Olanzapine Long-Acting Injection: A 24-Week, Randomized, Double-Blind Trial of Maintenance Treatment in Patients With Schizophrenia
Abstract
Objective
The purpose of the present study was to evaluate the efficacy and tolerability of olanzapine long-acting injection for maintenance treatment of schizophrenia.
Method
Outpatients with schizophrenia who had maintained stability on an oral regimen of olanzapine (10, 15, or 20 mg/day) for 4 to 8 weeks were randomly assigned to 24 weeks of double-blind treatment with "low" (150 mg every 2 weeks; N=140), "medium" (405 mg every 4 weeks; N=318), or "high" (300 mg every 2 weeks; N=141) doses of olanzapine long-acting injection; a very low reference dose of olanzapine long-acting injection (45 mg every 4 weeks; N=144); or their stabilized dose of oral olanzapine (N=322). Rates of and time to psychotic exacerbation were estimated using Kaplan-Meier methodology.
Results
At 24 weeks, the majority of oral olanzapine-treated patients (93%), as well as most olanzapine long-acting injection-treated patients receiving high (95%), medium (90%), low (84%), and very low doses (69%), remained exacerbation free, with the therapeutic 4-week regimen (medium dose) and pooled 2-week regimen (low and high doses) demonstrating efficacy similar to that of oral olanzapine as well as to each other. The three standard long-acting doses were superior to the very low reference dose based on time to exacerbation. Incidence of weight gain ≥7% of baseline was 21% for oral olanzapine compared with 21%, 15%, 16%, and 8% for the high, medium, low, and very low olanzapine long-acting treatment groups, respectively. No clinically significant differences were observed between the long-acting injection and oral olanzapine groups in general safety parameters. Few injection-site reactions occurred (3%). Two patients experienced sedation and delirium consistent with olanzapine overdose following possible accidental intravascular injection.
Conclusions
Olanzapine long-acting injection was efficacious in maintenance treatment of schizophrenia for up to 24 weeks, with a safety profile similar to oral olanzapine except for injection-related adverse events.
For patients with schizophrenia, nonadherence to medication is a major risk factor for relapse and rehospitalization (1, 2). Depot antipsychotics can mitigate nonadherence, possibly reducing the risk of relapse (3–6). Olanzapine long-acting injection is a depot formulation composed of a salt of olanzapine and pamoic acid suspended in an aqueous vehicle for intramuscular injection. Doses of olanzapine long-acting are described by the amount of olanzapine in each injection and provide an approximate daily dose that can be estimated by dividing the number of milligrams by the number of days in the prescribed injection interval (for example, 405 mg divided by 28 days). Pharmacokinetics enable therapeutic levels to be available upon the first injection, with concentrations increasing with subsequent injections until a steady state is achieved at approximately 3 months (7).
To assess the suitability of this new formulation as a maintenance treatment for schizophrenia, we conducted a relapse prevention study to determine whether stable patients who switched directly to olanzapine long-acting injection from oral olanzapine could maintain clinical stability with rates of relapse no greater than those for patients who remained on the oral formulation. In addition to comparing the long-acting injection formulation with the oral formulation, we also compared the efficacy of 2-week and 4-week injection intervals as well as the relative efficacy of the following four different injection doses: 1) a "low" dose of 150 mg given every 2 weeks (300 mg every month, comparable to approximately 10 mg/day of oral olanzapine); 2) a "medium" dose of 405 mg given every 4 weeks (405 mg every month, comparable to approximately 15 mg/day of oral olanzapine); 3) a "high" dose of 300 mg given every 2 weeks (600 mg every month, comparable to approximately 20 mg/day of oral olanzapine); and 4) a very low reference dose (45 mg given every 4 weeks [or 45 mg every month], comparable to approximately 1.5 mg/day of oral olanzapine) that was included in lieu of placebo. Primary efficacy measures were time to and rate of psychotic exacerbation after up to 24 weeks of double-blind treatment. Safety and tolerability were also assessed.
