Patterns of Psychotropic Drug Prescription for U.S. Patients With Diagnoses of Bipolar Disorders
Bipolar disorders are prevalent, major psychiatric illnesses with high rates of morbidity, comorbidity, disability, and mortality, even early in the course of the illness ( 1 , 2 , 3 , 4 , 5 ). A growing number of innovative treatments for patients given a diagnosis of bipolar disorder—by increasingly broad diagnostic conceptualizations ( 6 , 7 )—have become available in recent years ( 8 , 9 ). Treatments approved by the U.S. Food and Drug Administration, as well as widespread off-label use of such treatments, provide opportunities for complex medication regimens with unpredictable and largely untested effects ( 10 ).
Even though medication treatments have demonstrated short-term efficacy and some evidence of long-term prophylactic effects in bipolar disorder ( 9 ), levels of unresolved morbidity are high ( 2 , 11 , 12 , 13 , 14 , 15 , 16 , 17 ). A large proportion of residual illness is accounted for by major depression, dysthymia, and dysphoric mixed states. Such depressive illness accounts for a third of weeks spent in long-term follow-up with clinical treatment early in the course of bipolar disorder ( 2 , 14 ) or later ( 11 , 12 , 13 , 14 , 15 , 16 , 17 ) and is a major contributor to disability ( 5 ) and to mortality, mainly as a result of suicide ( 3 ). The difficulty of resolving long-term depressive morbidity in patients with bipolar disorder encourages use of antidepressants ( 18 ), despite evidence of inferior benefits in bipolar depression compared with recurrent major depression ( 18 , 19 ) and despite the lack of specific FDA approval of these drugs for the treatment of bipolar disorder ( 8 ).
Not surprisingly, given the many treatment options in the face of clinical challenges of treating patients with bipolar disorder, more varied and complex regimens appear to be increasingly common. This clinical impression is supported by recent surveys in relatively small, selected research samples that may or may not be representative of broader community practices ( 2 , 10 , 20 , 21 , 22 , 23 , 24 ). Specific research data on utilization rates for the growing number of psychotropic drugs used in the treatment of bipolar disorder in broad community samples have not been reported.
Medication utilization data are available in pharmacy claims databases maintained by health maintenance and insurance organizations. If an effective method of identifying patients with probable bipolar disorder can be implemented, utilization data summarizing prescribing patterns of agents used in their treatment should be informative. Large pharmacy databases with nationwide scope can be useful sources of data for investigating utilization rates for specific treatments ( 25 , 26 , 27 ).
In this article we report on a study of psychotropic drugs prescribed for patients diagnosed as having a bipolar disorder, in which we employed a replicable algorithm to identify such patients in a nationwide medical and pharmacy claims database during defined exposure periods for each patient in order to estimate prescription rates for selected classes of drugs. This study addressed three questions: Which agents are selected for initial monotherapy? How long are they continued? How do they later change? Eligible patients who started long-term treatment with multiple psychotropic agents were excluded.
Methods
Data source
We obtained data from the MarketScan research databases for calendar years 2002 and 2003, including the Commercial Claims and Encounters and Medicare Coordination-of-Benefits databases. MarketScan data have been used in many recent research studies, including analyses related to psychiatric treatments ( 26 , 27 ). The Commercial Claims database includes medical and pharmacy claims of employees and their dependents covered by health benefit programs of large employers and for early retirees. The other database contains the same information for Medicare-eligible retirees with employer-provided Medicare Supplemental Plans. Together these files contain fully adjudicated claims information for the sampled years for nearly five million commercially insured lives; about 85% of the beneficiaries in the sample were under age 65 when prescriptions were dispensed. The prescription drug data files included information on the prescription fill date, days of drug supply dispensed, and quantity dispensed, based on National Drug Codes.
MarketScan research databases meet or exceed requirements of the U.S. Health Insurance Portability and Accountability Act (HIPAA) of 1996, including criteria for limited-use datasets, and contain no data elements prohibited by HIPAA. Thomson-Medstat subjected the databases to a third-party statistical analysis to verify that HIPAA requirements for fully deidentified datasets were met. Data use also met ethical standards for anonymous and aggregate research analysis and reporting of data derived from clinical records not requiring specific patient consent.
