A Pilot Study of Telephone Care Management and Structured Disease Self-Management Groups for Chronic Depression
Much of the burden of chronic or recurrent depression could be prevented by organized and sustained treatment—psychotherapy, pharmacotherapy, or both. Randomized trials clearly demonstrate the efficacy of antidepressant medications and structured psychotherapies for patients with recurrent depression and dysthymia, challenging traditional views that chronic depression represents character pathology unlikely to respond to treatment ( 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 ). Recent evidence supports the use of combined treatments (psychotherapy and pharmacotherapy) for more severe or treatment-resistant depression ( 15 , 16 , 17 , 18 , 19 , 20 ). Unfortunately, patients treated for chronic depression seldom receive either vigorous pharmacotherapy or evidence-based psychotherapy ( 21 , 22 , 23 , 24 ).
Efficacy trials among patients in specialty settings demonstrate that organized treatment programs (including structured pharmacotherapy or cognitive-behavioral psychotherapy or both) can significantly improve outcomes ( 11 , 12 , 16 , 17 , 18 , 25 ). Effectiveness trials in primary care settings demonstrate that systematic care programs can significantly improve the process and outcomes of acute-phase depression treatment ( 26 ). Proven models include the collaborative care models developed by Katon and colleagues ( 27 , 28 ), and Simon and colleagues' telephone care management ( 29 ). It is not clear, however, whether similar systematic care programs can improve the quality of treatment or clinical outcomes of patients receiving specialty care in community practice ( 30 , 31 ).
This report describes a pilot effectiveness trial of telephone care management and structured group self-management support for chronic or recurrent depression. The interventions incorporate key elements of the chronic care model ( 32 , 33 , 34 ) and build on our earlier experience with systematic telephone care management for affective disorders ( 35 , 36 , 37 ). We evaluated two group self-management programs, one a peer-led chronic-disease self-management group based on work by Lorig and colleagues ( 38 ) and the other a professionally led group emphasizing more traditional psychoeducation and cognitive-behavioral techniques. In a four-arm pilot trial, we compared telephone care management alone and in combination with each of these group modalities with usual care in order to evaluate the feasibility and acceptability of each intervention program, provide a preliminary test of the effectiveness of each program compared with usual care, and inform the design of a subsequent large-scale effectiveness trial.
Methods
Participants and recruitment
Study participants were enrolled between December 2003 and May 2004. Follow-up data were collected until July of 2005. Participants were recruited from the Central Behavioral Health Clinic of Group Health Cooperative (GHC), a health maintenance organization that serves approximately 500,000 individuals in Washington State. The clinic serves about 8,500 patients per year, of whom approximately 1,700 (20%) are treated for chronic or persistent depression.
We identified patients who had persistent symptoms of depression despite at least six months of antidepressant treatment prescribed in specialty care. Computerized data systems were used to identify GHC members aged 18 and older who had initiated antidepressant treatment at least 180 days previously, had a visit diagnosis of major depressive disorder at the time of initial antidepressant prescription, were continuously enrolled in GHC for at least the previous 180 days, and had no diagnosis of bipolar disorder or psychotic disorder or prescription for a mood stabilizer or antipsychotic medication in the past two years.
All eligible patients received an invitation letter that included a detailed study description and an option to decline further contact. A telephone call approximately seven days later included a 20-item depression scale extracted from the 90-item Hopkins Symptom Checklist (SCL-90) ( 39 ). Patients who scored above .75 on the SCL-90, indicating significant current symptoms, were invited to complete an in-person baseline assessment. All invited participants were offered $20 compensation for completing the baseline assessment.
Inclusion criteria assessed at the baseline interview (see below) required at least one major depressive episode in the past two years as diagnosed by a structured interview and a history of either recurrent major depression (more than three episodes in the past five years) or dysthymia. In other words, all patients met criteria for recurrent major depression or dysthymia, but (consistent with our effectiveness design) patients were heterogeneous with respect to current mood state (dysthymia, chronic major depression, partial remission, relapse, or recurrence) and current antidepressant treatment. Exclusion criteria included history of mania or hypomania, cognitive impairment, near-terminal medical illness, intent to disenroll from GHC within the next 12 months, and emergent clinical needs (for example, risk of harm to self or others). Alcohol or drug use disorders were not exclusion criteria.