Method
This double-blind, randomized, multicenter study was conducted from July 2004 to September 2006 at 112 sites in 26 countries. The study protocol was approved by institutional review boards at each site. After receiving a complete description of the study, all patients and/or their authorized legal representatives provided written informed consent before participation.
Participants were 18 to 75 years of age, with a DSM-IV or DSM-IV-TR diagnosis of schizophrenia. Patients were clinically stable, defined as having outpatient status for at least 4 weeks before the first study visit, with a Brief Psychiatric Rating Scale (BPRS [8]) positive symptom subscale score ≤4 (range: 1–7) on each of the following items: conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content. Patients treated previously with a depot antipsychotic were required to have received their last injection at least 2 weeks or one injection interval before entry (4 weeks for injectable risperidone). Exclusion criteria included significant suicidal or homicidal risk; pregnancy or breastfeeding; acute, serious, or unstable medical conditions; or substance dependence (except nicotine or caffeine) within the past month.
Procedures
Conversion/stabilization phase
After a 2- to 9-day screening period, patients entered a 4- to 8-week period in which they were switched from their previous antipsychotic to open-label oral olanzapine monotherapy (10, 15, or 20 mg/day, per investigator's discretion) and required to demonstrate maintenance of clinical stability. Patients had to meet the following stabilization criteria for 4 consecutive weeks to be eligible for randomization: 1) no dose change of oral olanzapine; 2) a Clinical Global Impressions-Improvement of Illness (CGI-I [8]) score ≤4; and 3) a BPRS positive symptom score ≤4 on conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content.
Double-blind maintenance phase
Patients who met the stabilization criteria were randomly assigned, in a 1:1:2:1:2 ratio, to very low, low, medium, or high fixed doses of long-acting olanzapine or to remain on their stabilized dose of oral olanzapine for up to 24 weeks. Fewer patients were randomly assigned to the very low reference dose arm in order to minimize the number of individuals exposed to a potentially subtherapeutic dose. Moreover, fewer patients were randomly assigned to the high-dose and low-dose regimen arms to ensure that these two 2-week regimen groups, when combined, would have a number of patients similar to that of the 4-week medium-dose arm, which enabled us to compare all patients on a 2-week regimen with all patients on a 4-week regimen (excluding the very low-dose reference regimen).
Patients and study personnel were blind to treatment assignment. All patients received four oral tablets (drug or placebo) each day and an injection (drug or placebo) every 2 weeks. The staff administering injections were not part of the study team and provided no clinical ratings.
No oral antipsychotic supplementation was allowed. Concomitant medications with primarily CNS activity were prohibited, except for benzodiazepines and sedative-hypnotics as sleep aids (≤2 mg/day lorazepam equivalents) and anticholinergic medications (≤6 mg/day biperiden equivalents) not used as prophylaxis for potential treatment-emergent extrapyramidal symptoms.
Measures
The primary efficacy measures were rates of and time to psychotic exacerbation. Exacerbation was defined as 1) an increase of any BPRS positive symptom item to a score >4, with an absolute increase ≥2 for the specific item since randomization; 2) an increase of any BPRS positive symptom item to a score >4, with an absolute increase ≥4 on the positive symptom subscale since randomization; or 3) hospitalization as the result of worsening of positive psychotic symptoms.
Symptom severity was assessed using the 30-item Positive and Negative Syndrome Scale (PANSS [9]), the PANSS-derived BPRS, and the Clinical Global Impressions-Severity of Illness (CGI-S [8]). Efficacy assessments were performed weekly for the first 12 weeks and every 2 weeks thereafter.
Tolerability and safety were assessed using reports of adverse events, standard laboratory tests (including fasting glucose and lipid levels), ECG, and physical examination. Treatment-emergent categorical changes in lipid parameters were defined using National Cholesterol Education Program Adult Treatment Panel III (10) criteria, and changes in glucose were defined using American Diabetes Association (11) criteria. Extrapyramidal symptoms were assessed using the Barnes Akathisia (12), Simpson-Angus (13), and Abnormal Involuntary Movement (8) scales. Safety data were collected at each visit.
