Strategies to Decrease Costs of Prescribing Selective Serotonin Reuptake Inhibitors at a VA Medical Center
Abstract
OBJECTIVE: The authors examined the efficacy of a multifaceted intervention designed to contain the cost of prescribing selective serotonin reuptake inhibitors (SSRIs) to inpatients and outpatients served by a Veterans Affairs (VA) medical center. METHODS: Elements of the intervention included identification of a preferred agent, tablet splitting, education and feedback for prescribers, and an electronic record and ordering system to facilitate changes in prescriber behaviors. VA databases were searched for information on use and costs of antidepressants. RESULTS: Over 35 months the number of patients treated with SSRIs and the amount spent on SSRIs increased. However, the mean monthly cost per patient decreased from $57.12 to $42.19. The projected cost savings over the 35 months was approximately $700,000; one-fourth of the savings was due to tablet splitting and three-fourths to changes in the proportions of the various SSRIs prescribed. A survey of the top 75 antidepressant prescribers showed that after the educational interventions, 91 percent were aware that citalopram was the medical center's preferred antidepressant, and 59 percent identified it as their own preferred first-line treatment. DISCUSSION AND CONCLUSIONS: The results suggest that multifaceted interventions can influence antidepressant costs through provider education and changes in pharmacy and computerized information processes, resulting in substantial cost savings for institutions.
Depression results in considerable societal costs in terms of morbidity, direct health care costs, and indirect costs due to lost productivity (1). Although treatment of depression may decrease the use of some services (2,3,4), efforts to improve treatment of depression have generally not decreased net costs to institutions and payers (5). There are thus two important but competing aims: identification and treatment of depressed patients and cost containment.
National spending for all antidepressants increased by 600 percent during the 1990s (6), and annual expenditures for selective serotonin reuptake inhibitors (SSRIs) in the United States exceeded $7 billion in 2000 (7). SSRIs and other newer antidepressants are as effective as older, less expensive agents and are better tolerated, easier to use, and less toxic in overdose than the older drugs (8,9,10). Most of the recent increase in spending for antidepressant treatment reflects increased use rather than increases in drug prices (6,11).
A number of interventions have been used by institutions and payers to influence drug costs, including eliminating drugs from formularies, negotiating contracts with pharmaceutical companies, using generic products, and encouraging use of less expensive products. Organizations have also used cost-sharing strategies (6,12), utilization controls (such as formulary barriers) (6), attempts at dosage reduction (13), flags requiring consultation with a mental health specialist (14), and identification of preferred agents (15). Recent studies have described the potential for decreasing costs through the use of tablet splitting (16,17).
Depression is common in the veteran patient population (18), and the Veterans Healthcare Administration (VHA) recently mandated screening for depression as one of several preventive care initiatives. Nationally, the Department of Veterans Affairs (VA) spent $78 million on antidepressants to treat 650,000 veterans—19 percent of all VA outpatients—in the second half of fiscal year 2000 (19). During 1998, at the Portland VA Medical Center, monthly acquisition costs for all antidepressants increased by 50 percent. A total of $1.1 million was spent on SSRIs in 1998, representing 87 percent of acquisition costs for all antidepressants.
In early 1999, a work group was formed at the medical center to analyze the costs associated with antidepressant prescription and contain them as much as possible. In this paper, we examine the multifaceted intervention developed by the work group and the outcomes associated with the intervention.
Methods
Setting
The patient population of the Portland VA Medical Center is approximately 95 percent male and 95 percent white. About 20,000 patients are followed in the primary care division. The primary care clinics have 47 staff providers (38 physicians and nine nurse practitioners and physician assistants) and 47 internal medicine residents linked with staff physicians. The mental health clinic treats approximately 8,000 patients annually and has 42 staff who prescribe antidepressants (24 physicians and 18 nurse practitioners). Primary care and mental health clinic staff have separate monthly administrative meetings. Providers regularly use an internal VA e-mail system (VISTA) to communicate about administrative and patient-care issues.
Developing the interventions
The work group included psychiatrists, internists, pharmacists, and computer information specialists and met for an hour every other month. During the period of intervention development and implementation, no members of the work group received support from pharmaceutical companies.