Diagnoses
Diagnoses of bipolar disorder and subtypes were based on ICD-9 ( 28 ) diagnostic codes 296.x (296.4, bipolar I mania; 296.5, bipolar I major depression; 296.6, bipolar I mixed state; 296.7 or 296.8, bipolar disorder not otherwise specified; and 296.89, bipolar II disorder). Diagnoses and diagnostic subgroups were based on the first or preponderant psychiatric diagnostic categories assigned (in circumstances involving more than one diagnosis) in the claims history of each patient during the study sampling period in inpatient or outpatient settings between July 1, 2002, and November 30, 2003 (latest date of intake), with maximum follow-up to December 31, 2003. We excluded approximately 15,500 patients with bipolar disorder diagnoses who used the psychotropic drugs of interest in the preceding six months to help ensure that patients in the sample were initiating treatment, although possible earlier treatment exposures could not be determined.
This process identified 12,237 patients with bipolar disorder in a base population of 4,211,218 beneficiaries with continuous medical and pharmacy coverage in 2002. Among these 12,237 cases, we identified those with a claim for any drug of interest between July 1, 2002, and November 30, 2003 (the dates of study inclusion) and assigned them to one of eight psychotropic drug groups on the basis of the initial drug provided. Those who were started on two or more psychotropic drugs were excluded. This procedure generated a study sample of 7,760 patients with presumptive bipolar disorder who received a new psychotropic monotherapy, after we excluded 4,477 who received no pharmacotherapy or initial polytherapy.
Drug classes
We employed a retrospective cohort study design to determine the proportions of patients who received prescriptions for psychotropic drugs of interest among four major classes (eight subclasses) of psychotropic agents used clinically to treat patients with bipolar disorder, whether they were FDA approved for such indications or not ( 8 , 9 ). The first drug class, representing proved or putative mood stabilizers, included lithium or certain anticonvulsants (divalproex, the most prevalent option, and the pooled subgroup of carbamazepine, gabapentin, lamotrigine, levetriacetam, oxcarbazepine, topiramate, and zonisamide). The second category, antipsychotic-antimanic agents, included second-generation antipsychotics (clozapine, olanzapine, quetiapine, risperidone and long-acting risperidone, and ziprasidone) and older neuroleptics (chlorpromazine, fluphenazine and fluphenazine decanoate, perphenazine, trifluoperazine, thiothixene, and haloperidol and haloperidol decanoate). The third drug class of interest, antidepressants, included modern agents (serotonin reuptake inhibitors and the atypical agents bupropion, mirtazapine, nefazodone, and trazodone) and older antidepressants (tricyclic antidepressants and monoamine oxidase inhibitors). The fourth class included sedative-anxiolytics of any type (barbiturates, benzodiazepines, and buspirone). Patients were assigned to one of these four mutually exclusive cohorts on the basis of the drug first used by the patient during the sampling period.
Drug use patterns
Drug use is reported as frequency of use and time to a change in regimen. Each patient with bipolar disorder in the sample was followed for a maximum of 365 days, and the case was censored at the date of a first change in treatment. Drug use patterns were defined in three ways. First, discontinuation was considered to have occurred when the days of medication dispensed ended and no refill was provided within another 30 days (and dated at that 30-day point); in addition, only patients for whom drugs were not added or changed before these time limits were reached were included. We also ascertained time gaps in the recorded treatment patterns in order to identify persons likely to have discontinued treatment or to have engaged in intermittent treatment. The second pattern of drug use was switching medication, which was timed as the date of receiving a prescription for a new drug (within the same or another class) within 30 days of the last prescription of the initial drug prescribed. The third pattern was augmentation, or adding a new drug, which was timed at the addition or first prescription of a different type of medication in the same or another class before the initial treatment was discontinued.
Discontinuing, switching, and augmenting were considered mutually exclusive events in a Kaplan-Meier competing-risks survival analysis. Cases were censored at one-year of unaltered treatment or at a first new or altered prescription event of interest. In essence, the analysis describes the initial monotherapy experience of sampled patients with bipolar disorder.