Measurements of olanzapine plasma concentrations following oral or long-acting olanzapine administration were obtained from 346 patients during months 1, 3, and 6 of double-blind treatment, with samples generally collected prior to the next injection.
Statistical Methods
All analyses were performed on an intent-to-treat basis using SAS, version 8.2 (SAS Institute, Inc., Cary, N.C.). Continuous data were evaluated using analysis-of-variance (ANOVA) models, with terms for treatment and either investigator or geographic region. Categorical data were evaluated using Fisher's exact or chi-square tests. Time to psychotic exacerbation and 24-week cumulative exacerbation rates were evaluated using Kaplan-Meier methodology and the log-rank test. Hazard ratios and corresponding 95% confidence intervals (CIs) based on Kaplan-Meier estimates were generated using a Cox proportional hazards model. All hypotheses were tested with an alpha of 0.05 (two-sided).
A noninferiority test was used to compare Kaplan-Meier exacerbation rates for the oral olanzapine group with those of the 2-week combined group (high and low dose) of long-acting olanzapine as well as with those of the 4-week (medium dose) group. The combined 2-week group was similarly compared with the 4-week (medium dose) group to assess the effect of dose interval. Two groups could be declared noninferior to each other (i.e., sufficiently similar) if the upper limit of the 95% CI for the difference between the groups was <20%. The 20% criterion was established based on one-half of the expected difference in exacerbation rates between olanzapine and placebo using the lower limit of the 80% CI of the difference found in a previous olanzapine study (14).
Additional efficacy analyses were conducted for mean change from baseline to last-observation-carried-forward endpoint on the PANSS (total, positive, and negative subscales), BPRS total, and CGI-S scores using an ANOVA model with terms for treatment and investigator.
Safety analyses for the double-blind period used time of randomization as the baseline rather than first exposure to oral olanzapine, unless otherwise specified.
Results
Conversion/Stabilization Phase
At study entry, 60% of patients were receiving olanzapine, 49% were receiving an antipsychotic other than olanzapine, and 8% were not receiving any antipsychotic. During the 4- to 8-week conversion/stabilization phase, 45% of patients maintained clinical stability on 10 mg/day of oral olanzapine, 22% on 15 mg/day, and 33% on 20 mg/day. The mean baseline PANSS total score was 62.6, which decreased significantly to 57.3 (p<0.001) over this period. (Patient flow through the study is shown in the data supplement accompanying the online version of this article.)
A total of 35% of patients reported ≥1 treatment-emergent adverse event, with the most frequent being increased weight (6%), somnolence (5%), insomnia (4%), anxiety (3%), increased appetite (3%), and headache (2%). Nineteen patients (2%) experienced ≥1 serious adverse event, with 13 events related to worsening of schizophrenia. Mean weight gain was 1.1 kg (SD=2.4). Patients newly switching to olanzapine gained more weight on average during the conversion/stabilization period than those who were already taking olanzapine before study entry (1.4 kg [SD=2.6] versus 0.8 kg [SD=2.3], p=0.002), with patients already taking olanzapine having a higher mean study entry weight relative to those not yet taking olanzapine (77.2 kg [170 lb] versus 74.8 kg [165 lb]). Small but statistically significant mean increases in aspartate aminotransferase (+1.63 IU/liter [SD=12.20]), alanine aminotransferase (+2.58 IU/liter [SD=19.95]), alkaline phosphatase (1.35 IU/liter [SD=13.70]), and P-R interval (1.08 msec [SD=11.97]) were observed but not considered clinically significant.
Double-Blind Maintenance Phase
No statistically significant differences were observed for baseline physical characteristics and illness history (Table 1). Small but statistically significant differences were observed for some measures of baseline illness severity (Table 2), but these were not considered clinically significant, and controlling for these differences did not alter the findings of the study.
 |
 |
Of the 322 patients randomly assigned to oral olanzapine, 44% received 10 mg/day, 25% received 15 mg/day, and 31% received 20 mg/day, resulting in a mean dose of 14.3 mg/day. No significant differences were observed for concomitant benzodiazepine or anticholinergic use.
