Impact of High-Deductible Health Plans on Medication Use Among Individuals With Bipolar Disorder
Abstract
Objective:
High-deductible health plans (HDHPs) require substantial out-of-pocket spending for most services, although medications may be subject to traditional copayment arrangements. This study examined effects of HDHPs on medication out-of-pocket spending and use and quality of care among individuals with bipolar disorder.
Methods:
This quasi-experimental study used claims data (2003–2014) for a national sample of 3,532 members with bipolar disorder, ages 12–64, continuously enrolled for 1 year in a low-deductible plan (≤$500) and then for 1 year in an HDHP (≥$1,000) after an employer-mandated switch. HDHP members were matched to 18,923 contemporaneous individuals in low-deductible plans (control group). Outcome measures were out-of-pocket spending and use of bipolar disorder medications, psychotropics for other disorders, and all other medications and appropriate laboratory monitoring for psychotropics.
Results:
Relative to the control group, annual out-of-pocket spending per person for bipolar disorder medications increased 20.8% among HDHP members (95% confidence interval [CI]=14.9%–26.7%), and the absolute increase was $36 (95% CI=$25.9–$45.2). Specifically, out-of-pocket spending increased for antipsychotics (27.1%; 95% CI=17.4%–36.7%) and anticonvulsants (19.2%; 95% CI=11.9%–26.6%) but remained stable for lithium (−3.7%; 95% CI=–12.2% to 4.8%). No statistically significant changes were detected in use of bipolar disorder medications, other psychotropics, or all other medications or in appropriate laboratory monitoring for bipolar disorder medications.
Conclusions:
HDHP members with bipolar disorder experienced a moderate increase in out-of-pocket spending for medications but preserved bipolar disorder medication use. Findings may reflect individuals’ perceptions of the importance of these medications for their functioning and well-being.
HIGHLIGHTS
High-deductible health plan (HDHP) members with bipolar disorder experienced a moderate increase in out-of-pocket spending for medications but no change in use of bipolar disorder medications and psychotropics for other disorders.
HDHP members with bipolar disorder might elect to pay more out of pocket to maintain use of medications essential for their functioning and well-being.
The switch to an HDHP was not associated with changes in the already low rate of appropriate laboratory monitoring for psychotropic medications.
HDHP members might make trade-offs to preserve important care, but increases in cost-sharing could be a financial strain for some people, and the consequences of such trade-offs warrant further research.
Bipolar disorder has a 12-month prevalence of 2.8% and a lifetime prevalence of 4.4% in the United States (1) and carries a high risk of morbidity and mortality (2, 3). Bipolar disorder is a chronic illness. Many individuals have persistent symptoms and difficulty functioning and experience episodic exacerbations of the illness (4). Individuals with bipolar disorder require medications to treat manic and depressive episodes (5–7). Maintenance use of bipolar disorder medications is recommended to prevent mood exacerbations, hospitalization, and suicide (8–10).
In the United States, high-deductible health plans (HDHPs) have become the predominant commercial health insurance arrangement. In 2019, 55% of workers with employer-sponsored health insurance had deductibles of $1,000 or more, and 45% of workers in small firms and 22% of workers in large firms had deductibles of $2,000 or more (11).
Switching to HDHPs is a real-world natural experiment that involves several types of patient cost-sharing changes. HDHPs require substantially higher out-of-pocket spending than traditional plans. Members pay full cost for most nonpreventive care (e.g., specialist visits, emergency department visits, and diagnostic tests) until reaching the deductible amount. After this, patient cost-sharing declines. HDHPs typically cover prescription medications under a traditional copayment structure, similar to low-deductible plans, except for health savings account–eligible HDHPs (11), which subject medications to the deductible.
Reductions in medication use could occur for HDHP members for several reasons: small-to-moderate average increases in medication cost-sharing; overall increased levels of out-of-pocket payments that exceed HDHP members’ personal health care budget, causing reduced prescription medication purchasing; the increased cost burden of HDHPs, reducing contact with physicians and providing fewer opportunities to receive prescriptions; and complex HDHP benefit structures that may confuse enrollees who might believe that medications are subject to high out-of-pocket costs (12).