After a full description of study procedures, risks, and benefits, all participants provided written consent before the baseline assessment and again before enrollment in the randomized trial. Participants were advised that some would be offered additional treatment services, but willingness to accept any intervention was not a requirement for participation. All study procedures were reviewed and approved by GHC's Institutional Review Board.
Baseline measures
Current and lifetime mood disorder diagnoses were assessed with the depression and mania modules of the Structured Clinical Interview for DSM-IV (SCID) ( 40 ). Comorbid anxiety disorders were assessed with the panic disorder and generalized anxiety disorder modules of the SCID ( 40 ).
Borderline personality disorder was assessed by using the Structured Clinical Interview for DSM-IV Axis II Personality Disorders ( 41 ). The Patient Satisfaction Index ( 42 ), focused on mental health care, was used to assess satisfaction with mental health treatment before implementation of the study interventions to provide a measure for later comparisons.
Randomization
Within one week after the baseline interview the study data manager assigned eligible and consenting participants to one of four treatment groups (usual care, telephone care management, telephone care management plus a peer-led chronic-disease self-management program, or telephone care management plus a professionally led group) using computer-generated block randomization. Participants assigned to one of the three intervention groups were notified by the telephone care manager. Those assigned to usual care were notified by mail. Twenty-six patients were assigned to each of the four groups.
Interventions
The chronic care model ( 32 , 33 ) guided the overall design of the intervention programs. Important components of this model include information systems to monitor treatment quality and treatment adherence, decision support through treatment algorithms and appropriate specialty consultation, practice redesign to ensure appropriate follow-up care, and patient education and activation to promote effective self-management. The telephone care management system was directed at the first three elements, and the two group programs were directed at the fourth. Patients in all groups were also free to use any nonstudy services normally available.
Telephone monitoring and care management. A computerized care manager decision support system supported systematic tracking of patient contacts, scripted clinical assessments, automatic application of treatment algorithms, and generation of feedback reports. The care manager (a master's-level counselor) contacted each patient at specified intervals—at least monthly during the first three months, then at intervals that varied according to symptoms, medication adherence, and side effects. During the first session the care manager helped each participant create a written care plan. Each contact included a structured five- to ten-minute assessment of depressive symptoms, medication use, and side effects. Following computer-assisted scripts, the care manager provided education about medication adherence and management of side effects and incorporated motivational enhancement strategies to address ambivalence about medication use when patients had discontinued medication treatment or were taking dosages lower than those prescribed. Information collected about symptoms, side effects, and current medication dosage generated specific recommendations to the patient's usual-care treating provider.
After each contact, the care manager sent the treating provider a report of current symptoms, medication use, side effects, prior treatment, and algorithm-based recommendations. In the case of moderate or severe symptoms (or if algorithm-generated recommendations suggested urgent intervention), the care manager communicated with the treating provider by telephone within 24 hours. The care manager also provided any needed outreach and care coordination, including facilitation of follow-up care.
Care management training included four hours of didactic training, four hours of role-play, and direct observation of two care management contacts before certification. The care manager received weekly supervision by the study psychologist and psychiatrist. Cases reviewed at supervision meetings included all patients overdue for monitoring calls, all patients with moderate or greater levels of depressive symptoms, and any cases requested for review by the care manager.
Peer-led chronic-disease self-management program. The peer-led program is an evidence-based program shown to relieve symptoms (such as pain and depression), reduce use of health services ( 38 , 43 , 44 , 45 , 46 , 47 , 48 ), and reduce activity limitations ( 49 ) across a range of chronic conditions. The six-week workshop includes several core components: disease-related goal setting and problem solving, cognitive symptom management (relaxation, distraction, self-talk, and visualization), communication skills, medication management, development of a patient-physician partnership, and use of community resources.
The program incorporates strategies that are based on self-efficacy theory ( 50 ) and evidence that positive role models (that is, lay leaders with experience) increase patients' confidence for disease management. The chronic-disease self-management program aims to enhance self-efficacy for disease management by promoting self-directed application of newly acquired skills, reinterpreting symptoms as multidetermined and modifiable, modeling coping behaviors, and using guided rehearsal and social persuasion. The program follows a highly structured and detailed protocol that includes a structured method for group problem solving and weekly action planning. We supplemented the six-week workshop with ongoing bimonthly groups focused on continued goal setting and problem solving in order to reinforce skill mastery and problem-solving abilities. Each group had two leaders, and at least one leader had prior experience teaching the course. Peer leaders referred all clinical concerns to treating providers or the care manager.
