Risks for Depression Onset in Primary Care Elderly Patients: Potential Targets for Preventive Interventions
Abstract
Objective: Prevention of late-life depression, a common, disabling condition with often poor outcomes in primary care, requires identification of seniors at highest risk of incident episodes. The authors examined a broad range of clinical, functional, and psychosocial predictors of incident depressive episodes in a well-characterized cohort of older primary care patients. Method: In this observational cohort study, patients age ≥65 years without current major depression, recruited from practices in general internal medicine, geriatrics, and family medicine, received annual follow-up assessments over a period of 1 to 4 years. Of 617 enrolled subjects, 405 completed the 1-year follow-up evaluation. The Structured Clinical Interview for DSM-IV (SCID) determined incident major depressive episodes. Each risk indicator’s predictive utility was examined by calculating the risk exposure rate, incident risk ratio, and population attributable fraction, leading to determination of the number needed to treat in order to prevent incident depression. Results: A combination of risks, including minor or subsyndromal depression, impaired functional status, and history of major or minor depression, identified a group in which fully effective treatment of five individuals would prevent one new case of incident depression. Conclusions: Indicators routinely assessed in primary care identified a group at very high risk for onset of major depressive episodes. Such markers may inform current clinical care by fostering the early detection and intervention critical to improving patient outcomes and may serve as the basis for future studies refining the recommendations for screening and determining the effectiveness of preventive interventions.
The individual, family, and societal costs of depressive conditions in later life are enormous (1) and will rise considerably in the years ahead as the population ages. Although the best available treatment strategies substantially improve the lives of many who are afflicted, too many suffer incomplete improvement or rapid relapses or recurrences (2 , 3) . Moreover, real-world effectiveness outcomes are even poorer (4) , reflecting a confluence of patient, provider, and sociocultural barriers to optimal implementation of therapies. The net result is that ≥10% of older adults seen in primary care settings suffer from current major or minor depressive disorders (5) , with considerable symptom and functional morbidity and risk for mortality from suicide and general medical causes (6 , 7) .
Growing attention is being paid to the potential for preventing incident depression. One approach is to intervene in patient subgroups at extremely high risk for depression (e.g., as a result of a particular medical illness or its therapy). However, to have a broader impact, it is important to identify patients who are at high risk for depression from among larger, more heterogeneous populations. Study of new-onset depression in community dwelling elderly in the Netherlands has demonstrated the potential cost-effectiveness of preventive interventions for depression based on favorable indices of the potential health gains in focusing on those with depressive symptoms, functional deficits, and a small social network size (8) . Additional risks were female gender, low education, and chronic medical disease burden. In another Netherlands cohort comprising noninstitutionalized elderly registered with a primary care provider, similar analyses led to the conclusion that preventive interventions should focus on those with subsyndromal depression (9) . Although groundbreaking, the former study did not include any measure for categorical diagnosis of depression, relying on a self-report depressive symptom scale, and the latter, although it did use a measure for categorical diagnosis of depression analogous to that of DSM-IV, did not assess a range of other putative clinical and psychosocial risks for depression onset. In addition, neither study recruited subjects directly from primary care, an important setting for future broadly targeted preventive interventions because most older persons with clinically significant depressive symptoms do not seek mental health specialty care but do see primary care providers (10) .
Accordingly, we sought to examine predictors of incident major depressive episodes in a cohort of older primary care patients well-characterized with regard to major, minor, and subsyndromal depression, along with a broad range of clinical, functional, and psychosocial variables that have conferred risk for depression outcome in prior work. By examining several epidemiological indicators of health effect in this cohort, we planned to identify the high-risk groups for which depression prevention would likely yield the greatest health benefit at the lowest cost.
Method
Subjects and Inclusion Criteria
Subjects were recruited in Monroe County, N.Y., from private practices and University-affiliated clinics in internal medicine, geriatrics, and family medicine. As described previously (11) , all patients aged ≥65 years presenting for care on selected recruitment days and capable of giving informed consent were eligible for study enrollment. After complete description of the study, written informed consent was obtained using procedures approved by the University of Rochester Research Subjects Review Board. For the present analyses, only subjects without a diagnosis of current or partially remitted major depression at study intake were included.