Results
Patient characteristics
From a total sample of 4,211,218 insured persons, we identified 12,237 patients with a bipolar disorder diagnosis made between July 1, 2002, and November 30, 2003, for whom the diagnosis was not present for the preceding six months dating back to January 1, 2002, and we excluded another 15,500 who were prescribed a psychotropic drug, as noted above. These numbers ([12,237±15,500]/4,211,218) suggested a one-year observed prevalence of ICD-9 bipolar disorder of approximately .7%. Because we excluded established and treated cases of bipolar disorder, this rate cannot be considered representative of the prevalence of bipolar disorder in the study population.
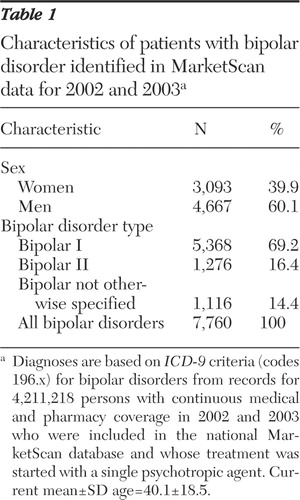
Of the patients with bipolar disorder, 7,760 (63.4%) were prescribed an initial psychotropic monotherapy at some time during the sampling (intake) period of July 1, 2002, through November 30, 2003; these patients formed the study pool. As shown in Table 1 most patients (60.1%) were men, and the mean age was 40.1 years. Diagnoses were as follows: bipolar I, 69.2%; bipolar II, 16.4%; and bipolar disorder not otherwise specified, 14.4%. These figures suggest underrecognition of patients with bipolar II illness, which should be nearly as prevalent as bipolar I disorder ( 1 , 4 ).
 |
Use of specific drug classes
We first determined overall numbers and proportions of patients prescribed the four major classes (eight subclasses) of drugs for initial psychotropic monotherapy ( Table 2 ). Rates of use were as follows: antidepressants, 49.8% (modern, 47.4%, and older agents, 2.4%); mood stabilizers, 24.6% (divalproex, 8.3%; other anticonvulsants, 8.8%; and lithium salts, 7.5%); sedative-anxiolytics, 14.8%; and antipsychotics, 10.7% (second-generation, 10.1%, and older neuroleptics, .6%).
 |
Incidence of polytherapy
We selected January 1, 2003, a midpoint of the two-year study, for a cross-sectional analysis of psychotropics prescribed per patient for each of five major categories of initial monotherapies: antidepressants, lithium, antipsychotics, anticonvulsants, and sedative-anxiolytics. As Table 3 shows, a majority of patients who had started with a single drug were receiving two or more psychotropics on the selected date (55.6%: 56.7% of the patients with bipolar I and 50.6% of those with bipolar II disorder). This rate of use of two or more psychotropic drugs probably underestimates the rate that would be found after a longer follow-up period or with more inclusive sampling, because the sampling method excluded patients treated for more than 12 months as well as those who started with more than one psychotropic agent.
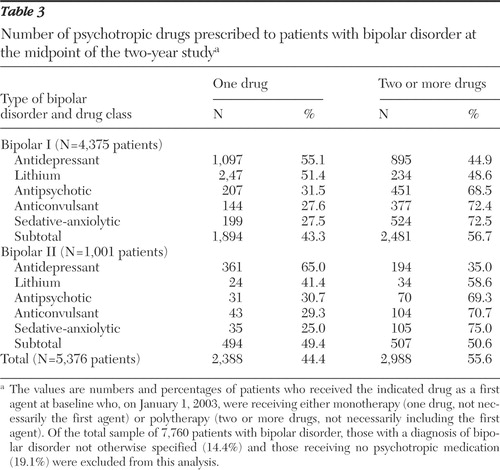 |
As shown in Table 3 , for patients with bipolar I disorder and those with bipolar II disorder the respective proportions who remained on an initial monotherapy were as follows: antidepressants, 55.1% and 65.0%; lithium, 51.4% and 41.4%; antipsychotics, 31.5% and 30.7%; anticonvulsants, 27.6% and 29.3%; and sedative-anxiolytics, 27.5% and 25.0%. That is, antidepressants were the preferred monotherapy for bipolar II disorder (mainly depressed subtype) as well as for bipolar I disorder, whereas lithium was the preferred mood-stabilizing agent for monotherapy among patients with either type I or type II bipolar disorder.