The 1971–1986 RAND Health Insurance Experiment (13) found that high patient cost-sharing reduced both appropriate and inappropriate utilization among all individuals, including hospitalizations, outpatient visits, and preventive services. Studies of HDHPs suggest that reductions in medication and health care utilization do not occur in all clinical situations (13–17) or for all subgroups of people (16, 17). However, prior research has found that insurance features that create financial or administrative barriers (e.g., cost-sharing, prior authorization, and capped coverage) are associated with reduced medication adherence and increased use of emergency services among individuals with mental illness (18–23). It remains unclear how individuals with severe mental illness respond to HDHP enrollment.
Therefore, the purpose of this study was to examine the impact of HDHPs on medication out-of-pocket spending and use and appropriate laboratory monitoring for psychotropic medications among individuals with bipolar disorder.
Methods
Data Source and Study Population
We drew our study population from a large commercial and Medicare Advantage health insurance claims database. Data included medical and pharmacy claims and enrollment information. The study population comprised individuals with bipolar disorder in low-deductible plans whose employers either mandated a switch to an HDHP during our 2003–2014 study period (HDHP group) or kept their employees in low-deductible coverage (control group). This design was used to minimize member self-selection bias.
As in our previous work (24, 25), we defined employers with low- and high-deductible coverage as those offering exclusively plans with annual deductibles of $0–$500 and $1,000 or more, respectively (further details are available in an online supplement to this article).
Qualifying HDHP group employers were those with at least 1 year of low-deductible-only coverage followed by at least 1 year of HDHP-only coverage. We defined the index date for employers that switched to HDHPs as the beginning of the month when the switch occurred. For employers that did not switch plans, the index date was the beginning of the month when their yearly account renewed. Some members had multiple eligible index dates (e.g., low-deductible year to low-deductible year and low-deductible year to HDHP year); we randomly assigned these enrollees to the HDHP pool or the control pool. For members assigned to the control pool who had multiple low-deductible year to low-deductible year spans, we randomly selected one of their potential index dates (and their corresponding before-after enrollment years).
The study cohort was drawn from 53 million members ages 0–64 who were enrolled between January 1, 2003, and December 31, 2014. As in prior research (16, 26), we used an established algorithm and outpatient and inpatient bipolar disorder diagnoses as defined by ICD-9-CM codes to classify members regarding bipolar type (I, II, or other) (see online supplement). We identified 355,700 members with bipolar disorder.
We limited the qualifying population to a prematch sample of 3,619 HDHP members and 39,953 individuals in the control group who were continuously enrolled for at least 2 years in the above-defined employers, were ages 12–64 years at the time of the index date, had their most recent bipolar disorder diagnosis occur between 5 years and 7 months prior to the index date, and had employers that did not carve out mental health benefits.
Matching Strategy and Covariates
We ensured balance between the HDHP and control groups by using coarsened exact matching (24, 25, 27–30) with employer- and member-level baseline covariates (see online supplement). These included quartile for employer baseline out-of-pocket cost/standardized cost ratio, four categories of baseline total out-of-pocket spending, and quartile for a member’s baseline total standardized cost. Matching variables also included employer- and member-level propensity to join HDHPs. The employer-level propensity score included employer size; proportion of women; proportions of members in each of four U.S. regions and in race-ethnicity, age, education, and income categories; baseline total standardized cost; the employer’s mean adjusted clinical groups (ACG) score; median copay; index month and year; and type of insurance plan. The member-level propensity score included age category, U.S. region, employer size, year of first qualifying diagnosis, baseline count of prescription medication categories, and baseline quarterly pharmacy out-of-pocket spending. After matching, the final study population included 3,532 HDHP members and 18,923 matched individuals in the control group.
Outcome Measures
We defined and classified psychotropic medications for bipolar disorder and for other disorders on the basis of National Drug Codes from pharmacy claims linked to First Data Bank and the American Hospital Formulary Service therapeutic classification hierarchy, which are gold standards for therapeutic classification.
We created three primary medication categories: medications for bipolar disorder, including lithium, selected anticonvulsants, and selected antipsychotics (see online supplement); psychotropic medications for other disorders, including anxiolytics, antidepressants, dementia medications, substance abuse medications, benzodiazepines, sedative-hypnotics, and attention-deficit hyperactivity disorder medications; and all other medications, which included the remaining medications, such as those for cardiovascular disease, diabetes, and pain relief.