Procedures
Subjects underwent semistructured research interviews, conducted by trained raters, at study intake. Measures were completed based on data obtained from these interviews and from review of subjects’ primary care medical charts. Annual follow-up in-person research interviews were conducted up to 4 years after study intake, with data collected from these interviews supplemented by information obtained from interim 6-month contact via telephone and annually repeated medical chart review. Study recruitment was staggered between 2001 and 2005, with annual follow-up interviews through 2006, and thus the number of subjects eligible for the follow-up interviews decreased at each annual time point (i.e., the number of subjects eligible for the 1-year follow-up interview was higher than that for the 2-, 3-, and 4-year follow-up interviews). The study outcome was incident major depression, while the other variables assessed were examined as putative risk indicators.
Measures
Depression diagnoses were assigned by investigators and raters at consensus conferences and were based on the Structured Clinical Interview for DSM-IV (SCID) (12) , a well-validated tool for establishing psychiatric diagnoses in research settings. Based on SCID data, subjects without current or partially remitted major depression at study intake who developed major depression at any point during the follow-up period were defined as having experienced an episode of incident major depression. We examined incident depressive episodes rather than incident major depressive disorder, allowing us to assess the role of a history of depression as a predictor variable.
Demographic, psychopathological, functional, and psychosocial variables were chosen as the predictive models based on prior literature demonstrating their association with depression. These predictor variables were dichotomized to facilitate the identification of at-risk subgroups, using established cut-points, when possible, or based on a median split. Demographic characteristics were age, gender, years of education, and race. Each participant’s race, obtained for descriptive purposes, was self-reported based on response choices listed in accordance with reporting required by the National Institutes of Health.
Severity of depressive symptoms was assessed with the 24-item Hamilton Depression Rating Scale (HAM-D) (13) , an examiner-rated instrument that has been widely used and validated in older and medically ill populations. Participants with minor or subsyndromal depression were those with either current minor depressive disorder, defined using DSM-IV criteria, or current subsyndromal depression, defined as ≥2 depressive symptoms present at subthreshold or threshold levels according to SCID (with one or more being depressed mood or decreased interests/pleasure) and not meeting criteria for current major or minor depression, as described previously (14) . Although, in principle, the present definitions for minor and subsyndromal depression would include subjects with partially remitted major depression (as defined by DSM-IV and SCID criteria), these individuals were excluded from the study. Interrater reliability for depression assessment was high. Kappa coefficients for diagnoses of mood disorders ranged from 0.66 to 0.86 (p=0.0003, based on six raters and three subjects), and the intraclass correlation coefficient for HAM-D scores was 0.93 (based on six raters and five subjects). SCID also was used to record any history of major or minor depressive disorder. We chose to include patients with a history of major or minor depressive disorder (recognizing that the study sample consequently would include those with fully remitted major depression and fully or partially remitted minor depression) in order to make our findings of maximum relevance to the broad practices of primary care providers who may not be fully aware of a patient’s entire history of depressive episodes. If a history of depression proved to be an important risk indicator, it would reinforce the need for clinicians to elicit such history before making decisions about monitoring or treating their practice population.
Coding of “other psychiatric disorder” was made for those with a SCID diagnosis of any current alcohol-related or anxiety disorder. Overall cognitive function was assessed with the Mini-Mental State Examination (15) . Patients with dementia were included, except for severely impaired subjects who were unable to give informed consent. The diagnosis of dementia was assigned by an experienced geriatric psychiatrist (Dr. Lyness) based on subject interview, record review, and performance on the Mini-Mental State Examination as well as on the Trail Making Test, Part A and Part B (16) , and the initiation/perseveration subscales of the Mattis Dementia Rating Scale (17) .