The work group focused on identifying strategies likely to reduce the cost per patient treated for depression. Because substantially more money was being spent on SSRIs than on other antidepressants, the group targeted SSRI prescriptions, specifically citalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline. The first strategy was aimed at optimizing the use of tablet splitting. For example, it is less expensive to use half a 40 mg tablet of paroxetine than a whole 20 mg tablet, as shown in Table 1. The second strategy was aimed at modifying provider behavior by emphasizing the use of a preferred drug. The goal was to increase the "market share" of the preferred antidepressant—that is, the use of the preferred SSRI in proportion to total SSRI use.
The work group chose not to eliminate access to any particular antidepressant or class of antidepressant: overly stringent formulary restrictions have been shown to negatively affect outcomes (20). Nor did we attempt to switch patients from one antidepressant to another. Although there are no known significant differences in efficacy among antidepressants (21), we did not want to create instability among patients who were stable on their current antidepressants. We focused primarily on influencing the choice of antidepressant for patients who were beginning treatment for depression.
We also did not want to encourage an increase in tricyclic antidepressant use. VA outpatients are often on complex medication regimens and have worse health status, and more comorbid cardiac conditions, than do non-VA patients (22). Additionally, although SSRIs cost more than tricyclic antidepressants, total health care costs have been shown to be at least offset by reductions in other costs associated with the use of SSRIs (10,23,24).
The work group identified citalopram as the preferred antidepressant on the basis of its lower cost, as shown in Table 1, and an efficacy and safety profile at least comparable to that of other SSRIs.
During the last quarter of 2000, 80 of 440 antidepressant prescribers at the Portland VA Medical Center (18 percent) wrote 3,300 of 4,000 antidepressant prescriptions a month (83 percent). The work group focused on influencing the prescribing behaviors of these top 80 antidepressant prescribers. Of these 80 top prescribers, 43 (54 percent) were primary care providers and 29 (36 percent) were mental health clinicians.
Intervention types
Tablet splitting.Portland VA Medical Center pharmacists began tablet splitting for selected medications in early 1999, and the work group identified sertraline, paroxetine, and citalopram as targets for tablet splitting. Higher-dose tablets of these medications are scored in the middle. Patients were provided with tablet splitters, which cost $2.18 each, and given instructions by pharmacists on how to split their own tablets. Although the pharmacy was able to fairly consistently substitute half-dose tablets when appropriate on initial fills, there were barriers to developing a process to substitute higher-dose tablets after initial fills. Most refills in the VA system are done not locally but through centralized mail-out pharmacies. Providers often increase the dose of antidepressants gradually and thus frequently continue to prescribe lower-dose tablets after initial fills.
To help address this problem, in June 2000 the work group sent individual mailings to providers. Providers were given a list of all their patients who were taking tablets of paroxetine 20 mg, sertraline 50 mg, and citalopram 20 mg. The providers were asked whether some patients could appropriately be switched to half-tablet dosing and were requested to make this change for those patients. Clinical pharmacists sometimes helped with the changes. Beginning in June 2001, the pharmacy dedicated staff time to reviewing refill records for patients receiving low-dose SSRI tablets and substituting higher-dose tablets when appropriate. Patients and providers were notified when a change in tablet strength would take place.
Provider education and feedback.In April 1999, members of the work group began discussing the costs of SSRIs at meetings of the primary care division and the mental health division. In April 2000, in these meetings and through an e-mail message, citalopram was identified as the Portland VA Medical Center's preferred antidepressant. In August 2000, condensed VA depression treatment guidelines (25), including information about costs, were distributed to primary care and mental health providers. At various meetings in January 2001, the work group further designated certain antidepressants as first-line or second-line agents (primarily for cost reasons). Citalopram, sertraline, and paroxetine were labeled as first-line, and bupropion, fluoxetine, fluvoxamine, mirtazapine, and nefazodone were identified as second-line agents.
In June 2001, a total of 75 of the 80 top prescribers were sent anonymous surveys (five of the 80 prescribers had left the institution). The surveys asked about their prescribing practices and asked whether they were aware that the institution had identified a preferred antidepressant. Providers were subsequently sent an e-mail message with the results of the survey and an educational message about citalopram. In August 2001 these providers were sent confidential individual feedback about their use of the various SSRIs and another educational message about citalopram.