Survival analysis of initial monotherapy
On the basis of Kaplan-Meier survival methods, we computed times (and 95% confidence intervals [CIs]) to augmentation or discontinuation of each of the eight subcategories of initially prescribed drug types. Changing agents was rare, and patients in this category were excluded from the analysis.
As shown in Table 4 the rank order of drugs in terms of median weeks to discontinuation of the initial drug were as follows: lithium (58.3 weeks), antipsychotics (older neuroleptics, 57.1, and new antipsychotics, 29.1), antidepressants (modern antidepressants, 43.6, and older antidepressants 28.3), anticonvulsants (divalproex, 36.1, and 29.1 for other agents), and sedatives (30.0 weeks).
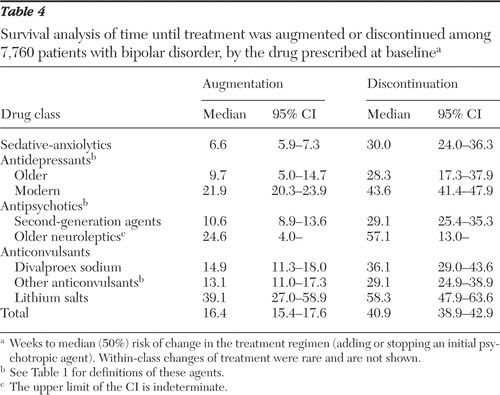 |
As shown in Table 4 adding one or more drugs was the earliest option selected by prescribing physicians; the median time to supplementing treatment was 2.5 times less than the median time to discontinuing an initially prescribed agent—16.4 weeks compared with 40.9 weeks. Treatment augmentation occurred in the following order of timing (weeks to median [50%] risk in the survival analysis): lithium (39.1 weeks), serotonin reuptake inhibitors and other modern antidepressants (21.9), divalproex (14.9), anticonvulsants other than divalproex (13.1), second-generation antipsychotics (10.6), older antidepressants (9.7), and sedative-anxiolytics (6.6).
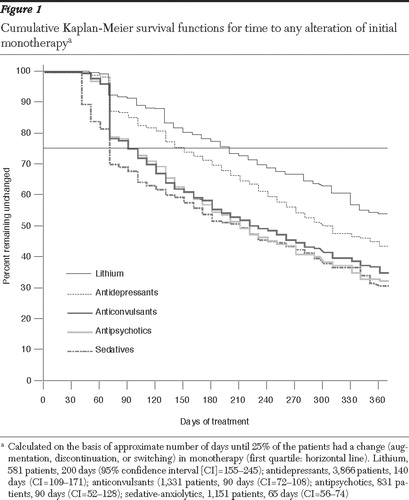
For illustration, we computed Kaplan-Meier survival functions for time to any alteration (augmentation, discontinuation, or switching) of initial monotherapy with lithium, antidepressants, anticonvulsants, antipsychotics, or sedatives. In terms of initial monotherapy, days until 25% of patients (first quartile) experienced an alteration in treatment (augmentation or discontinuation) were ranked as follows: lithium (200 days, CI=155-245), antidepressants (140, CI=109-171), anticonvulsants (90, CI=72-108), antipsychotics (90, CI=52-128), and sedative-anxiolytics (65, CI=56-74). Retention times (days) were unrelated to the frequency (percent) of initial drug selection; for example, lithium was selected relatively infrequently (7.5% of all patients) but retained as a monotherapy longer than other drugs (200 days to 25% alteration). Some drugs, particularly anticonvulsants, showed sharp decrements of monotherapy after one or two months, evidently reflecting typical prescription refill cycles of 30 days or multiples thereof ( Figure 1 ). Overall, these findings indicate a tendency to retain lithium and antidepressants longer than any other initial monotherapy for bipolar disorder.