Medical illness burden was assessed using the Cumulative Illness Rating Scale (18) . Measures of overall functional status were the Instrumental Activities of Daily Living and Physical Self-Maintenance Scale (19) . The Instrumental Activities of Daily Living assesses higher-order activities of daily living, such as shopping, driving, and preparing meals, whereas the Physical Self-Maintenance Scale assesses more basic self-care tasks, such as grooming, feeding, and bathing. For both of these scales, higher scores indicate poorer functioning. Global Assessment of Functioning assessed functional disability as a result of psychiatric causes. Similarly, the Karnofsky Performance Status Scale (20) measured functional disability as a result of physical causes. For both of these scales, lower scores indicate poorer functioning. Self-rated health was assessed by a validated single question, which was the first item of the general health subscale of the Medical Outcomes Study Short Form—36 (21) .
Social support was measured using three subscales of the Duke Social Support Index (22) , assessing perceived social support, social interaction, and instrumental support. A modified version of the Louisville Older Persons Event Scale (23) assessed the presence and severity of any stressful life event in the preceding 6 months. Perceived family criticism was assessed using the self-report Family Emotional Involvement and Criticism Scale (24) . Antidepressant treatment was measured by the Composite Antidepressant Scale (25) .
Statistical Analysis
Analyses were carried out via STATA—9 (StataCorp LP, College Station, Tex.). Descriptive statistics were means and standard deviations for continuous variables and counts and proportions for categorical variables. Generalized linear models with a logistic link were used to model the relationship between the predictor variables (history of depression and demographic, psychopathological, functional, and psychosocial variables) and depression outcome (an episode of incident major depression). Based on the logistic models, the following epidemiological indicators of health effect were used to assess the importance of each predictor variable as a risk indicator: exposure rate, incident risk ratio, population attributable fraction, and number needed to treat (26 , 27) .
The exposure rate was the percentage of the study sample exposed to the risk indicator (i.e., the prevalence of the risk indicator in the study population). The incident risk ratio compared the rate of incident major depression episodes among those participants exposed to the risk indicator with the rate of incident major depression episodes among unexposed participants. The incident risk ratio was computed by regressing the depression outcome onto the risk indicator via a Poisson log-linear model. Values >1 represented an increased risk level for the exposed group. (For variables with a significant incident risk ratio <1, the variable’s complement with a significant incident risk ratio >1 are reported, since the objective was to reduce increased episodes of incident major depression by eliminating the effect of the putative risk marker.) The population attributable fraction in the present study indicates the amount of percentage points the current episodes of incident major depression would be reduced if the adverse effect of the risk indictor were completely eliminated, thus representing an upper bound for the potential health gain in the group exposed to the risk indicator. The population attributable fraction was obtained for each risk indicator that conferred a higher risk for depression (using the AFLOGIT procedure in STATA based on a Poisson log-linear model), with adjustments for other competing risks. The number needed to treat was the number of participants with a given risk indicator who would need to receive a fully effective preventive intervention in order to prevent one new case of an episode of incident major depression. The number needed to treat was calculated as the inverse of the absolute risk difference based on the regression results from modeling the incident risk ratio as a function of a risk indicator, using the DPROBIT procedure in STATA.
In addition to regressing the incident major depression outcome onto each risk indicator individually, we performed multivariate regression including the risk indicators in a model to identify high-risk individuals for whom prevention was likely to have the greatest health benefit against the lowest implementation burden. Since there were many predictors chosen on the basis of prior evidence, backward selection procedure was used to trim the model. This approach was used to ensure that no important predictor was omitted, controlling for any potential selection bias. Starting from the full model, the predictor variable with the highest p value was removed. This process was continued until all p values were <0.2 in the final model. Using the resulting model, we also assessed the potential health benefits in targeting prevention among those exposed to joint risk indicators. We started with the risk indicator associated with the highest incident risk ratio and attributable fraction but the lowest number needed to treat. Next, assessing those subjects with the first-identified risk indicator, we determined the second risk indicator associated with the highest incident risk ratio and attributable fraction and lowest number needed to treat. We repeated this process to determine the optimal combination of risk indicators (i.e., the combination of risk indicators most predictive of an episode of incident major depression).
