressive symptoms between participants who completed the trial and those who did not. However, a higher percentage of women compared with men completed the trial (women, N=144 of 176, 82%; men, N=44 of 71, 62%; χ2=9.90, df=1, p<.001). Comparable percentages of male and female participants were randomly assigned to each study arm.
Symptom burden
Participants in both arms experienced on average a 4-point drop in depressive symptoms (BDI), with improvements sustained over two years of follow-up. Black and white participants demonstrated similar patterns of responses to PST-PC and dietary coaching on measures of depressive symptoms (Figure 1).
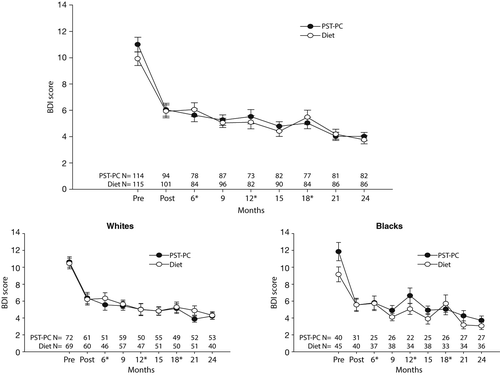
a Participants in both conditions demonstrated similar improvement in depressive symptoms, as measured with the Beck Depression Inventory (BDI). Asterisks indicate when a booster session was given. The top panel is for the overall group of 247, which included three Asian participants. There was a significant quadratic effect and linear time effects (F=75.91, dfs=1 and 1,356, p<.001, and F=159.57, dfs=1 and 1,356, p<.001, respectively) but no significant intervention or intervention × time effects. Examination of race as a moderator (bottom panels) showed baseline racial differences between interventions, and covarying the model for baseline score (Pre) resulted in a significant time effect (F=26.78, dfs=1 and 1,115, p<.001) for postintervention (Post) through two years, but no other significant effects. BDI scores can range from 0 to 63, with higher scores indicating more depressive symptoms. Vertical lines represent standard errors.
Both interventions were associated with similar and sustained improvements on total scores of the SPSI, a composite measure of active coping and negative problem orientation (avoidant coping, impulsivity, and rational problem solving) (30,32) before and after treatment. SPSI score was a significant covariate in our longitudinal model of BDI scores. An increase (improvement) in SPSI score was associated with a decrease in depressive symptoms (β=–.030±.003, t=–9.80, df=799, p<.001). Conversely, when examining SPSI scores as outcome using the same model of treatment, time, and treatment × time effects, and including BDI as a covariate, we found a bidirectional relationship such that depressive symptom scores were also a significant time-varying covariate of SPSI. A decrease in depression symptoms was associated with an increase (improvement) in SPSI score (β=–.654±.062, t=–10.56, df=799, p<.001).
Discussion
Both PST-PC and dietary coaching are potentially effective in protecting older black and white adults over a two-year period from the persistence of depressive symptoms (average of 4-point drop in BDI scores) and from the concomitant risk posed by persistent subsyndromal depressive symptoms for incident episodes of major depression. However, in the absence of a concurrent, usual-care control, this conclusion should be regarded as preliminary.
Compared with previously published rates of incident major depression among persons with subsyndromal symptoms receiving usual care (20%–25% over one year) (24–27), the apparent protective effect against major depression is noteworthy. We made a pragmatic decision not to control for care as usual (in effect a control for time’s passage, because treatment as usual is often no treatment at all) for several reasons, namely that many black participants lacked primary care services, our community advisory board warned that it could be a barrier to participation, and other studies of treatment as usual, including our own (33), have observed that subsyndromal depressive symptoms tend to persist under conditions of usual care—not improving and putting individuals at risk of major depressive disorder and deteriorating quality of life (33–37).
For example, in our study of suicide prevention for the primary care elderly population (34), older adults with subsyndromal symptoms under conditions of usual care had greater than a fivefold increased risk of conversion to major depressive disorder within one year, compared with those without such symptoms (33,35). Similarly, in a Dutch study of 170 older primary care patients aged 75 and older with subthreshold symptoms of depression and anxiety, a stepped-care intervention (which included problem-solving therapy) reduced the incidence of depressive and anxiety disorders by 50% over one year relative to care as usual (24% versus 12%) (36). A similar result was reported in the MANAS trial (25% versus 12.3%) in a mixed-aged sample of primary care patients in Goa, India (37). Our data showed an incidence of major depression among 21 of 247 persons (9%) over two years and among 13 of 247 persons (5%) over one year, similar to the Dutch and Indian observations. This observation contrasts with a published rate of 20%−25% having major depressive episodes over two years, based on the studies cited above, in which participants were recruited mainly in primary care settings.