Group leaders each attended a four-day training workshop that used an explicit training manual developed by the Stanford Patient Education Research Center ( 51 ). The study psychologist provided ongoing biweekly supervision during the first six weeks of the group and as-needed supervision thereafter to the senior peer leader. The first six sessions of the program were audiotaped, and a continuation session was directly observed for quality assurance and treatment fidelity.
Professionally led group program. A psychologist delivered the manualized group intervention over ten consecutive weeks, followed by six months of twice-monthly "booster" sessions. Selection of specific intervention elements was informed by Jacobson and colleagues' ( 52 , 53 ) randomized trial demonstrating that therapy limited to behavioral activation and identification and interruption of negative automatic thoughts was as effective as "complete" cognitive-behavioral treatment (that is, treatment that includes exploration and modification of core schema).
Traditional acute-phase cognitive-behavioral therapy components were adapted to address persistent depression ( 54 ) and emphasized setting reasonable goals, implementing lifestyle changes (such as regular aerobic exercise), enhancing medication adherence, increasing positive reinforcement (such as developing daily activity schedules), managing cognitive distortions, and using other behavioral strategies (such as social skills rehearsal). Session content explicitly addressed self-management, encouraging participants to identify and evaluate current and potential coping strategies ( 55 ). For example, substance use and suicide attempts were discussed as potential but maladaptive strategies for coping with depression. Booster sessions emphasized self-directed goal setting and problem solving and attention to long-term self-care planning and provided sustained social support.
Continuation cognitive-behavioral therapy of this type delivered in an individual format has been shown to improve outcomes for patients with recurrent depression and incomplete recovery between episodes ( 13 ). In contrast to some cognitive-behavioral therapy interventions designed as stand-alone interventions, the group program explicitly addressed medication adherence and effective collaboration with prescribing providers.
A psychologist (DM), who was trained in cognitive-behavioral therapy and who had eight years of experience treating depression, led the groups. The study psychologist provided ongoing weekly or biweekly supervision during the first ten weeks of the group program and as-needed supervision thereafter. All sessions were audiotaped for assessment of protocol adherence and quality assurance.
Usual care. Participants assigned to usual care were free to use any primary care or specialty services normally available inside or outside GHC. No additional services were provided, but no services normally available were withheld.
Outcome assessments
Participants were contacted by telephone by interviewers blinded to treatment assignment for outcome assessments at three, six, nine, and 12 months after randomization. Participants were paid $30 at the completion of the 12-month interview. Each assessment included the current depression module of the SCID, the 20-item SCL depression scale, the Patient Satisfaction Index, and the Patient-Rated Global Improvement (PGI) ( 56 ). The PGI is a 7-point rating of treatment effectiveness from the patient's perspective and was used to measure changes since the baseline assessment.
Antidepressant medication use was assessed with automated prescription refill data. Receipt of "adequate" antidepressant treatment for 90 days or more was calculated by using algorithms developed and validated by our group ( 57 ). Adequate treatment was defined by means of a moderate dosing standard (reflecting dosages generally considered adequate by psychiatrists) ( 58 ).
Analyses
Feasibility and acceptability. Because the primary aim of the study was to test the feasibility of delivering each intervention in regular practice, primary analyses examined participation in each of the intervention programs and compared rates of treatment dropout in the three groups.
Preliminary evaluation of intervention effectiveness. We compared each intervention program to the usual-care group using an intent-to-treat approach—that is, individuals were included in the analyses in the group to which they were randomly assigned regardless of the degree of intervention participation. Our primary outcome measure was the mean SCL depression score from month 6 to month 12 (the expected time of maximal intervention effect). Secondary measures included percentage with major depression based on SCID diagnosis and a PGI rating of much improved or better. Cross-sectional analyses examined each time point individually, and longitudinal analyses examined all follow-ups together. Cross-sectional analyses used t tests to compare group means for ordinal and continuous outcomes (such as SCL score), and chi square tests compared proportions for binary outcomes. Analyses adjusting for baseline differences on outcomes were conducted by using ordinary least-squares and logistic regression. Longitudinal analyses assessed the average effect over the six-, nine-, and 12-month follow-ups, using generalized estimating equations. Additional analyses compared the proportion of patients receiving adequate antidepressant treatment and satisfaction with treatment.