Medication use was measured as the average monthly number of standardized medication doses (SMD). We created this metric of treatment intensity on the basis of the typical dose among users in the overall population, allowing capture of change in both frequency and dose of medication use for comparison over time and between study groups (see online supplement). We also assessed whether individuals had any fills for at least one bipolar disorder medication in the baseline and follow-up years.
To assess overall cost-sharing increases among HDHP members relative to the control group, we estimated mean annual out-of-pocket spending per person as the sum of copayment, coinsurance, and deductible amounts for all health services (medical and pharmacy services) in a study period divided by the number of persons in a given study group. We also calculated monthly and yearly out-of-pocket spending per person for the above medication categories. Moreover, we assessed out-of-pocket spending per 30-day drug fill (in contrast to per person) as a proxy for the cost faced by individuals who used medications. Fills were spread across the days covered (i.e., a 30-day fill claim on January 16th would be spread as one dose per day from January 16th through February 14th).
Finally, we examined laboratory monitoring, a quality-of-care measure that consensus guidelines recommend at least once a year for people taking specific medications (31). We created medication-specific measures for lithium, carbamazepine, valproic acid, and second-generation antipsychotics (see online supplement). Individuals were included in this analysis only if they used the above medications for at least 6 months in any given year of observation (baseline or follow-up year or one of each). We also created a summary measure to examine the annual proportion of individuals receiving all appropriate laboratory monitoring for all medications that they were taking in the year (1,038 and 5,310 members in HDHP and control groups, respectively). For example, monitoring for members taking carbamazepine and lithium would qualify as compliant only if they received required monitoring for both medications.
Study Design and Analysis
We used a standardized differences approach to compare baseline characteristics of our study groups (32). We used an interrupted-time-series with comparison-series design and a pre-post with control group design to estimate the effects of being switched by employers into HDHPs (33–35).
We aligned relative time for all members at their index dates. We constructed controlled interrupted-time-series plots with monthly points adjusted for the coarsened exact weights to display trends in our matched study groups. Segmented linear regression models demonstrated that the study groups had parallel baseline trends in all outcome measures (data not shown), justifying our use of a pre-post with control group design. Applying a person-level difference-in-differences analytic approach, we used generalized estimating equations to compare changes in outcomes in the year before and the year after the index date among HDHP versus control group members. Models used a negative binomial distribution for count outcomes, such as out-of-pocket spending, with log link function. The term of interest was the two-way interaction between indicators for cohort (HDHP or control group) and study time periods (the year before or after the index date).
We then applied marginal-effects methods to calculate mean out-of-pocket spending and SMD during baseline and follow-up years as well as absolute and relative changes (36). We adjusted all regression models for the variables used in matching. We analyzed data with SAS, version 9.3, and Stata, version 14, software. The study was approved by the Harvard Pilgrim Healthcare Institutional Review Board.
Results
After matching, all standardized differences between HDHP and control group characteristics were well below 0.2, indicating minimal baseline differences (Table 1) (32). The average age of HDHP and control members was 38, and 60%−61% of each group was female. About 44% lived in low-income neighborhoods, 6%−7% lived in low-education neighborhoods, 4%−5% were Hispanic, and the mean ACG morbidity score was 2.2, indicating higher morbidity than the overall US commercially insured population.