Time to all cause discontinuation is shown in the online data supplement.
Efficacy
Figure 1 illustrates the Kaplan-Meier survival curves showing the proportion of patients remaining free of psychotic exacerbation over the 24-week double-blind treatment period. For patients receiving oral olanzapine, 93% remained free of exacerbation at 24 weeks. For patients receiving long-acting injection, 95% of the high-dose group remained exacerbation free, as well as 90% of the medium-dose group, 84% of the low-dose group, and 69% of the very low reference dose group. Time to exacerbation was longer for all three standard long-acting injection groups relative to the very low reference dose group (all log-rank test p values <0.01), with no statistically significant differences among the therapeutically dosed groups except for a shorter time to exacerbation for the low-dose injection group relative to the high-dose (p=0.005) and the oral olanzapine (p=0.004) groups.

aHigh dose=300 mg every 2 weeks; medium dose=405 mg every 4 weeks; low dose=150 mg every 2 weeks; very low dose [reference used in lieu of placebo]=45 mg every 4 weeks. Stabilized oral dose=10, 15, or 20 mg/day.
Comparison of 2-week, 4-week, and oral regimens
Comparison of exacerbation rates using Kaplan-Meier 24-week percentages indicated no significant difference between the pooled 2-week (high and low doses combined) and therapeutic 4-week (medium dose) regimens (difference=0 [95% CI=–5 to 5]; hazard ratio=1.0 [95% CI=0.6 to 1.8], p=0.89). There was also no significant difference between the pooled 2-week regimen and the oral formulation (difference=3 [95% CI=–2 to 8]; hazard ratio=1.5 [95% CI=0.8 to 2.7], p=0.17) or between the therapeutic 4-week regimen and the oral formulation (difference=3 [95% CI=–2 to 7]; hazard ratio=1.4 [95% CI=0.8 to 2.6], p=0.21). All comparisons met criteria for noninferiority.
Long-acting olanzapine dose comparisons
All three standard long-acting doses were superior to the very low reference dose. Patients treated with the reference dose had a higher risk of psychotic exacerbation relative to patients in the low dose (hazard ratio=2.1 [95% CI=1.2–3.7], p=0.007), medium dose (hazard ratio=3.5 [95% CI=2.2–5.8], p<0.001), and high dose (hazard ratio=7.4 [95% CI=3.1–17.5], p<0.001) groups. Patients treated with the low dose had a higher risk of exacerbation relative to those treated with the high dose (hazard ratio=3.5 [95% CI=1.4–8.7], p=0.008). There were no other significant differences in risk of exacerbation between the long-acting dose groups.
Symptom scores
Analysis of mean baseline-to-endpoint change in the PANSS total score showed superiority of the three standard long-acting injection groups relative to the very low reference dose group (all p values ≤0.001). The standard long-acting injection groups and the oral treatment group all maintained mean PANSS total scores in the mid-50s, indicating sufficient symptom control throughout the study. However, the very low reference dose group showed statistically significant worsening over time, with mean PANSS total scores increasing into the mid-60s (see the online data supplement). Similar patterns were generally observed for scores on the PANSS subscales, the BPRS, and the CGI-S.
Pharmacokinetics
The online data supplement illustrates the decrease in olanzapine concentrations after the first injection followed by the increase and attainment of steady-state conditions by the 12th week, after which concentrations were sustained through the last 3 months of the study. The low, medium, and high doses of long-acting olanzapine produced 10th- to 90th-percentile steady-state plasma olanzapine concentrations (5–41 ng/ml, 8–51 ng/ml, 7–73 ng/ml, respectively) similar to those for 10, 15, and 20 mg/day of oral olanzapine (13–48 ng/ml, 21–63 ng/ml, 21–85 ng/ml, respectively).