A separate but related observation is that our sample was recruited from both primary care clinic and community sites (in order to oversample black participants). Because incidence may differ according to locus of recruitment, we stratified the randomization to intervention group by locus of recruitment. We did not, however, detect different incidence as a function of primary care, community-based, or mental health specialty recruitment. Moreover, our community-referred participants were mostly black, and black participants carried a higher burden of risk of major depression than white participants did (Table 2).
Contrary to our study hypothesis, we observed in both PST-PC and dietary coaching comparable and sustained reductions in depressive symptoms over time. Dietary coaching provided more than a control for face-to-face contact. It was by design an active control intervention in its own right, coaching participants to address the challenges of implementing healthy dietary practices, with homework assignments. Participants in this group reported both improvements in depressive symptoms and in problem-solving skills. Dietary coaching’s active-coping component, as well as social contact, may have protected against depression. Participants received assistance in tackling a problem associated with managing health issues. With the higher positive problem-solving orientation of black participants, dietary coaching fit culturally with life experience of having to problem solve and cope even in the absence of many resources. Dietary coaching also did not pose the issues of safety, stigma, and financial burden associated with long-term antidepressant pharmacotherapy.
In our longitudinal modeling of covariation between BDI scores and SPSI scores, we observed that increasing (improving) scores on the SPSI predicted lower depression scores and vice versa—that falling depression scores predicted increasing (improving) scores on the SPSI. This finding suggests the possibility of a bidirectional effect (that is, better problem solving leads to improvement in depression, and improvement in depression leads to better problem solving). However, this inference should be seen as preliminary, because SPSI scores and BDI scores are very likely to have shared variance based on their intrinsic definitions and constructs.
This study breaks new ground in indicated depression prevention research with an active control condition for the effects of attention, face-to-face time, and support, two years of follow-up, and an adequate number of black participants to explore effects of race on patterns of incident depression, trajectory of symptoms, and changes in health-related quality of life over two years. Most studies of depression prevention have not used an active comparator, have followed patients for shorter periods (generally one year), and have not had sufficient racial or ethnic diversity in their study groups to examine variability related to sociocultural characteristics. Both interventions in this trial were found to be acceptable to blacks and whites, with comparably low rates of nonadherence and dropout over two years.
Conclusions
Recruitment and retention of black participants were facilitated by partnerships with community champions for the study, the nonuse of antidepressant medication, low respondent burden, and conduct of the study in community settings (including participants’ homes), rather than in a medical setting. Lifestyle interventions such as dietary coaching may be more culturally appropriate and acceptable in racial-ethnic minority communities, regardless of income. These are important strategic considerations for reaching underserved individuals at risk of major depression, given that cultural beliefs and stigma contribute to low utilization of mental health care among older individuals from racial-ethnic minority groups. At a time of increasing shortages of mental health professionals dedicated to working with older adults (36), it is plausible that PST-PC and dietary coaching may be amenable to delivery by lay health counselors (peer supporters) with the same racial-ethnic background as the community they serve, increasing the scalability of these interventions in impoverished areas and utility to federally qualified community health centers or other primary care settings where nurses or health educators could fill this role. Thus the results of this study may be particularly pertinent to the integration of primary care and behavioral health services, especially for older patients whose increasing general medical comorbidity places them at high risk of developing major depressive disorders.