Results
Participants
Of 297 patients identified from computerized records, 189 (64%) completed the telephone eligibility-screening interview. Of those, 153 (81%) had significant residual symptoms of depression and were invited to the baseline interview. A total of 119 attended the baseline assessment, and 107 were found eligible to be invited into the randomized trial. Of those, 104 agreed to participate.
Baseline characteristics of study participants assigned to the four groups are shown in Table 1 . Participants in each of the four groups did not significantly differ on any characteristics measured at baseline.
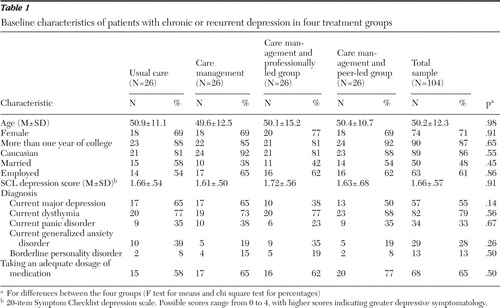 |
Usual-care group participants completed 92% of all blinded follow-up interviews (at three, six, nine, and 12 months), the care management group completed 82%, the professionally led group completed 94%, and the peer-led group completed 83%.
Treatment participation
Table 2 shows participation rates in telephone care management in the three intervention groups. Acceptance of this treatment component was high, and no significant differences were found between the groups in any measure of participation.
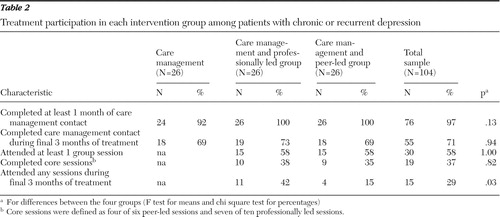 |
Table 2 also shows rates of group participation. A majority of participants randomly assigned to a group intervention attended at least one session, but only 37% completed the acute phase—that is, four of the six chronic-disease self-management program sessions or seven of the ten cognitive-behavioral therapy sessions. More participants in the professionally led group attended sessions during the past three months of treatment.
Clinical outcomes and adequacy of treatment
Figure 1 shows the mean SCL depression scores over time in the four treatment groups. In a repeated-measures model comparing the average effect over months 6, 9, and 12, no significant differences were found among the groups. Results for ratings on the PGI were similar (data not shown); 22% of those assigned to usual care rated themselves as much or very much improved averaged across the six-, nine-, and 12-month follow-ups compared with 35% in the care management group, 42% in the professionally led group, and 34% in the peer-led group.
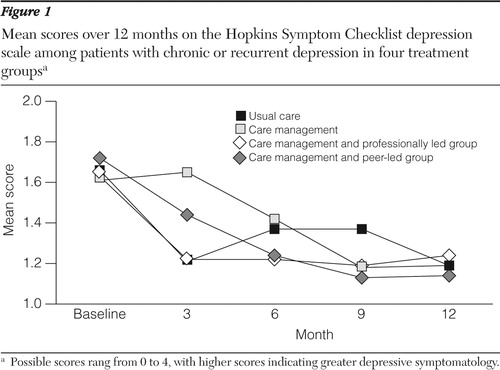
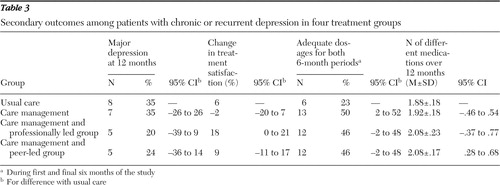
Table 3 presents the results by treatment group for the other primary and secondary outcomes, with a 95% confidence interval (CI) for the difference between each group and usual care. The professionally led group had the lowest rate of major depression at 12 months (20%), but the CI was wide and included zero, indicating no statistically significant difference. Similarly, this group scored highest on the Patient Satisfaction Index (average score for the six-, nine-, and 12-month follow-ups). Compared with usual care, all three intervention groups had a larger proportion of participants receiving adequate antidepressant medication over 12 months (46%–50%, compared with 23% for usual care), but the CIs were very wide and, with the exception of the care management group, included zero. The mean number of different prescription medications taken over the 12-month follow-up period (a proxy for antidepressant switch or augmentation) was similar in the four groups, ranging from 1.88 to 2.08 per person.
 |
Discussion
This pilot study supports the feasibility and acceptability of a systematic care management and self-management support program for patients with persistent or recurring depression. We found outreach telephone-based care management to be an accessible and acceptable alternative to programs requiring one-on-one, in-person contact. Almost all participants assigned to receive telephone outreach accepted the care manager's calls and completed the structured assessments of symptoms, medication adherence, and side effects as well as the written self-care plan.