Computerized information interventions. In 1999 the medical center began using an electronic patient record. The software contains a medication-ordering package with drop-down menus and the ability to send comments to users. In mid-2000, comments on the value of using half tablets when possible began to be sent to users as they ordered antidepressants. In February 2001, providers began to be notified about the preferred antidepressant when any antidepressant was ordered. Antidepressants other than citalopram could still be ordered, but only after the prescriber viewed the message about citalopram's being the preferred antidepressant. The February 2001 update also automatically implemented half-tablet dosing, when appropriate, whenever a medication was ordered.
Analysis
The primary source of data was the Consumer Health and Information Performance Sets (CHIPS) database of the Veterans Integrated Service Network 20. CHIPS is a relational database that can be accessed by means of structured query language (SQL) and contains relevant data from the medical center's computer (VISTA). Initial queries about prescription fills for all SSRIs and tablet strengths were made. Records were then sorted by date and patient, and patient records were counted to determine the number of individual patients. Costs were determined by multiplying the quantity of each SSRI tablet strength prescribed by the cost per tablet recorded in the database.
As a form of verification, reports were obtained from VISTA with a software routine, Fileman, by another investigator. These reports provided outpatient pharmacy prescription data on a per-fill basis. Data were extracted monthly on each dosage of antidepressant, the number of fills, the total number of tablets, and the cost per tablet. Data obtained by using the two approaches were found to be consistent.
Because the first interventions began in early 1999, we defined our study period as January 1999 through November 2001. To correct for variations over the study period in prescription of 90-day supplies of antidepressants, cost per patient per month was defined as cost per patient per 30-day equivalent of medication. Market share for each SSRI was defined as the ratio of the number of patients taking a specific SSRI to the number of patients taking all SSRIs.
To assess the implementation of tablet splitting, we calculated ratios of higher-dose to lower-dose tablets prescribed per month. By calculating these ratios, we controlled for changes in market share and changes in the number of patients for whom medications were prescribed over the study period.
We determined projected cost savings due to tablet splitting by calculating the proportion of the total monthly antidepressant dosage for each tablet strength used in January 1999 and projecting those proportions forward to determine what the costs would have been had the proportions not changed. We calculated projected cost savings due to changes in market share by calculating the proportions of patients taking the various SSRIs in January 1999 and projecting these proportions forward to determine what costs would have been had market share changes not taken place.
Results
Over the study period, total monthly SSRI costs increased, from a minimum of $97,858 in October 1999 to a maximum of $124,883 in October 2001. The number of patients per month for whom SSRIs were prescribed also increased, from a minimum of 1,892 in February 1999 to a maximum of 2,909 in October 2001. During that time, the total number of patients at the medical center for whom any type of medication was prescribed increased from 13,177 to 17,213 per month. SSRI acquisition costs remained essentially stable, as shown in Table 1.
The mean cost per patient per month for SSRIs declined over the entire study period, from $54.52 in January 1999 to $42.19 in November 2001. This cost reached a maximum of $57.12 in March 1999 and a minimum of $40.43 in September 2001.
The ratios of higher-dose to lower-dose tablets prescribed increased over the study period. In January 1999 the ratio of the number of sertraline 100 mg tablets to 50 mg tablets prescribed was 6.5. By November 2001, this ratio had increased to 11.4. The ratio of the number of paroxetine 40 mg tablets to 20 mg tablets prescribed in January 1999 was .34; by November 2001 it had increased to 3.0. Citalopram 40 mg tablets were not introduced at the medical center until January 2000. The ratio of the number of citalopram 40 mg to 20 mg tablets prescribed increased from .79 in January 2000 to 3.5 in November 2001.
The market shares of the various SSRIs prescribed also changed in important ways (Figure 1). Citalopram was first prescribed at the medical center in January 1999, and its use steadily increased. In November 2001, citalopram was prescribed for 1,275 patients at a cost of $36,694, representing one-third of the dollars spent on SSRIs. The market share of citalopram among SSRIs steadily increased to a maximum of 47 percent in November 2001. Meanwhile, the number of patients receiving sertraline decreased from a maximum of 1,020 in March 1999 to a minimum of 572 in November 2001, a decrease in SSRI market share from 49 percent to 21 percent. The SSRI market shares of fluoxetine, paroxetine, and fluvoxamine also decreased somewhat.