Discussion
This study of computerized medical benefits data found a one-year prevalence of bipolar disorder of .7% compared with the expected U.S. one-year prevalence of 2.6±.2% ( 4 ). This low rate probably reflects exclusion of patients who were already receiving polytherapy and a sample that consisted only of insured patients with pharmacy benefits; most were employed or otherwise relatively high functioning, so that severely ill or disabled patients with bipolar disorder, as well as elderly, very young, or untreated persons with bipolar disorder, may not have been well represented. The prevalence of bipolar II disorder was only about one-quarter that of bipolar I disorder ( Table 1 ), whereas current estimates suggest that the prevalence of bipolar II disorder may be at least as high as the prevalence of bipolar I disorder and possibly similar to the rate for major depression ( 29 , 30 ). In addition, patients with bipolar disorder may have been misdiagnosed or undiagnosed. Patients with bipolar disorder who have relatively moderate symptoms, particularly those with bipolar II disorder, are likely to be misdiagnosed as having (unipolar) major depression or other disorders, whereas misdiagnosis of nonbipolar major depression as bipolar disorder seems much less likely ( 30 , 31 ).
That a majority of patients were men is surprising because women usually predominate in treated samples of patients with mood disorders ( 2 , 11 , 12 , 13 , 14 , 15 , 16 , 17 ). This finding may again reflect the insured status of patients in the databases used for study. The average age of 40 years for patients nominally starting new treatment regimens may seem much higher than the median onset age of 20 years for bipolar disorder ( 32 ), but delays of five to ten years in diagnosis and institution of sustained treatment among patients with bipolar disorder are typical internationally ( 3 , 33 ). However, the possibility of previous treatment trials more than six months before study entry cannot be excluded.
A striking finding was a high prevalence (49.8%) of antidepressant treatment as an initial monotherapy for patients with bipolar I disorder as well as those with bipolar II disorder; serotonin reuptake inhibitors and other modern antidepressants were preferred over older agents, such as tricyclics, by 20 to 1 ( Table 2 ). Monotherapy with modern antidepressants also persisted longer (a median of 21.9-43.6 weeks) than any other treatment except lithium (39.1-58.3 weeks) ( Table 4 ). High rates of antidepressant use among patients with bipolar disorder also have been noted in other recent American studies ( 18 , 19 , 20 , 26 , 27 , 29 , 31 , 34 , 35 ). Such treatment contributes substantially to the cost of care for patients with bipolar disorder ( 26 ) and carries risks of adverse clinical effects ( 7 , 18 , 19 ), although quantitative risks of inducing mania or mood destabilization remain uncertain among patients with bipolar I disorder ( 34 ) and may be more limited among patients with bipolar II disorder ( 35 ).
The high observed rates of antidepressant use probably reflect the clinically compelling nature of depression, dysthymia, and dysphoria as the major residual morbidity among patients with bipolar disorder, despite ongoing treatment ( 11 , 12 , 13 , 14 , 15 , 16 , 17 ), making it a clinically plausible target for treatment with modern, relatively well-tolerated antidepressants. Nevertheless, use of antidepressants for patients with bipolar disorder is not specifically FDA approved, may be relatively ineffective and behaviorally risky ( 7 , 18 , 19 ), and requires more research to evaluate its efficacy and safety in bipolar depression.
Initial treatment of patients identified as having bipolar disorder was least likely to involve antipsychotic drugs (10.7%); when such drugs were used, second-generation agents were preferred by 17 to 1 over older neuroleptics ( Table 2 ). Anticonvulsants with antimanic or putative mood-stabilizing actions were selected at intermediate rates (17.1%), and a substantial minority of patients (14.8%) started with a benzodiazepine or other sedative. However, anticonvulsants and second-generation antipsychotics, as well as sedative-anxiolytics, were maintained as monotherapies for much less time than either lithium or antidepressants. Adding one or more psychotropics to an initial monotherapy was the most common type of alteration of initial treatment regimens. The median time to augmenting anticonvulsants was 14.0 weeks; the time for antipsychotics was 11.4 weeks, and for sedatives, only 6.6 weeks ( Table 4 ).