Results
Among participants recruited, 617 met the study inclusion criteria. An additional 133 subjects who were enrolled in the parent study were excluded from the present study because they had current depression at the time of study intake. As reported previously (28) , the cohort consisting of both those patients who did and did not meet study criteria (i.e., those who completed intake assessments) represented 50.1% of the primary care patients invited to participate in the study enrollment. Based on available data, patients who elected to enroll did not differ from those who did not enroll with regard to age, gender, or scores on the 15-item Geriatric Depression Scale (29) . Among those patients who elected to enroll, 405 completed the 1-year follow-up interview, 338 completed the 2-year follow-up interview, 259 completed the 3-year follow-up interview, and 54 completed the 4-year follow-up interview. Those subjects who did complete at least one annual follow-up assessment did not differ from those subjects who did not complete at least one annual follow-up assessment with regard to demographic variables and HAM-D and Cumulative Illness Rating Scale scores but were more likely to be Caucasian (30) ( Table 1 ).
Predicting Episodes of Incident Major Depression
Thirty-three participants (5.3%) developed an episode of incident major depression over the course of the follow-up assessment period. The exposure rates for each predictor variable in the entire sample and in the group of subjects who developed an episode of incident major depression are shown in Table 2 . As expected, exposure rates were frequently elevated in the group of subjects who experienced an episode of incident major depression. Results from the multiple regression model and subsequent calculations, yielding incident risk ratios, attributable fractions, and the number needed to treat (among those subjects with incident risk ratios >1) to prevent an episode of incident major depression, are also reported. (Of note, some of the estimated incident risk ratios in the model were <1 even though the individual exposure rate was >1 as a result of adjustments for the effects of the other predictors in the model.)
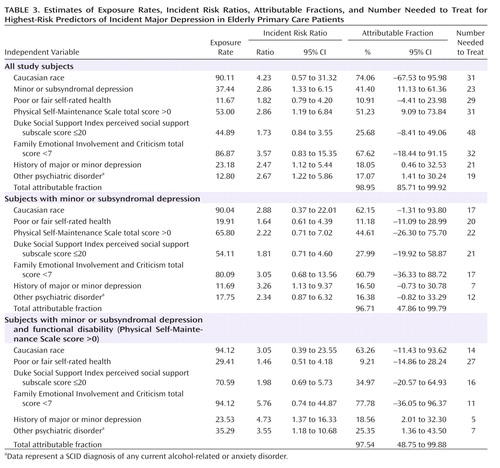
These results were then used in the backward selection procedure to define the high-risk subgroups that might be best targeted for preventive intervention ( Table 3 ). The optimal combination of risk variables (those variables with the lowest exposure rate and number needed to treat and highest incident risk ratios and attributable fractions) from the entire panel of predictors was entered into a multiple regression, yielding estimates of exposure rates, incident risk ratios, attributable fractions, and the number needed to treat. Since the first regression revealed that minor and subsyndromal depression were the predictor variables with the highest attributable fractions and lowest number needed to treat, the next regression examined the other predictor variables among those subjects with minor or subsyndromal depression. The same regression procedure was repeated for subjects who had minor or subsyndromal depression as well as functional disability (Physical Self-Maintenance Scale score >0), since subjects with a Physical Self-Maintenance Scale score >0 had high attributable fractions and a low number needed to treat (and because of the ease of clinically screening subjects for physical self-maintenance disability relative to, for example, a high perceived family criticism). The final model revealed that a number needed to treat as low as 5 was obtained for subjects with the combined predictor variables of minor or subsyndromal depression, functional disability, and a history of major or minor depression.
Discussion
Although prior studies have demonstrated the association of many putative risk indicators with depression outcome, our study is the first, to our knowledge, to identify optimal risk indicators for incident major depressive episodes in a cohort of older primary care patients well-characterized as to current minor or subsyndromal depression. Moreover, we used a wider range of operationalized measures of psychopathology, medical conditions, and psychosocial factors than prior investigators (8 , 9 , 31) . We found that a combination of risks, including minor or subsyndromal depression, impaired functional status, and history of major or minor depression, did identify a group at very high risk for incident depression. The number needed to treat was 5, a figure low enough to make an effective preventive intervention potentially cost effective. It is, perhaps, not surprising that our results point to the importance of “indicated” prevention strategies (32) (i.e., targeting persons already somewhat symptomatic and with a history of depression, but these data demonstrate the usefulness of focusing on these specific factors together with functional impairment compared with other putative risks).