Factors limiting the dissemination of evidence-based psychosocial treatments for chronic or recurring depression, such as cognitive-behavioral psychotherapy, include the significant resources required and the limited training and expertise available in most health care settings. In our trial, more than half the patients who were offered participation were willing to at least try group self-management training programs, both peer led and professionally led. We were able to offer participants only two meeting time options for each type of group; we expect rates of engagement would have been higher if more choices were available. Over the study's full year, we observed greater long-term participation among participants in the professionally led group. This group continues to meet regularly—as a peer support group—several months after the study's completion.
The main limitation of this study is its small sample. We cannot reach any definitive conclusions about the additional clinical benefit of the active interventions. For example, although the results are consistent with up to a 39% reduction in the proportion of participants with major depression in the professionally led group, the results are also consistent with no effect or even a worsening of symptoms. These interventions were implemented within a single prepaid health system, and we cannot be certain how results would generalize to other settings or patient populations. Clinicians' nonadherence to the care manager's recommendations could also have had an impact on depression outcome measures in the intervention groups. A larger trial of organized self-management support for chronic depression among patients receiving care in more than one type of practice setting will be necessary for a full evaluation of program effectiveness.
Even though the generic chronic-disease self-management program was not developed to address the unique characteristics of depression, we chose to evaluate this generic peer-led program because of its growing popularity and availability. We are not aware of any explicit evaluations of the program with patients with depression, but it has been successfully delivered to mixed groups of individuals with a variety of chronic conditions, including depression. Compared with a depression-specific intervention, a more generic chronic-disease self-management program has the potential to integrate care for depression with care for other chronic conditions, increasing availability of support and reducing stigma.
Some of our program participants, however, gave us feedback that they would have preferred a program with more content addressing the unique aspects of struggling with chronic or recurrent depression and a less structured format. Conversely, among our cognitive-behavioral therapy group participants there was great appreciation for the support and guidance of others who had experienced similar challenges with depression. It is possible that a combination of initial professionally taught skills training and long-term peer support and mentorship holds the greatest promise for feasibility and effectiveness.
Conclusions
A systematic program of telephone care management and group self-management support is a feasible and acceptable addition to outpatient psychiatric care for patients with chronic depression.
Acknowledgments and disclosures
This work was supported by grant MH-065530 from the National Institute of Mental Health. The authors thank Paul Landraitis, M.A., care manager, for his contribution to this project.
The authors report no competing interests.
1. Nierenberg AA: Long-term management of chronic depression. Journal of Clinical Psychiatry 62:17–21, 2001Google Scholar
2. Kocsis JH, Friedman RA, Markowitz JC, et al: Maintenance therapy for chronic depression: a controlled clinical trial of desipramine. Archives of General Psychiatry 53:769–774, 1996Google Scholar
3. Keller MB, Kocsis JH, Thase ME, et al: Maintenance phase efficacy of sertraline for chronic depression: a randomized controlled trial. JAMA 280:1665–1672, 1999Google Scholar
4. Miller NL, Kocsis JH, Leon AC, et al: Maintenance desipramine for dysthymia: a placebo-controlled study. Journal of Affective Disorders 64:231–237, 2001Google Scholar
5. Frank E, Kupfer DJ, Perel JM, et al: Three-year outcomes for maintenance therapies in recurrent depression. Archives of General Psychiatry 47:1093–1099, 1990Google Scholar
6. Reynolds CJ, Frank E, Perel JM, et al: Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA 281:39–45, 1999Google Scholar