Lithium was selected for only 7.5% of patients with bipolar disorder initially, but it was retained in monotherapy much longer than other treatments, including antidepressants, continuing for approximately 200 days (28.6 weeks) before being altered for 25% of patients (first quartile) and 58.3 weeks before being altered for 50% of patients (median) ( Figure 1 and Table 4 ). The tendency for lithium monotherapy to be retained longer than other treatments and to be the only agent with substantial long-term use as a monotherapy has been noted in other smaller U.S. samples of clinically treated patients with bipolar disorder ( 2 , 13 ). In international practice, and particularly in specialized mood disorder clinics, lithium continues to be a cornerstone of long-term prophylactic treatment ( 8 , 23 , 33 , 36 , 37 ). Moreover, lithium is the only treatment with strong and consistent evidence of reducing the risk of suicide and attempts and of limiting the potential lethality of suicidal acts ( 3 , 38 , 39 , 40 ) in a disorder with prominent residual depressive morbidity ( 11 , 12 , 13 , 14 , 15 , 16 , 17 ).
At the midpoint of the study a majority of patients (55.6%) were being treated with two or more psychotropic agents simultaneously, indicating that most U.S. patients with bipolar disorder (both type I and II) eventually received polytherapy ( Table 3 ). This rate, although high, probably underestimates polytherapy at later times, particularly because some patients who were sampled at the study midpoint had been treated for less than a year. It is also noteworthy that at the midpoint 19.1% of patients with bipolar disorder were receiving no medication ( Table 3 ).
Although this study is based on a large community sample of patients diagnosed as having bipolar disorder, it has notable limitations. First, the sample is observational and nonrandomized. Second, it is derived from a national medical benefits database involving insured persons, most of whom were or had been working. As such, it is likely to underrepresent indigent, severely ill, disabled, elderly, or very young patients with bipolar disorder. Diagnoses are based on clinical categorization for insurance purposes and were not verified by direct examination. Prescription-based data on drugs dispensed and available for use may not closely parallel actual drug utilization rates. We could not evaluate clinical benefits or adverse effects of specific treatments, nor could we exclude treatment trials that occurred more than six months before study intake. Finally, we selected only patients who were started on one psychotropic drug in order to address the specific aims of this study to identify agents selected first for monotherapy and to determine how long this continued and how it changed. Additional studies are required to evaluate clinical practices in regard to when and how polytherapy is employed in contemporary community samples of patients with bipolar I disorder and those with bipolar II disorder ( 10 , 20 , 23 ).
Despite the limitations of the findings, they add to other recent, congruent observations in smaller clinical samples and they support clinical impressions. They document recent clinical practices and suggest experimental hypotheses and topics for potential quality improvement testing in regard to the relative utility of antidepressants and specific mood-stabilizing agents. Such studies are urgently needed in view of the high levels of persisting depressive morbidity among patients with bipolar disorder ( 11 , 12 , 13 , 14 , 15 , 16 , 17 ) and the poorly studied status of the therapeutics of bipolar depression ( 7 ). It is also noteworthy that lithium was used relatively infrequently as an initial monotherapy but was sustained longer without supplementation than other antimanic or putative mood-stabilizing anticonvulsant or second-generation antipsychotic drugs. These findings add to the impression that lithium has been undervalued and remains underutilized in American psychiatry. Finally, the findings add to the strong impression that most U.S. patients with bipolar disorder are treated over the long term with various combinations of psychotropic agents, even though most have not been tested empirically for effectiveness or safety ( 8 , 10 , 20 ) and despite the high cost of most modern mood-stabilizing drugs other than lithium.
Acknowledgments and disclosures
The study was supported in part by a research contract from Novartis Pharmaceuticals Corporation (JH and RJB), by a grant from the Bruce J. Anderson Foundation, and by the McLean Private Donors Research Fund for Bipolar Disorder and Psychopharmacology (RJB). This report is dedicated to the memory of the authors' esteemed, late colleague, senior biostatistician John Hennen, Ph.D. During the study Dr. Baldessarini and Dr. Hennen were consultants to Novartis Corporation, and Dr. Zhang was under contract by Novartis Corporation. Dr. Leahy, Dr. Arcona, and Dr. Gause are employees of Novartis Corporation.