Also of note is the presence of functional disability, rather than medical illness burden, in the final risk indicator models. Medical burden long has been associated with depression in later life, and specific medical illnesses may be of particular etiological or prognostic significance (e.g., the role of small vessel cerebrovascular disease [33] ) in selected subgroups. However, growing evidence suggests that the effects of medical illnesses may more broadly, perhaps mediated through functional disability, play the greatest roles in the heterogeneous populations found in primary care settings (34) . Although none of the other psychosocial measures appeared in the final risk models, perceived family criticism had predictive characteristics comparable with that of functional disability ( Table 3 ). We opted to make the next-level risk model conditional on functional disability rather than family criticism because functional disability is commonly addressed in primary care and other clinical settings, while family criticism is rarely assessed formally. Future work should continue to examine the potential role of family and other psychosocial factors in predicting incident depression, in part to identify mechanisms of depression onset that might inform innovative approaches to preventive psychosocial interventions.
Several study limitations must be acknowledged. Our findings may not generalize to other populations, including groups with greater representation of non-Caucasian seniors, although it is not clear how our findings might differ in other settings. Further, there was no evidence of systematic bias in our recruitment methods, and the inclusion of race as a covariate supports the validity of the results based on any “missing at random” data. Our study cohort may reflect biases introduced during the recruitment and retention phases, although the inclusion rates compare favorably with other studies using such labor-intensive assessment methodologies. The absolute number of incident depression cases was relatively modest, limiting power to detect predictors. However, this makes the present findings more striking. Our analyses assumed that risk indicators exerted the same effects on outcome across the entire cohort (i.e., we did not examine particular subgroups). Last, the numbers needed to treat were based on the assumption of a fully effective preventive intervention. Although useful to guide the optimal targeting of interventions, our findings underestimate the number needed to treat values for any interventions likely to be available for the foreseeable future. It also must be noted that elimination of a risk marker may not necessarily improve outcomes (i.e., the marker may be a proxy for the actual pathogenic factors, hence our use of the terms risk indicator or marker as distinguished from true risk factors). However, our identification of risks that suggest an “indicated” prevention strategy (e.g., current minor or subsyndromal depression) increases the likelihood that reduction of a risk will improve outcomes.
Preventive interventions research might focus fruitfully on at-risk subject groups identified by the predictors identified in the present study. However, it remains unclear what interventions are most likely to be efficacious. Additionally, to achieve effectiveness in actual practice, a preventive intervention must be acceptable to persons who largely do not identify themselves as being depressed. Antidepressant medications might be considered, but psychosocial treatments may be somewhat more effective than medications for treating milder forms of depression, such as subsyndromal depression (35) . One approach might be to adapt evidence-based psychotherapy for depression (e.g., interpersonal psychotherapy or problem solving therapy) to the context of persons suffering from subsyndromal depression rather than full-fledged major depression. Alternatively, or in tandem with depression-specific therapy, an intervention that focuses on coping with functional disability or that involves increasing physical activity or exercise (36) may be more acceptable to these patients and may hold promise of preventive efficacy.
Pending the development and empirical validation of preventive interventions, the present study may inform current clinical practice by fostering early detection and intervention critical to improving patient outcomes for depression. The risk markers identified are clinical domains routinely assessed, at least informally, in primary care evaluations, although quantification and chart documentation of these risks in practice remain quite variable. As practices move toward adopting chronic disease management approaches based on electronic medical records, it will be straightforward to identify and “flag” patients at risk for incident depression. Such patients could be followed closely over time using cost-effective methods, such as telephone-based depression symptom scales (37) . Treatment outcomes for patients who go on to develop diagnosable depressive disorders may be maximized by the use of evidence-based depression care management paradigms, administered within primary care practices (38 , 39) or via telephone (40) . Future research must refine the recommendations for such screening and demonstrate its cost effectiveness as well as determine the effectiveness of preventive interventions. Further, the methodologies of the present analyses should be applied to more narrowly defined populations for whom depression poses particularly grave risks, such as those with specific chronic medical conditions (e.g., heart failure, stroke), with similar goals of identifying those at greatest risk for developing depression and elucidating potential targets for intervention.
