Preference-Weighted Health Status of PTSD Among Veterans: An Outcome for Cost-Effectiveness Analysis Using Clinical Data
Posttraumatic stress disorder (PTSD) is a chronic, disabling, and increasingly common mental health problem, with prevalence rates ranging from 6% to 36% in primary care ( 1 , 2 , 3 , 4 , 5 ) and up to 19% among soldiers returning from Iraq ( 6 , 7 , 8 ). Persons diagnosed as having PTSD use health services at a higher rate than persons with diagnoses of most other mental disorders ( 9 ), and persons with PTSD experience significant decrements in health-related quality of life ( 10 ).
Effective pharmacological ( 11 , 12 , 13 ) and psychotherapeutic ( 14 , 15 ) treatments for PTSD are readily available; however, treatment effectiveness is typically assessed by disease-centered interviews, such as the Clinician-Administered PTSD Scale (CAPS) ( 16 ). Measures of health status that do not focus on specific diseases—that is, disease-generic metrics—such as the Medical Outcomes Survey Short-Form–36 (SF-36) ( 17 ), and that assess self-reported functioning are commonly used outcome measures, but they fail to capture how people value health status. The domains of these generic health status measures are combined into an index score without consideration of what domains people value most.
Policy makers can allocate resources based in part on how interventions maximize preferred outcomes ( 18 ). However, measures of health status that are not based on preferences and measures focused on clinical symptoms are predominantly clinician tools, not health policy tools per se ( 19 , 20 , 21 , 22 ). In contrast, preference-weighted health status measures provide quantitative and theoretically sound estimates of the desirability (or lack thereof) of various health states. Preference-based data inform health care policy makers because the preferences serve as a proxy for disease burden. In addition, preference-based data can be used for consumer advocacy because these data reflect how patients rate the burden of various disease states. Put simply, when diseases are more burdensome, more services are needed to treat them ( 18 ).
Preference-based measurements (a broad category of metrics that encompasses health utilities) exist for hundreds of specific diseases, but relatively few have been developed for mental disorders ( 23 ). Researchers from the Global Burden of Disease Study estimated the disability weight associated with a PTSD health state ( 24 ). (Disability weight is another of the many types of preference-based metrics.) Other researchers have more directly assessed the disability weight associated with PTSD ( 25 ). The PTSD disability weight from both methods was determined by expert consensus. However, the disability weight for PTSD was not determined by those who matter most: the community that is potentially affected by health policy decisions. To date, no community- or patient-valued preference-based measures exist for PTSD.
In practical terms, preference-based measurements are integral in calculating the measure of effectiveness in cost-effectiveness analysis—the quality-adjusted life year (QALY). A U.S. Public Health Service expert panel recommended that cost-effectiveness analyses use QALYs as the primary endpoint ( 18 ). A year in perfect health is worth one QALY, and death is worth zero. Hoch and Smith ( 26 ) stated that health care decisions without cost considerations assume that there are not any alternative uses for the resources consumed, and they recommended that outcomes and costs be considered at the population level. Drummond and colleagues ( 27 ) have described in more detail how preference-based metrics are used in cost-effectiveness analysis.
There are community-based preference weights for health states defined by the SF-36 ( 17 , 28 ), a self-report measure of health-related quality of life. Brazier and colleagues ( 28 ) condensed the SF-36 and isolated 249 health states. They asked a community sample in Great Britain to weight the health states between anchors of perfect health (equal to 1) and death (equal to 0) by using standard econometric instruments designed to elicit preference weights. The investigators intended for these weights to be applied to any SF-36 data set, so that other researchers would not have to re-elicit them. A health utility is a probability (p) as to whether a person is indifferent when it comes to making two choices. In the first choice, the person prefers living in his or her current state of health with certainty. In the second choice, the person prefers an intervention that would cure the state of health with a probability of success equal to 1-p and certain death equal to p.
For example, Pyne and colleagues ( 29 ) administered the SF-36 to a sample of patients receiving treatment for substance use disorders. They weighted patient responses on this generic health status measure with the preference weights published by Brazier and colleagues ( 28 ) and estimated a preference-weighted health status score (PWHS score) for each participant. (We use the term "PWHS score" in this article, even though we recognize that other terms exist, such as health utility or preference score.) Specifically, in a six-month follow-up of patients treated for substance use disorders, average PWHS scores increased by .073 after six months of abstinence from a reference case of .448 associated with heavy drinking, all other variables being equal in their model. Pyne and colleagues demonstrated how PWHS scores could be used to calculate QALYs (by weighting the PWHS score by the time a person has spent in the condition) and therefore could be used as a single measure of effectiveness in cost-effectiveness analyses of treatments for substance use disorders.