7. Markowitz JC: Psychotherapy for dysthymic disorder. Psychiatric Clinics of North America 19:133–149, 1996Google Scholar
8. Jarrett RB, Basco MR, Risser R, et al: Is there a role for continuation phase cognitive therapy for depressed outpatients? Journal of Consulting and Clinical Psychology 66:1036–1040, 1998Google Scholar
9. Blackburn IM, Moore RG: Controlled acute and follow-up trial of cognitive therapy and pharmacotherapy in out-patients with recurrent depression. British Journal of Psychiatry 171:328–334, 1997Google Scholar
10. Fava GA, Grandi S, Zielezny M, et al: Four-year outcome for cognitive behavioral treatment of residual symptoms in major depression. American Journal of Psychiatry 153:945–947, 1996Google Scholar
11. Fava G, Rafanelli C, Grandi S, et al: Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Archives of General Psychiatry 55:816–820, 1998Google Scholar
12. Fava G, Rafanelli C, Grandi S, et al: Six-year outcome for cognitive-behavioral treatment of residual symptoms in major depression. American Journal of Psychiatry 155:1443–1445, 1998Google Scholar
13. Jarrett RB, Kraft D, Doyle J, et al: Preventing recurrent depression using cognitive therapy with and without a continuation phase: a randomized clinical trial. Archives of General Psychiatry 58:381–388, 2001Google Scholar
14. Teasdale JD, Segal ZV, Williams JM, et al: Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology 68:615–623, 2000Google Scholar
15. Keller MB, McCullough JP, Klein DN, et al: A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. New England Journal of Medicine 342:1462–1470, 2000Google Scholar
16. Frank E, Grochocinski VJ, Sanier CA, et al: Interpersonal psychotherapy and antidepressant medication: evaluation of a sequential treatment strategy in women with recurrent major depression. Journal of Clinical Psychiatry 61:51–57, 2000Google Scholar
17. Paykel ES, Scott J, Teasdale JD, et al: Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Archives of General Psychiatry 56:829–835, 1999Google Scholar
18. Scott J, Teasdale JD, Paykel ES, et al: Effects of cognitive therapy on psychological symptoms and social functioning in residual depression. British Journal of Psychiatry 177:440–446, 2000Google Scholar
19. Klein DN, Santiago NJ, Vivian D, et al: Cognitive-behavioral analysis system of psychotherapy as a maintenance treatment for chronic depression. Journal of Consulting and Clinical Psychology 72:681–688, 2004Google Scholar
20. Gelenberg AJ, Trivedi MH, Rush AJ, et al: Randomized, placebo-controlled trial of nefazodone maintenance treatment in preventing recurrence in chronic depression. Biological Psychiatry 54:806–817, 2003Google Scholar
21. Keller MB, Lavori PW, Klerman GL, et al: Low levels and lack of predictors of somatotherapy and psychotherapy received by depressed patients. Archives of General Psychiatry 43:458–466, 1986Google Scholar
22. Kotin J, Post RM, Goodwin FK: Drug treatment of depressed patients referred for hospitalization. American Journal of Psychiatry 130:1139–1141, 1973Google Scholar
23. Keller MB, Harrison W, Fawcett JA, et al: Treatment of chronic depression with sertraline or imipramine: preliminary blinded response rates and high rates of undertreatment in the community. Psychopharmacology Bulletin 31:205–212, 1995Google Scholar
24. Dawson R, Lavori PW, Coryell WH, et al: Course of treatment received by depressed patients. Journal of Psychiatric Research 33:233–242, 1999Google Scholar
25. Katon WJ, Von Korff M, Lin E, et al: Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Archives of General Psychiatry 56:1109–1115, 1999Google Scholar
26. Badamgarav E, Weingarten SR, Henning JM, et al: Effectiveness of disease management programs in depression: a systematic review. American Journal of Psychiatry 160:2080–2090, 2003Google Scholar
27. Katon W, Von Korff M, Lin E, et al: Collaborative management to achieve treatment guidelines: impact on depression in primary care. JAMA 273:1026–1031, 1995Google Scholar
28. Katon W, Robinson P, Von Korff M, et al: A multifaceted intervention to improve treatment of depression in primary care. Archives of General Psychiatry 53:924–932, 1996Google Scholar
29. Simon GE, Von Korff M, Rutter C, et al: Randomized trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. British Medical Journal 320:550–554, 2000Google Scholar
30. Trivedi MH, Rush AJ, Crismon ML, et al: Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Archives of General Psychiatry 61:669–680, 2004Google Scholar
31. Simon GE, Ludman EJ, Operskalski BH: Randomized trial of a telephone care management program for outpatients starting antidepressant treatment. Psychiatric Services 57:1441–1445, 2006Google Scholar