Safety
Adverse events
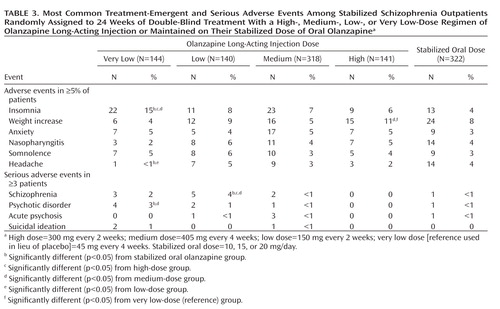
Two patients receiving long-acting olanzapine experienced events attributed to possible inadvertent intravascular injection. Both these patients experienced symptoms of dizziness and malaise within 10 to 20 minutes of injection, followed by gradual worsening of symptoms of excessive sedation and/or delirium consistent with olanzapine overdose. No changes in vital signs were noted. The two patients were hospitalized for management of symptoms and fully recovered approximately 60 hours after injection. One patient chose to discontinue the study after the event, and the other continued and had no further such events following subsequent injections. No deaths were reported during the study. The most common treatment-emergent adverse events were insomnia, weight gain, anxiety, and somnolence (Table 3). Incidence of local reactions at the injection site (e.g., pain, swelling) was low (N=30, 3%). Among the 57 patients (5%) who experienced ≥1 event that was reported as serious, the most common were schizophrenia (N=11), psychotic disorder (N=8), acute psychosis (N=5), and suicidal ideation (N=3).
 |
Weight and vital signs
No statistically significant group differences were observed for mean changes in blood pressure or pulse. Figure 2 illustrates mean weight change from baseline of the conversion/stabilization phase to the end of the maintenance phase. Weight gain was greater for the high-dose group than for the low-dose group (p=0.04) (Figure 2 [also see the online data supplement]). Incidence of weight gain ≥7% from the time of randomization in either the combined 2-week group (19%, p=0.42) or the medium 4-week dose group (15%, p=0.05) did not differ significantly from the oral olanzapine group (21%). The incidence of such weight gain was higher in the high-dose (21%, p=0.004) and low-dose (16%, p=0.05) groups relative to the very low reference dose group (8%).

aHigh dose=300 mg every 2 weeks; medium dose=405 mg every 4 weeks; low dose=150 mg every 2 weeks; very low dose [reference used in lieu of placebo]=45 mg every 4 weeks. Stabilized oral dose=10, 15, or 20 mg/day. Time from study entry to randomization could be between 4 and 8 weeks. One kilogram=2.2 pounds. Data represent the following: all doses (p<0.001) versus very low reference dose; high dose versus low dose (p=0.04); and high dose versus medium dose (p=0.06).
Laboratory values
Randomization-to-endpoint mean changes in laboratory analytes were small, with few statistically significant differences across groups. The online data supplement presents values for glucose, lipids, and prolactin. The very low reference dose group showed a greater mean decrease in total (-0.37 mmol/liter [SD=0.80]) and low-density lipoprotein cholesterol (–0.32 mmol/liter [SD=0.68]) relative to the other groups (all p values <0.05). The high-dose group showed a mean increase in prolactin (3.57 mg/liter [SD=33.77]), whereas the other groups showed a decrease (all p values <0.05). Categorical analyses of shifts in fasting glucose and lipid levels at any time revealed no significant group differences, except that the high-dose group had a higher incidence of shifting from normal to high triglyceride levels (25%) relative to the medium dose (10%, p=0.02), low dose (6%, p=0.008), and very low reference dose (3%, p=0.001) groups but not the oral treatment group (14%, p=0.09).
ECG
No clinically significant differences were observed in mean baseline-to-endpoint changes in ECG intervals or heart rate between the long-acting injection groups and the oral olanzapine group. Incidence of increases >60 msec or abnormally high QTc intervals was low (<1%) and did not differ significantly across treatment groups.
Extrapyramidal symptoms
Extrapyramidal symptoms were minimal at the time of randomization and throughout the double-blind period, with symptom scores generally indicating very small mean decreases in all groups (see the online data supplement).