Magruder and colleagues ( 3 ) administered the SF-36, as well as other survey and structured-interview measures, to a primary care sample of 888 veterans from four Department of Veterans Affairs (VA) hospitals. We applied Brazier and colleagues' ( 28 ) health utility weights to Magruder and colleagues' ( 3 ) data set and explored the relationship among PTSD, other anxiety disorders, and mood and substance use disorders on preference-weighted health status. Magruder and colleagues reported that 87% of the 12% of veterans who met DSM-IV criteria for PTSD also met DSM-IV criteria for a co-occurring mental disorder. Thus health status associated with PTSD was commingled with these disorders. No research has systematically compared PWHS scores associated with PTSD, other anxiety disorders, mood disorders, and substance use disorders.
In the study reported here, we determined the PWHS scores of veterans with PTSD and co-occurring mood, substance use, and anxiety disorders. We created a regression model that allows health policy researchers to estimate PWHS scores (that is, health utilities—a necessary component of QALYs) from existing PTSD data sets. Users of the model can calculate patient-level PWHS scores by adding or subtracting regression coefficients, based on the patient's demographic characteristics, psychiatric diagnoses, and PTSD symptom severity. When taken together with other epidemiological data (for example, prevalence) reported by Magruder and colleagues ( 3 ), researchers can begin to estimate the disease burden of PTSD within the VA primary care system. The regression coefficients can be a helpful tool for health care policy makers and decision makers.
Methods
Participants
We performed a secondary analysis of the data from 808 veterans collected by Magruder and colleagues ( 3 ). Magruder and colleagues interviewed veterans in four VA primary care clinics: in Tuscaloosa and Birmingham, Alabama, and in Charleston and Columbia, South Carolina. Veterans were screened for PTSD with the PTSD Checklist (PCL) ( 30 , 31 ) and the Trauma Assessment for Adults (TAA) ( 32 ). If criterion A of the PTSD diagnosis could possibly be met on the basis of the TAA, veterans were assessed with CAPS ( 16 ). PTSD "caseness" was determined by using the 1–2 rule, such that a PTSD symptom was clinically significant if the frequency was at least once per month and if it was at least moderately distressing. Other axis I disorders were assessed with the Mini-International Neuropsychiatric Interview ( 33 ). Health-related quality of life was assessed with the SF-36 ( 34 ). ICD-9 diagnoses for two years (with the interview date as the midpoint) were added to the data set from the electronic medical record.
Table 1 presents demographic characteristics of the sample. The majority of veterans were male (79%), married or cohabitating (66%), and Caucasian (62%). The mean±SD age was 60±12 years.
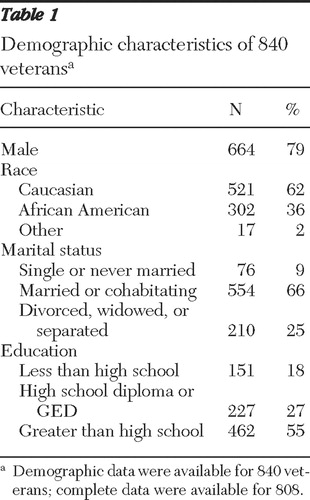 |
Of the 1,198 veterans invited to participate in the original study ( 3 ), partial data were available for 1,076 veterans. Demographic data were available for 840 veterans. Complete data were available for 808 veterans. Thus complete data from 268 (25%) of the 1,076 veterans were missing for the regression model (described below). Using missing data or no missing data as a dichotomous dependent variable, we constructed two logistic regression models to assess whether the missing cases were random through all categories. The first logistic regression model included all covariates considered in the ordinary least-squares model, and none of the regression coefficients were statistically significant. The second logistic regression, which reduced all mental illness variables and retained only three control variables (sex, work, and disability), identified sex as the only covariate with statistically significant effects on whether data were missing. In light of the aforementioned logistic regressions to examine whether data were missing in a systematic way, sex served as a control variable in our analyses. Therefore, we do not believe that it seriously affected our analysis.
Procedure
We calculated PWHS scores by transforming SF-36 scores using the methods outlined by Brazier and colleagues ( 28 ). We first condensed the SF-36 into the seven-item SF-6D. We then applied the transformation formula ( 28 ) to calculate PWHS scores. Preference-based metrics derived by using other methods can range from a low of 0 (equal to death) or lower (for states worse than death) to 1 (perfect health). When SF-6D data are used, the lowest PHWS score becomes .3. We created several ordinary least-squares regression models using diagnostic and demographic variables as predictors and PWHS scores as a dependent variable. The SAS, version 9.1.3, PROC REG procedure was used for the analyses.