1 : Preventing depression: a global priority. JAMA 307:1033–1034, 2012Crossref, Medline, Google Scholar
2 : Early intervention to reduce the global health and economic burden of major depression in older adults; in Annual Review of Public Health. Edited by Fielding JEBrownson RCGreen LW. Palo Alto, Calif, Annual Reviews, 2012Crossref, Google Scholar
3 : Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. British Journal of Psychiatry 202:329–335, 2013Crossref, Medline, Google Scholar
4 : Depressive symptoms among African American and white older adults. Journals of Gerontology: Series B, Psychological Sciences and Social Sciences 60:P313–P319, 2005Crossref, Medline, Google Scholar
5 : A comparison of the frequencies of risk factors for depression in older black and white participants in a study of indicated prevention. International Psychogeriatrics 22:1240–1247, 2010Crossref, Medline, Google Scholar
6 Mental Health: Culture, Race and Ethnicity: A Supplement to Mental Health: A Report of the Surgeon General. Rockville, Md, Department of Health and Human Services, 2001Google Scholar
7 : Education, cognitive test scores, and black-white differences in dementia risk. Journal of the American Geriatrics Society 54:898–905, 2006Crossref, Medline, Google Scholar
8 : The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurology 10:819–828, 2011Crossref, Medline, Google Scholar
9 : Race, stress, and mental health; in Minority Health in America: Findings and Policy Implications From Commonwealth Fund Minority Health Survey. Edited by Hogue CJRHargraves MACollis KS. Baltimore, Johns Hopkins University Press, 2000Google Scholar
10 : A review of racial differences in geriatric depression: implications for care and clinical research. Journal of the National Medical Association 89:731–736, 1997Medline, Google Scholar
11 : Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. Journal of Affective Disorders 79:71–79, 2004Crossref, Medline, Google Scholar
12 : Prevention of depression with primary care patients: a randomized controlled trial. American Journal of Community Psychology 23:199–222, 1995Crossref, Medline, Google Scholar
13 : Opportunities for cost-effective prevention of late-life depression: an epidemiological approach. Archives of General Psychiatry 63:290–296, 2006Crossref, Medline, Google Scholar
14 : Evidence-based review of risk factors for geriatric depression and brief preventive interventions. Psychiatric Clinics of North America 28:785–803, 2005Crossref, Medline, Google Scholar
15 : Prevention of late-life depression in primary care: do we know where to begin? American Journal of Psychiatry 163:1611–1621, 2006Link, Google Scholar
16 : Race, gender, and partnership in the patient-physician relationship. JAMA 282:583–589, 1999Crossref, Medline, Google Scholar
17 : Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303:47–53, 2010Crossref, Medline, Google Scholar
18 : Can people with nonsevere major depression benefit from antidepressant medication? Journal of Clinical Psychiatry 73:518–525, 2012Crossref, Medline, Google Scholar
19 : Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 288:2836–2845, 2002Crossref, Medline, Google Scholar
20 : Problem solving therapy. Journal of Psychotherapy Integration 11:187–205, 2001Crossref, Google Scholar
21 : Preventing depression in age-related macular degeneration. Archives of General Psychiatry 64:886–892, 2007Crossref, Medline, Google Scholar
22 : Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. JAMA 299:2391–2400, 2008Crossref, Medline, Google Scholar
23 : Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. American Journal of Geriatric Psychiatry 11:46–52, 2003Crossref, Medline, Google Scholar
24 : The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 1:385–401, 1977Crossref, Google Scholar
25 : Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II), Version 2.0. New York, New York State Psychiatric Institute, 1994Google Scholar
26 : “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12:189–198, 1975Crossref, Medline, Google Scholar
27 : An inventory for measuring depression. Archives of General Psychiatry 4:561–571, 1961Crossref, Medline, Google Scholar
28 : SF-36 Health Survey. Manual and Interpretation Guide 2. Boston, Health Institute, New England Medical Center, Nimrod Press, 1997Google Scholar
29 : Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Research 41:237–248, 1992Crossref, Medline, Google Scholar
30 : Development and preliminary evaluation of the Social Problem-Solving Inventory. Psychological Assessment 2:156–163, 1990Crossref, Google Scholar
31 : The Brief Symptom Inventory: an introductory report. Psychological Medicine 13:595–605, 1983Crossref, Medline, Google Scholar
32 : Problem-Solving Therapy: A Positive Approach to Clinical Intervention. New York, Springer, 2007Google Scholar
33 : Outcomes of minor and subsyndromal depression among elderly patients in primary care settings. Annals of Internal Medicine 144:496–504, 2006Crossref, Medline, Google Scholar
34 : Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA 291:1081–1091, 2004Crossref, Medline, Google Scholar
35 : Risks for depression onset in primary care elderly patients: potential targets for preventive interventions. American Journal of Psychiatry 166:1375–1383, 2009Link, Google Scholar
36 : Stepped-care prevention of anxiety and depression in late life: a randomized controlled trial. Archives of General Psychiatry 66:297–304, 2009Crossref, Medline, Google Scholar
37 : Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet 376:2086–2095, 2010Crossref, Medline, Google Scholar