Discussion
The 24-week efficacy and safety of olanzapine long-acting injection at low (150 mg every 2 weeks), medium (405 mg every 4 weeks), or high (300 mg every 2 weeks) doses were found to be similar to those at doses of 10, 15, or 20 mg/day of oral olanzapine. The most notable exception to this finding was the occurrence of two cases of a postinjection syndrome characterized by excessive sedation and/or delirium consistent with olanzapine overdose.
Efficacy
Patients stabilized on oral olanzapine showed maintenance of stability, with relatively few patients relapsing over the 24-week period following direct switching to the low, medium, or high olanzapine long-acting injection doses. The overall efficacy of these doses appeared comparable to that of oral olanzapine, with no apparent differences between the use of 2- and 4-week dosing intervals. However, there was evidence of a dose effect across the low, medium, and high doses, since the high dose was superior to the low dose. Efficacy findings for the oral and reference dose arms were as anticipated based on findings from previous oral olanzapine relapse-prevention studies (14, 15), suggesting that the present study was an adequate test of our hypotheses.
Pharmacokinetics
Pharmacokinetic data indicated that therapeutic olanzapine concentrations were present immediately following the first injection and did not reach a steady state until approximately 12 weeks. Olanzapine concentrations were in the range of those resulting from within-label oral olanzapine doses (16) at all times during the study. Oral antipsychotic supplementation was not allowed at any time.
Safety
With the exception of injection-related events, including postinjection delirium/sedation syndrome, safety and tolerability were otherwise comparable between the oral and long-acting formulations. The relatively small and infrequent changes in safety parameters in all treatment groups during the double-blind maintenance period likely reflected the fact that all patients had previously received oral olanzapine during the conversion/stabilization period, and 60% had already been receiving oral olanzapine before study entry. A few differences were observed between the therapeutic long-acting doses. The high-dose group experienced more weight gain, an increase in prolactin, and a greater incidence of change from normal to high triglyceride levels. Mean body weight increased by 1.06 kg (2.3 lb) during open-label treatment with oral olanzapine and increased further during double-blind treatment with therapeutically dosed long-acting or oral olanzapine for a total mean increase of approximately 2 to 3 kg (SD=4) (approximately 4 to 6 lb [SD=9]). Thus, strategies to mitigate weight gain and other changes in metabolic parameters should be considered, and physicians should conduct a careful benefit/risk assessment when prescribing either the oral or long-acting formulations of olanzapine.
Postinjection Delirium/Sedation Syndrome
The most notable difference between the two formulations was the risk of excessive sedation and/or delirium consistent with olanzapine overdose after possible inadvertent intravascular injection. Across all olanzapine long-acting clinical trials, such cases have occurred after 0.07% of injections, with all patients recovering within 72 hours (7). Injection or partial injection into a blood vessel can result in a more rapid breakdown of the olanzapine pamoate salt, leading to excessive olanzapine exposure. Symptoms following possible inadvertent intravascular injection have also been noted with other intramuscularly injected products (such as penicillin procaine G [17]), with clinical presentation dependent on the properties of the drug. Therefore, although this type of event is relatively rare, patients receiving olanzapine long-acting injection should be observed for signs or symptoms of such a reaction for 3 hours after each injection, and clinicians and patients will need to consider this when deciding whether to use this medication.
Study Limitations
It should be noted that our study design was somewhat biased in favor of oral olanzapine. All patients in the double-blind phase were, in effect, preselected for response (and therefore presumably adherence to oral olanzapine), as demonstrated by their maintenance of stability during the conversion/stabilization phase, thus potentially limiting the adherence-related advantage of the long-acting formulation over oral olanzapine in the present study. In addition, patients randomly assigned to oral olanzapine continued to receive their previously stabilized dose, whereas those randomly assigned to long-acting olanzapine could be assigned to a suboptimal dose that might increase the risk of exacerbation or to a higher than optimal dose that might decrease tolerability. Despite these biases, the therapeutic doses of olanzapine long-acting injection achieved similar efficacy to that of oral olanzapine.