Before determining that ordinary least-squares regression was our statistical method of choice, we performed a series of preliminary data analyses to examine the data structure and fit for ordinary least-squares regression. We constructed a regression model with the natural logarithm of the SF-6D data (that is, PWHS scores) treated as the dependent variable, assuming a skewed distribution. The skewness and kurtosis were -.212 and -.676 for the original PWHS scores, and -.630 and -.079 for the log-transformed variable. Both sets did not deviate significantly from normality.
We emphasize that our regression models and the selection of covariates were based on a specific theoretical framework and our research interest. We found no meaningful multicollinearity among covariates, nor did we find meaningful outliers. Although PTSD diagnosis and the PTSD severity score were moderately correlated, as are other variables ( Table 2 ), each provides additive information in our analysis because a dichotomous variable cannot completely capture severity variations and, likewise, a continuous variable cannot reflect a well-defined threshold to ascertain a disease.
 |
Given that PTSD diagnosis was highly correlated with the interaction between PCL score and PTSD diagnosis (.94), and to avoid part of the collinearity that might arise from forming an interaction term between two related PTSD variables, predictors in this analysis were rescaled to be centered about the sample means when both PTSD diagnostic and severity variables were included in the same model. We then multiplied the centered predictors to form the interaction.
We performed the collinearity diagnostics procedure on centered and noncentered models. No conditional index value, an indicator of problems with multicollinearity, was close to 30 (the highest value in the centered model was 8.15). For each root number, no more than one proportion was greater than .6, the standard for determining multicollinearity. As a result of centering, the correlation between PTSD diagnosis and the interaction term declined to .74. We therefore have evidence that multicollinearity posed no meaningful threat to our analysis. We present results of the centered model below. [Tables presenting the noncentered models are available as an online supplement to this article at ps.psychiatryonline.org .]
We explored which variables from the study by Magruder and colleagues ( 3 ) meaningfully and theoretically contributed to the model such that the resulting coefficients could be used to calculate PWHS scores in other data sets. To demonstrate the relative impact of PTSD on PWHS scores, we entered a medical condition comparator as an additional variable in the regression model. We chose an ICD-9 diagnosis of chronic obstructive pulmonary disorder (COPD) because it is common (20% of veterans in our sample) and chronic. Preference-based metrics (that is, health utilities) for COPD exist ( 35 ). We examined differences in population estimates of PWHS scores among veterans with and without COPD and PTSD.
This project was fully approved by the authors' affiliated institutional review boards. Informed consent was obtained as part of the original study ( 3 ). It was not needed for the study reported here because it is a secondary data analysis and met exempt review criteria.
Results
Regression coefficients and other statistics
We applied the ordinary least-squares regression model to estimate the effects of PTSD diagnosis and PCL score (our measure of PTSD severity), with patient demographic characteristics and mood, anxiety, and substance use diagnoses as control variables. We also included COPD as a comparator in a separate regression model.
Table 3 presents results of the four models. Model 1 shows the influences of PTSD diagnosis and PCL score (symptom severity) on a veteran's PWHS score while controlling for potential confounding effects of co-occurring mental disorders and demographic characteristics. The regression coefficients of PCL score and the interaction term between the two factors were statistically significant at .05, and all covariates that were included accounted for 39% of the variability in PWHS scores. Because the coefficient of the interaction term between PTSD diagnosis and PCL score was statistically significant and because the coefficient of the PCL score alone was statistically significant, the PTSD diagnosis coefficient alone was considered significant.
 |
In models 2 and 3 we removed PTSD diagnosis and PCL score, respectively. By doing so, the adjusted and unadjusted R 2 decreased and the model became less efficient. R 2 change was significant at .01, and results are presented in Table 3 . Models 2 and 3 are not centered because centering does not affect models without interaction terms.
We also entered three other interaction terms: PTSD diagnosis by mood disorder, PTSD diagnosis by substance use disorder, and PTSD diagnosis by other anxiety disorder, but none of these predictors were clinically or statistically significant. They were therefore not included in the regression models. Results of our preliminary data analysis to determine the applicability of ordinary least-squares regression to this data set are not presented but are available upon request. This preliminary analysis showed the absence of significant effects of outliers.
In model 1 the estimate of the intercept, .637, was the grand mean (the estimate of the population mean) of the PWHS score for the population the sample represented, since all covariates were centered. The regression coefficient of PTSD diagnosis (-.029) suggested that among those with this diagnosis, the PWHS score was on average .029 lower than among those without a PTSD diagnosis, other variables being equal. The regression coefficient of the PCL score was also negative (-.004), whereas that of the interaction term between PTSD and the PCL score was positive (.002). The combination of these two terms indicates a crossover in preference-weighted health status between those with a diagnosis of PTSD and those without this diagnosis, with increases in PCL scores. Specifically, among those without a diagnosis of PTSD, the PWHS score was expected to drop by .004 when the PCL score increased by 1 point. Among those with a PTSD diagnosis, this reduction rate was .002 (-.004+.002). The effects of the three co-occurring mental disorders (mood, anxiety, and substance use disorders) were all negative, as expected, but only the effect of a co-occurring anxiety disorder was statistically significant.