The one-month cost savings projected for November 2001 with January 1999 baseline data was $38,588. Tablet splitting contributed $7,622 (20 percent) to the cost savings that month, and market share changes contributed $30,966 (80 percent). Over the study period, tablet splitting accounted for about 25 percent of total cost savings, and changes in market share accounted for about 75 percent. Savings due to sertraline tablet splitting were generally less than $1,000 per month, whereas savings from paroxetine tablet splitting were more substantial.
In June 2001, the top 75 prescribers of antidepressants were mailed a survey to be filled out anonymously; it consisted of three questions: "Do you know which antidepressant is now considered the preferred choice for first-line treatment of depression at the Portland VA Medical Center (due to cost reasons)?" "Do you have a preferred first-line antidepressant medication?" and "If yes, why is this your preferred first-line agent?" Of the 75 providers, 64 (85 percent) returned surveys. Of these 64 providers, 58 (91 percent) were aware that the VA had identified a preferred antidepressant (citalopram), and 38 (59 percent) identified citalopram as their own preferred choice for first-line treatment. The most commonly cited reasons for citalopram preference were cost, efficacy, and safety profile. Other first choices were sertraline, three providers (about 5 percent); paroxetine, two providers (2.5 percent); and venlafaxine, one provider (about 1 percent). Twenty of the 64 providers (about 31 percent) did not identify a first choice.
Discussion and conclusions
Over a 35-month period, the total number of patients treated with SSRIs and the total amount spent by the institution on SSRIs increased. However, the monthly cost per patient treated with SSRIs decreased, resulting in a total projected cost savings of about $700,000 over the 35-month period. Savings were realized by persuading providers to use a preferred antidepressant and by instituting tablet splitting. Most of the projected cost savings was due to changes in market share. A survey of the medical center providers who prescribed most of the antidepressants showed that most were aware of the identification of a preferred antidepressant.
This multifaceted approach to influencing provider behavior has a number of advantages. First, it does not use stringent formulary restrictions. Instead, it relies to a large extent on education and on reminders about costs. Second, the approach can be readily generalized to other medication classes and clinical scenarios. Third, the interventions, which are supported by computer information system changes, are designed to minimize the use of staff hours for implementation.
One of the major limitations of this study was the absence of a clear comparison group. We know that national VA pharmacy costs have increased considerably in recent years and that antidepressants as a group have become one of the most frequently prescribed types of medications (19). However, we were not able to identify comparable data on trends in specific cost per treated patient over time for the VA nationwide. However, to provide a regional comparison, Oregon Medicaid data show an increase in cost per patient treated with SSRIs over a period almost the same as that of our study period: In January 1999 the average cost per claim for SSRIs (30-day fill) was $75; by September 2001, the average cost per claim had increased to $85 (unpublished data, Ketchum K, Oregon State University, consultant to State of Oregon, Office of Medical Assistance, 2001).
The study had other limitations. Which specific interventions most effectively influenced provider behavior was not examined. It is highly likely that citalopram would have increased in market share starting from its introduction at the medical center in January 1999 regardless of our intervention. It is possible that other factors contributed to changes in prescribing practices and costs over time.
Finally, the intervention was limited in focus. Aside from identifying a preferred antidepressant and labeling some antidepressants as second-line, the work group did not specifically target other new antidepressants. Indeed, between January 2000 and November 2001, monthly costs for venlafaxine, bupropion, mirtazapine, and nefazodone increased from $28,692 to $53,352, and the contribution of these medications to the overall antidepressant budget increased from 21 percent to 29 percent.
However, these changes in the use of non-SSRI antidepressants do not substantially weaken our findings, for several reasons. First, our calculations are not affected by changes in prescriptions of non-SSRI antidepressants, because we examined projected cost savings in the SSRI class alone. Second, despite an increase in the use of non-SSRI agents, there was still a net decrease in the total cost per month for all antidepressants over the study period: The mean cost per patient per month for all antidepressants was $53.04 in January 1999, reached a peak of $56.45 in March 1999, and decreased to $47.18 in November 2001.
Clearly, the increasing use and the costs of the newest antidepressants present an important problem to address. Our work group is currently studying whether the newest antidepressants are being used as first-line agents or are being tried after failed trials of SSRIs.