1. Charney DS, Reynolds CF 3rd, Lewis L, Lebowitz BD, Sunderland T, Alexopoulos GS, Blazer DG, Katz IR, Meyers BS, Arean PA, Borson S, Brown C, Bruce ML, Callahan CM, Charlson ME, Conwell Y, Cuthbert BN, Devanand DP, Gibson MJ, Gottlieb GL, Krishnan KR, Laden SK, Lyketsos CG, Mulsant BH, Niederehe G, Olin JT, Oslin DW, Pearson J, Persky T, Pollock BG, Raetzman S, Reynolds M, Salzman C, Schulz R, Schwenk TL, Scolnick E, Unutzer J, Weissman MM, Young RC: Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry 2003; 60:664–672Google Scholar
2. Unutzer J: Clinical practice: late-life depression. N Engl J Med 2007; 357:2269–2276Google Scholar
3. Lyness JM: Treatment of depressive conditions in later life: real-world light for dark (or dim) tunnels. JAMA 2004; 291:1626–1628Google Scholar
4. Cui X, Lyness JM, Tang W, Tu X, Conwell Y: Outcomes and predictors of late-life depression trajectories in older primary care patients. Am J Geriatr Psychiatry 2008; 16:406–415Google Scholar
5. Weyerer S, Eifflaender-Gorfer S, Kohler L, Jessen F, Maier W, Fuchs A, Pentzek M, Kaduszkiewicz H, Bachmann C, Angermeyer MC, Luppa M, Wiese B, Mosch E, Bickel H: Prevalence and risk factors for depression in non-demented primary care attenders aged 75 years and older. J Affect Disord 2008; 111:153–163Google Scholar
6. Gallo JJ, Bogner HR, Morales KH, Post EP, Ten Have T, Bruce ML: Depression, cardiovascular disease, diabetes, and two-year mortality among older, primary-care patients. Am J Geriatr Psychiatry 2005; 13:748–755Google Scholar
7. Lyness JM, Heo M, Datto CJ, Ten Have TR, Katz IR, Drayer R, Reynolds CF 3rd, Alexopoulos GS, Bruce ML: Outcomes of minor and subsyndromal depression among elderly patients in primary care settings. Ann Intern Med 2006; 144:496–504Google Scholar
8. Smit F, Ederveen A, Cuijpers P, Deeg D, Beekman A: Opportunities for cost-effective prevention of late-life depression: an epidemiological approach. Arch Gen Psychiatry 2006; 63:290–296Google Scholar
9. Schoevers RA, Smit F, Deeg DJ, Cuijpers P, Marcel Dekker J, van Tilburg W, Beekman AT: Prevention of late-life depression in primary care: Do we know where to begin? Am J Psychiatry 2006; 163:1611–1621Google Scholar
10. Gallo JJ, Coyne JC: The challenge of depression in late life: bridging science and service in primary care. JAMA 2000; 284:1570–1572Google Scholar
11. Sanders ML, Lyness JM, Eberly S, King DA, Caine ED: Cerebrovascular risk factors, executive dysfunction, and depression in older primary care patients. Am J Geriatr Psychiatry 2006; 14:145–152Google Scholar
12. Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for Axis I DSM-IV Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research Department, 1994Google Scholar
13. Williams JBW: A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry 1988; 45:742–747Google Scholar
14. Lyness JM, Kim J, Tang W, Tu X, Conwell Y, King DA, Caine ED: The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry 2007; 15:214–223Google Scholar
15. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
16. Reitan RM, Davison LA: Clinical Neuropsychology: Current Status and Applications. New York, John Wiley and Sons, 1974Google Scholar
17. Mattis S: Dementia Rating Scale. Odessa, Fla, Psychological Assessment Resources, 1989Google Scholar
18. Linn BS, Linn MW, Gurel L: Cumulative Illness Rating Scale. J Am Geriatr Soc 1968; 16:622–626Google Scholar
19. Lawton MP, Brody EM: Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9:179–186Google Scholar