Along these same lines, a criticism of this study could be the choice of population. The patients were already clinically stable and demonstrated continued stability (and thus adherence) on oral olanzapine in the supportive context of a clinical trial. This patient population may differ somewhat from those for whom a depot antipsychotic is typically prescribed. Nevertheless, more than one-third of patients in this study had ≥2 exacerbations in the previous 24 months, which can be an indicator of possible nonadherence.
Conclusions
Our findings support the efficacy of olanzapine long-acting injection at low (150 mg every 2 weeks), medium (405 mg every 4 weeks), and high (300 mg every 2 weeks) doses for up to 24 weeks of maintenance treatment for schizophrenia, with results similar to those of oral olanzapine. The safety profile of these long-acting doses did not differ significantly from that of oral olanzapine, except for injection-related events. Although injection-related reactions were rare, two patients experienced symptoms of sedation and delirium consistent with olanzapine overdose following possible inadvertent intravascular injection. Based on these results showing similarity to the effectiveness of oral olanzapine, olanzapine long-acting injection may represent an important option for the maintenance treatment of schizophrenia. However, given the specific safety profile and requisite precautions, clinicians will need to weigh the risks and benefits when deciding whether this treatment is appropriate for their individual patients.
1 : Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56:241–247 Crossref, Medline, Google Scholar
2 : Antipsychotic adherence over time among patients receiving treatment for schizophrenia: a retrospective review. J Clin Psychiatry 2006; 67:1542–1550 Crossref, Medline, Google Scholar
3 : Fluphenazine and social therapy in the aftercare of schizophrenic patients: relapse analyses of a two-year controlled study of fluphenazine decanoate and fluphenazine hydrochloride. Arch Gen Psychiatry 1979; 36:1283–1294 Crossref, Medline, Google Scholar
4 : Review of treatments that can ameliorate nonadherence in patients with schizophrenia. J Clin Psychiatry 2006; 67(suppl 5):9–14 Crossref, Medline, Google Scholar
5 : Relapse and rehospitalization: comparing oral and depot antipsychotics. J Clin Psychiatry 2003; 64(suppl 16):14–17 Crossref, Medline, Google Scholar
6 : A cross-sectional study of patients' perspectives on adherence to antipsychotic medication: depot versus oral. J Clin Psychiatry 2008; 69:1548–1556 Crossref, Medline, Google Scholar
7 Zypadhera European Public Assessment Report: Annex I Summary of Product Characteristics. http://www.emea.europa.eu/humandocs/Humans/EPAR/zypadhera/zypadhera.htm. (Accessed March 25, 2009) Google Scholar
8 : Early Clinical Drug Evaluation Unit Assessment Manual for Psychopharmacology–Revised (Publication ADM 76–338). Bethesda, Md, United States Department of Health, Education, and Welfare, 1976 Google Scholar
9 : Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY, Multi-Health Systems, 1986 Google Scholar
10 National Cholesterol Education Program: Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation 2002; 106:3143–3421 Crossref, Medline, Google Scholar
11 American Diabetes Association: Standards of medical care in diabetes. Diabetes Care 2004; 27(suppl 1):S15–S35 Crossref, Medline, Google Scholar
12 : A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154:672–676 Crossref, Medline, Google Scholar
13 : A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 1970; 212:S11–S19 Crossref, Google Scholar
14 : A double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol 2003; 23:582–594 Crossref, Medline, Google Scholar
15 : Standard olanzapine versus placebo and ineffective-dose olanzapine in the maintenance treatment of schizophrenia. Psychiatr Serv 1997; 48:1571–1577 Link, Google Scholar
16 : Population pharmacokinetics and plasma concentrations of olanzapine, in Olanzapine (Zyprexa): A Novel Antipsychotic. Edited by Tran PBymaster FTye NHerrera JBreier ATollefson G. Philadelphia, Lippincott, Williams and Wilkins, 2000 Google Scholar
17 : Systemic toxic reactions to procaine penicillin G. Sex Transm Dis 1978; 5:4–9 Crossref, Medline, Google Scholar