Our results suggest that multifaceted interventions can influence antidepressant costs through provider education and changes in pharmacy and information management processes, resulting in significant cost savings for institutions. Importantly, our approach was designed not to limit therapeutic options or create barriers to effective treatment of depression. Many of our approaches can be used for other drug classes and in institutions other than those of the Department of Veterans Affairs. A controlled study of these interventions would be useful.
Acknowledgments
This research was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service Project MHI 20-020-1. The authors thank the members of the antidepressant cost reduction workgroup; Ron Brown for assistance in obtaining local pharmacy data; Chester Good, M.D., M.P.H., and Robert Rosenheck, M.D., for information about VA costs; and Kathy Ketchum, R.Ph., M.P.A., for assistance in obtaining and understanding Oregon Medicaid data.
The authors are affiliated with the Portland Veterans Affairs Medical Center in Portland, Oregon. Dr. Dobscha, Dr. Hoffman, Dr. Turner, and Dr. Hauser are with the behavior health and clinical neurosciences division, Dr. Anderson and Dr. Winterbottom are with the division of hospital and specialty medicine, and Ms. Snodgrass is with the Portland Center for the Evaluation of Clinical Services, with which Dr. Winterbottom is also affiliated. Dr. Dobscha, Dr. Hoffman, Dr. Turner, and Dr. Hauser are also with the department of psychiatry at the Oregon Health and Sciences University in Portland, and Dr. Anderson is also with the department of medicine at the Oregon Health and Sciences University. Send correspondence to Dr. Dobscha at the Portland VA Medical Center, P.O. Box 1034 (P3MHDC), Portland, Oregon 97207 (e-mail, [email protected]).
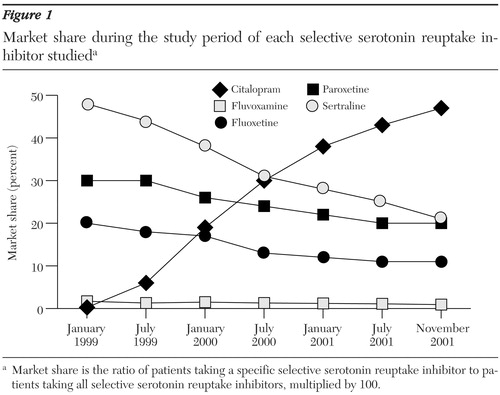
Figure 1. Market share during the study period of each selective serotonin reuptake inhibitor studieda
a Market share is the ratio of patients taking a specific selective serotonin reuptake inhibitor to patients taking all selective serotonin reuptake inhibitors, multiplied by 100.
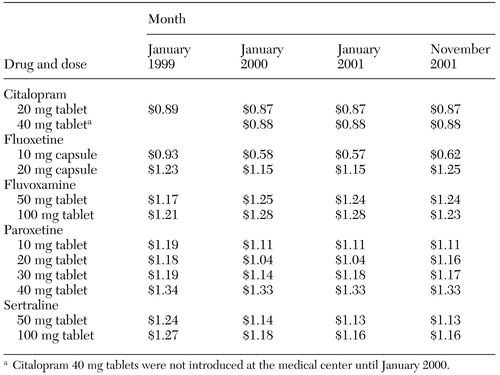 |
Table 1. Acquisition cost per tablet or capsule for selective serotonin reuptake inhibitors at the Portland (Oregon) Veterans Affairs Medical Center, January 1999 through November 2001
1. McCombs JS, Nichol MB, Stimmel GL, et al: The cost of antidepressant drug therapy failure: a study of antidepressant use patterns in a Medicaid population. Journal of Clinical Psychiatry 51:60-69, 1990Medline, Google Scholar
2. Simon G, Ormel J, VonKorff M, et al: Health care costs associated with depressive and anxiety disorders in primary care. American Journal of Psychiatry 152:352-357, 1995Link, Google Scholar
3. Simon GE, VonKorff M, Barlow W: Health care costs of primary care patients with recognized depression. Archives of General Psychiatry 52:850-856, 1995Crossref, Medline, Google Scholar
4. Unutzer J, Patrick DL, Simon G, et al: Depressive symptoms and the cost of health services in HMO patients aged 65 years and older: a 4-year prospective study. JAMA 277:1618-1623, 1997Crossref, Medline, Google Scholar
5. Von Korff M, Katon W, Bush T, et al: Treatment costs, cost offset, and cost-effectiveness of collaborative management of depression. Psychosomatic Medicine 60:143-149, 1998Crossref, Medline, Google Scholar
6. Croghan TW: The controversy of increased spending for antidepressants. Health Affairs 20 (2):129-135, 2001Google Scholar