32. Wagner EH, Austin BT, Von Korff M: Organizing care for patients with chronic illness. Milbank Quarterly 74:511–544, 1996Google Scholar
33. Von Korff M, Gruman J, Schaefer J, et al: Collaborative management of chronic illness. Annals of Internal Medicine 127:1097–1102, 1997Google Scholar
34. Andrews G: Should depression be managed as a chronic illness? British Medical Journal 322:419–421, 2001Google Scholar
35. Simon G, Ludman EJ, Tutty S, et al: Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: a randomized controlled trial. JAMA 292:935–942, 2004Google Scholar
36. Simon GE, Ludman EJ, Unützer J, et al: Randomized trial of a population-based care program for people with bipolar disorder. Psychological Medicine 35:13–24, 2005Google Scholar
37. Simon GE, Ludman EJ, Bauer MS, et al: Long-term effectiveness and cost of a systematic care program for bipolar disorder. Archives of General Psychiatry 63:500–508, 2006Google Scholar
38. Lorig K, Sobel DS, Stewart AL, et al: Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Medical Care 37:5–14, 1999Google Scholar
39. Derogatis L, Rickels K, Uhlenhuth EH, et al: The Hopkins Symptom Checklist: a measure of primary symptom dimensions, in Psychological Measurements in Psychopharmacology: Problems in Psychopharmacology. Edited by Pichot P. Basel, Switzerland, Kargerman, 1974Google Scholar
40. First M, Spitzer R, Gibbon M, et al: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version. Washington, DC, American Psychiatric Press, 1997Google Scholar
41. First M, Gibbon M, Spitzer RL, et al: Users Guide for the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II), Washington, DC, American Psychiatric Press, 1997Google Scholar
42. Nabati L, Shea N, McBride L, et al: Adaptation of a simple patient satisfaction instrument to mental health: psychometric properties. Psychiatry Research 16:51–56, 1998Google Scholar
43. Lorig K, Feigenbaum P, Regan C, et al: A comparison of lay-taught and professionally taught arthritis self-management courses. Journal of Rheumatology 13:763–767, 1986Google Scholar
44. Lorig KR, Mazonson PD, Holman HR: Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis and Rheumatism 36:439–446, 1993Google Scholar
45. Lorig K, Lubeck D, Kraines RG, et al: Outcomes of self-help education for patients with arthritis. Arthritis and Rheumatism 28:680–685, 1985Google Scholar
46. Lorig K, Holman HR: Long-term outcomes of an arthritis self-management study: effects of reinforcement efforts. Social Science and Medicine 29:221–224, 1989Google Scholar
47. Lorig K, Gonzalez VM, Ritter P: Community-based Spanish language arthritis education program. Medical Care 37:957–963, 1999Google Scholar
48. Lorig KR, Sobel DS, Ritter PL, et al: Effect of a self-management program on patients with chronic disease. Effective Clinical Practice 4:256–262, 2001Google Scholar
49. Von Korff M, Moore JE, Lorig K, et al: A randomized trial of a lay person-led self-management group intervention for back pain patients in primary care. Spine 23:2608–2615, 1998Google Scholar
50. Bandura A: Social Foundations of Thought and Action. Englewood Cliffs, NJ, Prentice Hall, 1986Google Scholar
51. Chronic Disease Self-Management Leader's Manual. Palo Alto, Calif, Stanford Patient Education Research Center, 1999Google Scholar
52. Jacobson N, Dobson K, Truax P, et al: A component analysis of cognitive-behavioral treatment for depression. Journal of Consulting and Clinical Psychology 64:295–304, 1996Google Scholar
53. Gortner E, Gollan J, Dobson K, et al: Cognitive-behavioral treatment for depression: relapse prevention. Journal of Consulting and Clinical Psychology 66:377–384, 1998Google Scholar
54. Thase ME: Psychotherapy of refractory depressions. Depression and Anxiety 5:190–201, 1997Google Scholar
55. Bauer M, McBride L: Structured Group Psychotherapy for Bipolar Disorder: The Life Goals Program. New York, Springer, 1996Google Scholar
56. Guy W: ECDEU Assessment Manual for Psychopharmacology. Pub no ADM 76-338. Rockville, Md, National Institute of Mental Health, 1976Google Scholar
57. Saunders K, Simon G, Bush T, et al: Assessing the accuracy of computerized pharmacy refill data for monitoring antidepressant treatment: a comparison of automated and self-report data. Journal of Clinical Epidemiology 51:883–890, 1998Google Scholar
58. Simon G, Lin EHB, Katon W, et al: Outcomes of "inadequate" antidepressant treatment in primary care. Journal of General Internal Medicine 10:663–670, 1995Google Scholar