Model 4 presents results of the ordinary least-squares regression with centered covariates, adding COPD diagnosis into the estimation process. Although the addition of COPD into model 4 significantly increased R 2 by .008 from model 1, the impact of this increase on PWHS score was trivial. PHWS score was minimally affected by the COPD coefficient (-.027), when compared with the affect of adding PTSD diagnosis and the interaction of PTSD and PCL score to model 1 over model 2. The impact on PWHS score was small because the metric unit of COPD is 0 or 1, with a range of 1. Thus, when the metric unit of COPD is multiplied by the coefficient, PWHS score can only change by -.027. In contrast, the impact of PTSD diagnosis on PWHS score is much larger. Although the metric unit of PTSD is the same as COPD, a 0 or 1, model 1 (compared with model 2) also adds the effect of the interaction between PCL score and PTSD diagnosis. Thus, even though the R 2 change from model 2 to model 1 (.009) is significant and nearly identical in the amount of the R 2 change from model 1 to model 4 (.008), the impact on PWHS score of the PTSD variables added is greater.
PTSD diagnosis and severity and preference-weighted health status
Table 3 suggests that the impact of PCL score on PWHS score was a function of PTSD diagnosis. Given the values of the regression coefficients in model 1, individuals with PTSD and lower PCL scores had lower PWHS scores than those who did not have a PTSD diagnosis; then with the increase in PCL score, the offsetting interactive effect (.002 × PTSD diagnosis × PCL score) would have increasingly important effects on determining an individual's PWHS score. The PWHS score among those with a diagnosis of PTSD crosses with those without a PTSD diagnosis at a PCL score of 43 (model 1, .029/.002+28.23, where 28.23 is the grand mean of the PCL score and .029/.002 is the value between the mean PCL score and the crossing point), when other variables remain constant. Beyond that PCL score, PWHS scores for those without a PTSD diagnosis were expected to be lower than for those with a PTSD diagnosis.
On the basis of results of model 1, we plotted two regression lines of PWHS scores against PCL scores for individuals with and without a diagnosis of PTSD ( Figure 1 ). When PCL scores were low, those with a PTSD diagnosis had considerably lower PWHS scores and the two regression lines were noticeably separated; then the separation narrowed with increases in PCL scores until the two lines converged. Finally, the two regression lines separated again in the opposite direction. PTSD diagnosis behaved as a dominant factor in determining an individual's health score among veterans with low PCL scores. However, regardless of PTSD diagnosis, it was the severity of PTSD symptoms that more accurately predicted a veteran's health status among those with high PCL scores.

PTSD, COPD, and preference-weighted health status
A useful approach to present results of an ordinary least-squares model is to predict scores of the outcome variable by using selected values of covariates. From model 4, which included both PTSD and COPD diagnoses, we calculated two sets of population estimates of PWHS scores for a given PTSD or COPD status. The first set of population estimates used values of the covariates that were fixed at status-specific covariate means for each of the PTSD or COPD states (presence or absence). The second set of population estimates used mean covariate values for the entire sample for all PTSD- or COPD-related PWHS scores. The first set of estimates yielded population estimates—that is, the PWHS score estimates for the population of veterans represented by our sampled data. The second set of estimates permitted a population comparison of PWHS scores for individuals with or without a PTSD or COPD diagnosis while simultaneously adjusting for the potentially confounding effects of other covariates (adjusted population estimates). Thus the effects of PTSD and COPD diagnoses on PWHS scores were effectively analyzed.
Table 4 presents two sets of predicted PWHS scores by PTSD and COPD status, with or without adjustment for co-occurring mental disorders and demographic characteristics (control variables). The population estimates showed much stronger variation in the effect of PTSD than that of COPD on the PWHS scores. The expected PWHS scores for veterans without PTSD and those with PTSD were, respectively, .652 and .535, with a considerable difference of .117, when the PCL score was fixed as the variable's sample mean. After control for confounding variables, the sizeable difference between veterans with and without PTSD was considerably reduced (.640 compared with .610). In contrast, veterans with a COPD diagnosis were expected to have a PWHS score of .615, about .03 lower than among those not diagnosed with COPD (.647). This difference remained almost the same when co-occurring mental disorders and demographic characteristics were controlled for (.642 –.615=.027).