Dr. Baldessarini is a consultant or research collaborator with Auritec, Biotrofix, IFI, Janssen, JDS, Lilly, Merck, NeuroHealing, Novartis, SK-Biopharmaceuticals, and Solvay Corporations, some of which produce treatments for mood disorders. Competing interests of other authors are noted above.
1. Goodwin FK, Jamison KR: Manic-Depressive Illness. New York, Oxford University Press, 2000Google Scholar
2. Tohen M, Zarate CA Jr, Hennen J, et al: McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. American Journal of Psychiatry 160:2099-2107, 2003Google Scholar
3. Tondo L, Isacsson G, Baldessarini RJ: Suicide in bipolar disorder: risk and prevention. CNS Drugs 17:491-511, 2003Google Scholar
4. Kessler RC, Chiu WT, Demler O, et al: Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey replication. Archives of General Psychiatry 62:617-627, 2005Google Scholar
5. Huxley N, Baldessarini RJ: Functional outcome in bipolar disorder. Bipolar Disorders, in pressGoogle Scholar
6. Baldessarini RJ: A plea for integrity of the bipolar disorder concept. Bipolar Disorders 2:3-7, 2000Google Scholar
7. Ghaemi SN, Baldessarini RJ: Kraepelin's ghost: revival of the manic-depressive spectrum. Psychotherapy and Psychosomatics, in pressGoogle Scholar
8. Baldessarini RJ: Drug therapy of depression and anxiety disorders, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed. Edited by Brunton LL, Lazo JS, Parker KL. New York, McGraw-Hill, 2005Google Scholar
9. Baldessarini RJ, Tarazi FI. Pharmacotherapy of psychosis and mania, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed. Edited by Brunton LL, Lazo JS, Parker KL. New York, McGraw-Hill, 2005Google Scholar
10. Ghaemi SN (ed): Polypharmacy in Psychiatry. New York, Dekker, 2002Google Scholar
11. Judd LL, Akiskal HS, Schettler PJ, et al.: Long-term natural history of the weekly symptomatic status of bipolar I disorder. Archives of General Psychiatry 59:530-537, 2002Google Scholar
12. Judd LL, Akiskal HS, Schettler PJ, et al: Prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Archives of General Psychiatry 60:261-269, 2003Google Scholar
13. Post RM, Denicoff KD, Leverich GS, et al: Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. Journal of Clinical Psychiatry 64:680-690, 2003Google Scholar
14. Baldessarini RJ, Salvatore P, Tohen M, et al: Morbidity from onset in first-episode bipolar I disorder patients: the International-300 study. Neuropsychopharmacology 29(suppl 1):S88, 2004Google Scholar
15. Joffe RT, MacQueen GM, Marriott M, et al: Prospective, longitudinal study of percentage of time spent ill in patients with bipolar I or bipolar II disorders. Bipolar Disorders 6:62-66, 2004Google Scholar
16. Judd LL, Akiskal HS, Schettler PJ, et al: Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Archives of General Psychiatry 62:1322-1330, 2005Google Scholar
17. Perlis RH, Ostacher MJ, Patel JK, et al: Predictors of recurrence in bipolar disorder: primary outcomes from the systematic treatment enhancement program for bipolar disorder (STEP-BD). American Journal of Psychiatry 163:217-224, 2006Google Scholar
18. Ghaemi SN, Hsu DJ, Soldani F, et al: Antidepressants in bipolar disorder: the case for caution. Bipolar Disorders 5:421-433, 2003Google Scholar
19. Ghaemi SN, Rosenquist KJ, Ko JY, et al: Antidepressant treatment in bipolar vs unipolar depression. American Journal of Psychiatry 161:163-165, 2004Google Scholar
20. Frye MA, Ketter TA, Leverich GS, et al: Increasing use of polypharmacotherapy for refractory mood disorders. Journal of Clinical Psychiatry 61:9-15, 2000Google Scholar