7. Latner AW: Top 200 drugs by retail sales in 2000. Drug Topics 6:18, 2001Google Scholar
8. Moller HJ: Pharmacotherapy of depressed patients: increasing the therapeutic arsenal with a new generation of antidepressive drugs [in German]. Fortschritte der Medizin 115:30-34, 36, 1997Medline, Google Scholar
9. Fairman KA, Teitelbaum F, Drevets WC, et al: Course of antidepressant treatment with tricyclic versus selective serotonin reuptake inhibitor agents: a comparison in managed care and fee-for-service environments. American Journal of Managed Care 3:453-465, 1997Medline, Google Scholar
10. Frank L, Revicki DA, Sorensen SV, et al: The economics of selective serotonin reuptake inhibitors in depression: a critical review. CNS Drugs 15:59-83, 2001Crossref, Medline, Google Scholar
11. Dubois RW, Chawla AJ, Neslusan CA, et al: Explaining drug spending trends: does perception match reality? Health Affairs 19 (2):231-239, 2000Google Scholar
12. Levine S, Campen D, Millares M, et al: Kaiser Permanente's prescription drug benefit. Health Affairs 19(2):185-190, 2000Google Scholar
13. Benkert O, Szegedi A, Wetzel H: Minimum effective dose for antidepressants: an obligatory requirement for antidepressant drug evaluation? International Clinical Psychopharmacology 11:177-185, 1996Google Scholar
14. Vodoor M, Southwell YP, Grubin M, et al: The management of depression: the implications for managed care--roundtable discussion: part 3. Managed Care Interface (suppl B):26-32, 2000Google Scholar
15. Singletary T, North DS, Weiss M, et al: A cost-effective approach to the use of selective serotonin reuptake inhibitors in a Veterans Affairs Medical Center. American Journal of Managed Care 3:125-129, 1997Medline, Google Scholar
16. Cohen CI, Cohen SI: Potential cost savings from pill splitting of newer psychotropic medications. Psychiatric Services 51:527-529, 2000Link, Google Scholar
17. Stafford RS, Radley DC: The potential of pill splitting to achieve cost savings. American Journal of Managed Care 8:706-712, 2002Medline, Google Scholar
18. Hankin CS, Spiro A III, Miller DR, et al: Mental disorders and mental health treatment among US Department of Veterans Affairs outpatients: the Veterans Health Study. American Journal of Psychiatry 156:1924-1930, 1999Abstract, Google Scholar
19. Rosenheck R, Leslie D: Cost and Prescription of Antidepressant Medication in VA. New Haven, Conn, VA Northeast Program Evaluation Center and VISN 1 MIRECC, 2001Google Scholar
20. Streja DA, Hui RL, Streja E, et al: Selective contracting and patient outcomes: a case study of formulary restrictions for selective serotonin reuptake inhibitor antidepressants. American Journal of Managed Care 5:1133-1142, 1999Medline, Google Scholar
21. Geddes JR, Freemantle N, Mason J, et al: SSRIs versus other antidepressants for depressive disorder. Cochrane Database System Review:CD001851, 2000Google Scholar
22. Kazis LE, Miller DR, Clark J, et al: Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Archives of Internal Medicine 158:626-632, 1998Crossref, Medline, Google Scholar
23. Mitchell J, Greenberg J, Finch K, et al: Effectiveness and economic impact of antidepressant medications: a review. American Journal of Managed Care 3:323-330, 1997Medline, Google Scholar
24. Saklad SR: Pharmacoeconomic issues in the treatment of depression. Pharmacotherapy 15 (suppl 6, pt 2):76S-83S, 1995Google Scholar
25. VHA Clinical Guideline for Major Depressive Disorder. Washington, DC, Veterans Health Administration, Mental Health and Strategic Health Care Group and MDD Working Group, 1998Google Scholar