 |
Discussion
Significance of PWHS for PTSD
This is the first study to estimate the PWHS scores (health utilities) associated with PTSD. Although researchers have assessed health-related quality of life of persons with PTSD ( 3 , 10 ), the results have not included a preference-weighted measure of health status. Preference-based measures are useful for policy makers ( 21 ) because they reflect the relative desirability of disease states and are not simply a description of functioning; thus they are recommended for resource allocation decisions in cost-effectiveness analysis ( 18 ). PWHS scores derived from model 1 can be compared with PWHS scores associated with other diseases and disorders. As we demonstrated in this study, veterans with PTSD had lower PWHS scores than those with COPD, a common chronic and distressing medical condition. The difference that we estimated may be conservative, because PTSD "caseness" in our study was based on a CAPS research diagnosis of PTSD rather than on a clinical diagnosis of PTSD. We would expect that individuals with a clinical diagnosis would be more disabled and thus have worse preference-weighted health status.
Inclusion of PTSD diagnosis and severity
The inclusion of two similar PTSD measures, CAPS (for diagnosis) and PCL (for severity), is necessary and can be explained from two perspectives. From the clinical perspective, the CAPS is a structured, clinician-administered interview and is considered the gold standard of PTSD assessment. In contrast, the PCL is a brief self-report PTSD screen. A correlation between the two measures is to be expected, but the measures are subject to different sources of variance. The CAPS is diagnostic, whereas the PCL adds a measure of severity. Used alone, the PCL is limiting.
The PCL does not fully assess criterion A for PTSD. The stem question on the PCL asks about a stressful event, but that event is open to wide interpretation by the respondent. This poses a problem, because a person could respond to a symptom question on the PCL without having been exposed to a trauma that meets criterion A1. In fact, researchers have found that after events that do not meet criterion A1, people often report PTSD symptoms of the same or greater severity reported by persons who actually have PTSD ( 36 ). Although symptom severity may look the same on the PCL, the functional impairment resulting from these symptoms may not. The PWHS scores are derived from the SF-36, a functional status measure. Not using a valid PTSD diagnosis may lead to gross over- or underestimates of PWHS scores.
Along with criterion A, the PTSD symptoms on the PCL are open for interpretation by the respondent, and there is also significant overlap between the symptoms described on the PCL and those of mental disorders. For example, loss of interest in pleasurable activities and irritability are symptoms of both depression and PTSD. The PCL may not discriminate between PTSD and depression in the same way that the CAPS can, because the CAPS specifically asks the interviewer to rate whether the symptom is trauma related. This discrimination between PTSD and other mental disorders is important, especially given the high co-occurrence among mental disorders ( 3 ).
To use only the dichotomous PTSD diagnosis would mean the loss of potentially important information. Veterans in this sample could have subclinical PTSD, which has been shown to be associated with functional impairments ( 37 ).
We believe that a model that uses PCL score as a proxy for PTSD diagnosis is incorrect. Similarly, diagnosis alone does not account for the range of symptom presentations, from mild to severe. From a statistical perspective, our regression model and the selection of covariates were based on a specific theoretical framework and our research interest. Even though there was a correlation between PTSD diagnosis and PCL score (with an even higher correlation between diagnosis of a mood disorder and PCL score), we retained both variables as predictor variables in our ordinary least-squares models because of their theoretical relevance ( 38 ).
The correlation between PTSD diagnosis and the PCL score was moderate (.48, p<.01). After the covariates were centered, the regression coefficient of PCL score remained relatively stable with the addition of PTSD diagnosis and the interaction term. Therefore, we had reason to believe that each PTSD variable—diagnosis and severity—provided additive information in our analysis, which was evidenced by a higher R 2 value with the additional PTSD variable.
Results of our regression model demonstrated that both PTSD diagnosis and PTSD severity were meaningful predictors. The significance of the interaction between PTSD diagnosis and PCL score demonstrated that both variables were important in predicting the PWHS score—a major objective of this study. We showed that the effect of PTSD diagnosis on the PWHS score relied on the level of PTSD severity; similarly the impact of the PCL score was conditional on whether or not an individual had been diagnosed as having PTSD. Both disease dimensions contributed jointly to the formation of an individual's PWHS score.
Application of the regression model
The regression coefficients (model 1, Table 3 ) can be easily used to estimate (via simple arithmetic) PWHS scores at the patient level on the basis of PTSD severity and diagnosis, the co-occurrence of other axis I mental disorders, and common demographic variables (sex, work status, and disability status). The model can be applied to existing research data sets in which the included variables were previously measured. Or the model can be applied to larger-scale populations in efforts to measure disease burden, as we have demonstrated in this VA population. As population demographic variables or PTSD prevalence or severity change, model 1 users can handily adjust for these changes on the basis of regression coefficients and sample means.