20. Karnofsky DA, Burchenal JH: The clinical evaluation of chemotherapeutic agents in cancer, in Evaluation of Chemotherapeutic Agents. Edited by MacLeod CM. New York, Columbia, 1949, pp 191–205Google Scholar
21. Ware JE Jr, Sherbourne CD: The MOS 36-Item Short-Form Health Survey (SF-36), I: conceptual framework and item selection. Med Care 1992; 30:473–483Google Scholar
22. George LK, Blazer DG, Hughes DC, Fowler N: Social support and the outcome of major depression. Br J Psychiatry 1989; 154:478–485Google Scholar
23. Murrell SA, Norris FH, Hutchins GM: Distribution and desirability of life events in older adults: population and policy implications. J Commun Psychol 1984; 12:301–311Google Scholar
24. Franks P, Campbell TL, Shields CG: Social relationships and health. Fam Process 1992; 36:25–41Google Scholar
25. Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Feder M, Einhorn A, Rosendahl E: Recovery in geriatric depression. Arch Gen Psychiatry 1996; 53:305–312Google Scholar
26. Clayton DG, Hills M: Statistical Models in Epidemiology. Oxford, Oxford University Press, 1993Google Scholar
27. Rothman KJ, Greenland S: Modern Epidemiology, 2nd ed. Philadelphia, Lippincott-Raven, 1998Google Scholar
28. Cui X, Lyness JM, Tu X, King DA, Caine ED: Does depression precede or follow executive dysfunction? Outcomes in older primary care patients. Am J Psychiatry 2007; 164:1221–1228Google Scholar
29. Sheikh JI, Yesavage JA: Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986; 5:165–173Google Scholar
30. Lyness JM, Chapman BP, McGriff J, Drayer R, Duberstein PR: One-year outcomes of minor and subsyndromal depression in older primary care patients. Int Psychogeriatr 2009; 21:60–68Google Scholar
31. Smits F, Smits N, Schoevers R, Deeg D, Beekman A, Cuijpers P: An epidemiological approach to depression prevention in old age. Am J Geriatr Psychiatry 2008; 16:444–453Google Scholar
32. Institute of Medicine Committee on Prevention of Mental Disorders: Reducing Risks for Mental Disorders. Washington, DC, National Academies Press, 1994Google Scholar
33. Taylor WD, Steffens DC, Krishnan KR: Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry 2006; 60:1299–1303Google Scholar
34. Lyness JM: Depression and comorbidity: objects in the mirror are more complex than they appear. Am J Geriatr Psychiatry 2008; 16:181–185Google Scholar
35. Pinquart M, Duberstein PR, Lyness JM: Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry 2006; 163:1493–1501Google Scholar
36. Timonen L, Rantanen T, Timonen TE, Sulkava R: Effects of a group-based exercise program on the mood state of frail older women after discharge from hospital. Int J Geriatr Psychiatry 2002; 17:1106–1111Google Scholar
37. Pinto-Meza A, Serrano-Blanco A, Penarrubia MT, Blanco E, Haro JM: Assessing depression in primary care with the PHQ-9: Can it be carried out over the telephone? J Gen Intern Med 2005; 20:738–742Google Scholar
38. Bruce ML, Ten Have TR, Reynolds CF 3rd, Katz II, Schulberg HC, Mulsant BH, Brown GK, McAvay GJ, Pearson JL, Alexopoulos GS: Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA 2004; 291:1081–1091Google Scholar
39. Unutzer J, Katon W, Callahan CM, Williams JW Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C: Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002; 288:2836–2845Google Scholar
40. Dietrich AJ, Oxman TE, Williams JW Jr, Schulberg HC, Bruce ML, Lee PW, Barry S, Raue PJ, Lefever JJ, Heo M, Rost K, Kroenke K, Gerrity M, Nutting PA: Re-engineering systems for the treatment of depression in primary care: cluster randomised controlled trial. BMJ 2004; 329:602Google Scholar