21. Centorrino F, Goren JL, Hennen J, et al: Multiple vs single antipsychotic agents for hospitalized psychiatric patients: case-control study of risks vs benefits. American Journal of Psychiatry 161:700-706, 2004Google Scholar
22. Centorrino F, Fogarty KV, Sani G, et al: Antipsychotic drug use: McLean Hospital, 2002. Human Psychopharmacology 20:355-358, 2005Google Scholar
23. Baethge C, Baldessarini RJ, Mathiske-Schmidt K, et al: Long-term combination therapy versus monotherapy with lithium and carbamazepine in 46 bipolar I patients. Journal of Clinical Psychiatry 66:174-182, 2005Google Scholar
24. Lin D, Mok H, Yatham KN: Polytherapy in bipolar disorder. CNS Drugs 20:29-42, 2006Google Scholar
25. Cohen FJ, Neslusan CA, Conklin JE, et al: Recent antihyperglycemic prescribing trends for US privately insured patients with type 2 diabetes. Diabetes Care 26:1847-1851, 2003Google Scholar
26. Matza LS, Rajagopalan KS, Thompson CL, et al: Misdiagnosed patients with bipolar disorder: comorbidities, treatment patterns, and direct treatment costs. Journal of Clinical Psychiatry 66:1432-1440, 2005Google Scholar
27. Harpaz-Rotem I, Rosenheck RA: Prescribing practices of psychiatrists and primary care physicians caring for children with mental illness. Child Care Health and Development 32:225-237, 2006Google Scholar
28. International Classification of Diseases, Ninth Revision, Clinical Modification. Geneva, World Health Organization, 1975Google Scholar
29. Akiskal HS, Benazzi F: Optimizing the detection of bipolar II disorder in outpatient private practice: toward a systematization of clinical diagnostic wisdom. Journal of Clinical Psychiatry 66:914-921, 2005Google Scholar
30. Bauer M, Pfennig A: Epidemiology of bipolar disorders. Epilepsia 46(suppl 4):8-13, 2005Google Scholar
31. Olfson M, Das AK, Gameroff MI, et al: Bipolar depression in a low-income primary care clinic. American Journal of Psychiatry 162:2146-2151, 2005Google Scholar
32. Faedda GL, Baldessarini RJ, Suppes T, et al: Pediatric-onset bipolar disorder: a neglected clinical and public health problem. Harvard Review of Psychiatry 3:171-195, 1995Google Scholar
33. Baethge C, Baldessarini RJ, Bratti IM, et al: Prophylaxis-latency and outcome in bipolar disorders. Canadian Journal of Psychiatry 48:449-457, 2003Google Scholar
34. Visser HM, Van der Mast RC: Bipolar disorder, antidepressants, and induction of hypomania or mania: a systematic review. World Journal of Biological Psychiatry 6:231-241, 2005Google Scholar
35. Altshuler LL, Suppes T, Black DO, et al: Lower switch rate in depressed patients with bipolar II than bipolar I disorder treated adjunctively with second-generation antidepressants. American Journal of Psychiatry 163:313-315, 2006Google Scholar
36. Tondo L, Baldessarini RH, Floris G: Long-term effectiveness of lithium maintenance treatment in types I and II bipolar disorders. British Journal of Psychiatry 178(suppl 40):184-190, 2001Google Scholar
37. Baldessarini RJ, Tondo L, Hennen J, et al: Is lithium still worth using? Update of selected recent research. Harvard Review of Psychiatry 10:59-75, 2002Google Scholar
38. Tondo L, Baldessarini RJ, Hennen J, et al: Lithium treatment and risk of suicidal behavior in bipolar disorder patients. Journal of Clinical Psychiatry 59:405-414, 1998Google Scholar
39. Cipriani A, Pretty H, Hawton K, et al: Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. American Journal of Psychiatry 162:1805-1819, 2005Google Scholar
40. Baldessarini RJ, Tondo L, Davis P, et al: Decreased suicidal risk during long-term lithium treatment: Meta-analysis. Bipolar Disorders 8:625-639, 2006Google Scholar