For example, the PWHS score for a veteran with PTSD who has a PCL score of 50 and a co-occurring mood disorder, whose substance use disorder or anxiety disorder status is unknown, and who is male, disabled, and not working is .542. This PWHS score was calculated with the following formula by using the actual value of each predictor (predictor), intercept, sample means (SM), and the regression coefficient (b) in Table 3 , where k is the total number of predictors included in the model: 
Because we do not know the person's status in terms of substance use or anxiety disorders in this example, we use the sample means to represent the likelihood of the hypothetical veteran having a diagnosis of these conditions. For our models, we used the value of 1 to indicate the presence of a status with respect to dichotomous variables. For example, 1 would be coded for a positive PTSD diagnosis, male sex, someone working, and someone disabled.
Once PWHS scores are estimated, researchers can compare them across populations. Among patients with PTSD, for example, researchers can compare PWHS scores across the various psychotherapies and pharmacotherapies. Similarly, policy makers can compare PWHS scores across various diseases and disorders in efforts to maximize improvement in health status by accounting for patient preference and disease prevalence. Sinnott and colleagues ( 39 ) summarized recent findings and presented a mean difference score of .041 (range -.011 to .097) as clinically important ( 40 , 41 , 42 ). These clinically important differences serve as benchmarks to help determine whether small differences in PWHS scores translate to actual improvements in an anchored measure of health status. For example, for someone with PTSD a difference of .04 in PWHS score reflects a change in PCL score of greater than 20 points, all else being equal. In contrast, for someone without PTSD, a .04 change is possible with only a 10-point change in PCL score. This difference is visually apparent in Figure 1 , because PTSD diagnosis and the interaction between PCL score and PTSD diagnosis affect the slope of the regression lines.
Limitations
Our findings are limited by four factors. First, the PWHS scores represent preferences of a British community sample ( 28 ) from a study conducted before the wars in Iraq and Afghanistan. Although some researchers may disagree (such as Drummond and colleagues [ 27 ]), Brazier ( 43 ) recommended that preference weights be elicited from the community of interest. O'Hagan and colleagues ( 44 ) found that a U.S. sample consistently valued generic health states higher than a British sample. Direct valuation of health states is useful but often time consuming. Second, the preference weights used in this study were attached to generic health states derived from the SF-36; they were not attached to disease-specific health states. Disease-specific health states are useful for monitoring changes across time in specific aspects of health within the context of a clinical trial ( 45 ). Generic health states may not be descriptive enough to convey the phenomenology of a PTSD diagnosis. Third, Magruder and colleagues ( 3 ) collected data in 1999, also before the wars in Iraq and Afghanistan, and from veterans who may be older than patients in current VA and military medical centers. In today's increasingly younger veteran population, PWHS scores might be even lower. Finally, there are floor effects associated with the SF-6D scoring method. Having floor effects refers to "the limited ability of the system to differentiate between expected low-value or poor health states" ( 39 ). It is possible that many veterans experienced significant pathology, and because of these floor effects, the model was not sensitive enough to distinguish among them.
Additional model parameters
The regression model accounted for 39% of the variance, which is a moderate amount but still leaves room for improvement. It is clear that mental health conditions, demographic characteristics, and disability status do not fully account for the variability in preference-weighted health status. It is possible that our model would have been stronger if it had accounted for severe or debilitating medical conditions, psychotic and personality disorders, and symptom severity associated with mood and substance use disorders and anxiety disorders other than PTSD. For example, researchers have demonstrated a relationship between PWHS scores (referred to as health utilities in their studies) and depression ( 46 ) and alcohol use severity ( 47 ). Unfortunately, we have no symptom distress data for any disorder other than PTSD, and as we reported, both PTSD diagnostic status and PTSD symptom severity were significant in our model. Thus it is likely that an assessment of symptom severity for the other mental disorders would have increased the R 2 .
We also deliberately chose not to explore other predictors, such as chronic medical conditions, with the available data. In contrast to the systematic and standardized assessment of PTSD, mood and substance use disorders, and anxiety disorders other than PTSD, medical conditions were diagnosed at the discretion of the veteran's provider using the ICD system. We have no way to determine the reliability or validity of the provider's diagnosis. For example, provider diagnosis of PTSD has been shown to be unreliable; in one study PTSD was grossly underdiagnosed ( 3 ). Model parameters derived from a mixed-methods assessment of symptoms (clinician judgment for medical conditions and standardized research criteria for mental health conditions) would likely invalidate the entire model.
Conclusions
This is the first study to present PWHS scores for PTSD. The regression model allows health policy researchers to measure preference-weighted health status associated with PTSD and adjust the estimates on the basis of symptom severity, common co-occurring disorders, and demographic characteristics of the sample. Although this study has notable limitations, the findings suggest that preference weights for PTSD are not uniform across all subpopulations, because PWHS scores were significantly altered by our modeled parameters. The model has practical implications for cost-effectiveness applications and for estimating disease burden of PTSD in community, veteran, and military health care settings.
Acknowledgments and disclosures
This work was partly supported by grant VCR-99-010-2 (to Dr. Magruder) funded by the Veterans Affairs Health Services Research and Development program. The authors thank Phoebe Kuesters, B.A., and Leah Russell, M.A., for their administrative support. The views expressed in this manuscript are those of the authors and do not necessarily represent the official policy or position of the Deployment Health Clinical Center, Walter Reed Army Medical Center, Uniformed Services University of the Health Sciences, Department of Defense, Department of Veterans Affairs, United States Government, or Medical University of South Carolina.
The authors report no competing interests.
1. Gore KL, Engel CC, Freed MC, et al: Test of a single-item posttraumatic stress disorder screener in a military primary care setting. General Hospital Psychiatry 30:391, 2008Google Scholar
2. Stein MB, McQuaid JR, Pedrelli P, et al: Posttraumatic stress disorder in the primary care medical setting. General Hospital Psychiatry 22:261–269, 2000Google Scholar
3. Magruder KM, Frueh BC, Knapp RG, et al: Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. General Hospital Psychiatry 27:169–179, 2005Google Scholar
4. Dobie DJ, Kivlahan DR, Maynard C, et al: Screening for post-traumatic stress disorder in female Veteran's Affairs patients: validation of the PTSD Checklist. General Hospital Psychiatry 24:367–374, 2002Google Scholar
5. Davidson JR, Weisler RH, Malik ML, et al: Treatment of posttraumatic stress disorder with nefazodone. International Clinical Psychopharmacology 13:111–113, 1998Google Scholar
6. Hoge CW, Castro CA, Messer SC, et al: Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine 351:13–22, 2004Google Scholar
7. Hoge CW, Auchterlonie JL, Milliken CS: Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA 295:1023–1032, 2006Google Scholar
8. Defense Medical Epidemiology Database (DMED 3.7). Silver Spring, Md, Army Medical Surveillance Activity, 2004. Available at afhsc.army.milGoogle Scholar
9. Gillock KL, Zayfert C, Hegel MT, et al: Posttraumatic stress disorder in primary care: prevalence and relationships with physical symptoms and medical utilization. General Hospital Psychiatry 27:392–399, 2005Google Scholar
10. Schnurr PP, Hayes AF, Lunney CA, et al: Longitudinal analysis of the relationship between symptoms and quality of life in veterans treated for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology 74:707–713, 2006Google Scholar
11. Davidson JR: Pharmacologic treatment of acute and chronic stress following trauma. Journal of Clinical Psychiatry 67(suppl 2):34–39, 2006Google Scholar
12. Ipser J, Seedat S, Stein DJ: Pharmacotherapy for post-traumatic stress disorder: a systematic review and meta-analysis. South African Medical Journal 96:1088–1096, 2006Google Scholar
13. Davis LL, Frazier EC, Williford RB, et al: Long-term pharmacotherapy for post-traumatic stress disorder. CNS Drugs 20:465–476, 2006Google Scholar
14. Bradley R, Greene J, Russ E, et al: A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry 162:214–227, 2005Google Scholar
15. Bisson JI, Ehlers A, Matthews R, et al: Psychological treatments for chronic post-traumatic stress disorder: systematic review and meta-analysis. British Journal of Psychiatry 190:97–104, 2007Google Scholar
16. Blake DD, Weathers FW, Nagy LM, et al: The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress 8:75–90, 1995Google Scholar
17. Ware JE, Sherbourne CD: The MOS 36-item Short-Form Health Survey (SF-36): I. conceptual framework and item selection. Medical Care 30:473–483, 1992Google Scholar
18. Gold MR, Siegel JE, Russell LB, et al: Cost-Effectiveness in Health and Medicine. New York, Oxford University Press, 1996Google Scholar
19. Bennett KJ, Torrance GW: Measuring health state preferences and utilities: rating scale, time trade-off, and standard gamble techniques; in Quality of Life and Pharmacoeconomics in Clinical Trials. Edited by Spiker B. Philadelphia, Lippincott-Raven, 1996Google Scholar
20. Berzon RA, Mauskop JA, Simeon GP: Choosing a health profile (descriptive) and/or patient-preference (utility) measure for a clinical trial; in Quality of Life and Pharmacoeconomics in Clinical Trials. Edited by Spiker B. Philadelphia, Lippincott-Raven, 1996Google Scholar
21. Garza AG, Wyrwich KW: Health utility measures and the standard gamble. Academy of Emergency Medicine 10:360–363, 2003Google Scholar
22. Tsevat J: What do utilities measure? Medical Care 38(suppl 9):160–164, 2000Google Scholar
23. CEA Registry. Boston, Center for the Evaluation of Value and Risk in Health, Tufts Medical Center Institute for Clinical Research, 2006. Available at research.tufts-nemc.org/cear/default.aspx Google Scholar
24. Murray CJL, Lopez AD (eds): The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020, Vol 1. Boston, Harvard University Press, 1996Google Scholar
25. Sanderson K, Andrews G: Mental disorders and burden of disease: how was disability estimated and is it valid? Australia New Zealand Journal of Psychiatry 35:668–676, 2001Google Scholar
26. Hoch JS, Smith MW: A guide to economic evaluation: methods for cost effectiveness analysis of person-level data. Journal of Traumatic Stress 19:787–797, 2006Google Scholar
27. Drummond MF, Sculpher M, Torrance G, et al: Methods for the Economic Evaluation of Health Care Programmes, 3rd ed. Oxford, United Kingdom, Oxford University Press, 2005Google Scholar
28. Brazier J, Roberts J, Deverill M: The estimation of a preference-based measure of health from the SF-36. Journal of Health Economics 21:271–292, 2002Google Scholar
29. Pyne JM, Booth BM, Farahati F, et al: Preference-weighted health status associated with substance use-disorders treatment. Journal of Studies on Alcohol 67:436–444, 2006Google Scholar
30. Weathers FW, Litz BT, Herman JA, et al: The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Presented at the Annual Convention of the International Society for Traumatic Stress Studies, San Antonio, Tex, Oct 24–27, 1993Google Scholar
31. Blanchard EB, Jones-Alexander J, Buckley TC, et al: Psychometric properties of the PTSD Checklist (PCL). Behaviour Research and Therapy 34:669–673, 1996Google Scholar
32. Resnick HS, Best CL, Kilpatrick DG, et al: Trauma Assessment for Adults, Interview Version. Charleston, SC, Crime Victims Research and Treatment Center, Medical University of South Carolina, 1993Google Scholar
33. Sheehan DV, Lecrubier Y, Sheehan KH, et al: The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59:22–33, 1998Google Scholar
34. Ware J, Snow KK, Kosiniski M, et al: SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI, QualityMetric Inc, 2003Google Scholar
35. O'Reilly J, Williams AE, Holt K, et al: Health status and health utility values for a cohort of COPD patients in primary care. Presented at the American Trauma Society International Conference. Seattle, 2003Google Scholar
36. Mol SS, Arntz A, Metsemakers JF, et al: Symptoms of post-traumatic stress disorder after non-traumatic events: evidence from an open population study. British Journal of Psychiatry 186:494–499, 2005Google Scholar
37. Stein MB, Walker JR, Hazen AL, et al: Full and partial posttraumatic stress disorder: findings from a community survey. American Journal of Psychiatry 154:1114–1119, 1997Google Scholar
38. Stolzenberg RM: Multiple regression analysis; in Handbook of Data Analysis. Edited by Hardy M, Bryman A. Thousand Oaks, Calif, Sage, 2004Google Scholar
39. Sinnott PL, Joyce VR, Barnett PG: Preference Measurement in Economic Analysis. Menlo Park, Calif, VA Palo Alto, Health Economics Resource Center, 2007Google Scholar
40. Walters SJ, Brazier JE: What is the relationship between the minimally important difference and health state utility values? The case of the SF-6D. Health Quality Life Outcomes 1:4, 2003Google Scholar
41. Walters SJ, Brazier J: Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Quality of Life Research 14:1523–1532, 2005Google Scholar
42. Marra CA, Woolcott JC, Kopec JA: A comparison of generic, indirect utility measures (the HUI2, HUI3, SF-6D, and the EQ-5D) and disease-specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Social Science and Medicine 60:1571–1582, 2005Google Scholar
43. Brazier J: Current State of the Art in Preference-Based Measures of Health and Avenues for Further Research. Sheffield, United Kingdom, University of Sheffield, 2005Google Scholar
44. O'Hagan A, Brazier KE, Khatapoush SA: A Comparison of United States and United Kingdom EQ-5D Health States Valuations Using Nonparametric Bayesian Method. Sheffield, United Kingdom, University of Sheffield, 2007Google Scholar
45. Llewellyn-Thomas HA: Health state descriptions: purposes, issues, a proposal. Medical Care 34:DS109–DS118, 1996Google Scholar
46. Revicki DA, Wood M: Patient-assigned health state utilities for depression-related outcomes: differences by depression severity and antidepressant medications. Journal of Affective Disorders 48:25–36, 1998Google Scholar
47. Kraemer KL, Roberts MS, Horton NJ, et al: Health utility ratings for spectrum of alcohol-related health states. Medical Care 43:541–550, 2005Google Scholar