| Before CEM | After CEM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HDHP | Control | HDHP | Control | |||||||
| (N=3,619) | (N=39,953) | Std. | (N=3,532) | (N=18,923) | Std. | |||||
| Characteristic | N | % | N | % | diff.b | N | % | N | % | diff.b |
| Age >40 on index date | 1,822 | 50.3 | 19,839 | 49.7 | .014 | 1,775 | 50.3 | 9,479 | 50.1 | .003 |
| Age on index date (M±SD) | 38.1±13.8 | 38.2±14.2 | −.005 | 38.1±13.8 | 38.1±13.7 | −.003 | ||||
| Female | 2,208 | 61.0 | 24,560 | 61.5 | −.010 | 2,163 | 61.2 | 11,409 | 60.3 | .019 |
| Enrolled in health savings account plan | 353 | 9.8 | 0 | — | .465 | 345 | 9.8 | 0 | — | .465 |
| Percentage of households below federal poverty level in neighborhood of residencec | .152 | .155 | ||||||||
| <5 | 989 | 27.3 | 10,969 | 27.5 | 964 | 27.3 | 4,991 | 26.4 | ||
| 5–9.9 | 1,012 | 28.0 | 11,698 | 29.3 | 985 | 27.9 | 5,520 | 29.2 | ||
| 10–19.9 | 1,051 | 29.0 | 11,516 | 28.8 | 1,027 | 29.1 | 5,665 | 29.9 | ||
| ≥20 | 564 | 15.6 | 5,751 | 14.4 | 553 | 15.7 | 2,740 | 14.5 | ||
| Missing | 3 | .1 | 19 | <.1 | 3 | .1 | 8 | <.1 | ||
| Percentage of persons with less than a high school education in neighborhood of residenced | .044 | .044 | ||||||||
| <15 | 2,766 | 76.4 | 30,888 | 77.3 | 2,699 | 76.4 | 14,623 | 77.3 | ||
| 15–24.9 | 588 | 16.2 | 6,470 | 16.2 | 574 | 16.3 | 3,073 | 16.2 | ||
| 25–39.9 | 216 | 6.0 | 2,174 | 5.4 | 213 | 6.0 | 1,035 | 5.5 | ||
| ≥40 | 46 | 1.3 | 403 | 1.0 | 43 | 1.2 | 185 | 1.0 | ||
| Missing | 3 | .1 | 18 | .0 | 3 | .1 | 7 | .0 | ||
| Race-ethnicitye | .091 | .049 | ||||||||
| Hispanic | 165 | 4.6 | 2,277 | 5.7 | 154 | 4.4 | 943 | 5.0 | ||
| Asian | 37 | 1.0 | 575 | 1.4 | 36 | 1.0 | 210 | 1.1 | ||
| Black neighborhood | 26 | .7 | 455 | 1.1 | 26 | .7 | 148 | .8 | ||
| Mixed neighborhood | 711 | 19.6 | 9,212 | 23.1 | 700 | 19.8 | 3,813 | 20.1 | ||
| White neighborhood | 2,680 | 74.1 | 27,434 | 68.7 | 2,616 | 74.1 | 13,809 | 73.0 | ||
| Adjusted clinical groups score (M±SD)f | 2.2±3.1 | 2.3±3.2 | −.016 | 2.2±3.1 | 2.2±3.0 | .015 | ||||
| U.S. region | .222 | .085 | ||||||||
| West | 456 | 12.6 | 5,982 | 15.0 | 447 | 12.7 | 2,632 | 13.9 | ||
| Midwest | 1,243 | 34.3 | 11,726 | 29.3 | 1,208 | 34.2 | 6,099 | 32.2 | ||
| South | 1,660 | 45.9 | 17,185 | 43.0 | 1,628 | 46.1 | 8,534 | 45.1 | ||
| Northeast | 257 | 7.1 | 5,046 | 12.6 | 246 | 7.0 | 1,652 | 8.7 | ||
| Missing | 3 | .1 | 14 | <.1 | 3 | .1 | 6 | <.1 | ||
| Outpatient copayment (M±SD $) | 19.2±5.7 | 16.8±6.7 | .382 | 19.2±5.6 | 19.0±5.9 | .031 | ||||
| Employer size (M±SD N of employees) | 259.0±740.6 | 8,261.7±21,490.7 | −.526 | 260.0±748.5 | 1,304.0±9,193.9 | −.160 | ||||
| Employer size (N of employees) | 1.316 | .086 | ||||||||
| 0–99 | 2,279 | 63.0 | 7,780 | 19.5 | 2,240 | 63.4 | 11,556 | 61.1 | ||
| 100–999 | 1,162 | 32.1 | 12,517 | 31.3 | 1,114 | 31.5 | 6,144 | 32.5 | ||
| ≥1,000 | 178 | 4.9 | 19,656 | 49.2 | 178 | 5.0 | 1,224 | 6.5 | ||
| Substance use disorder | 650 | 18.0 | 7,264 | 18.2 | −.006 | 635 | 18.0 | 3,506 | 18.5 | −.014 |
| Bipolar disorder type | .039 | .039 | ||||||||
| Type I | 2,653 | 73.3 | 29,232 | 73.2 | 2,588 | 73.3 | 13,800 | 72.9 | ||
| Type II | 421 | 11.6 | 5,097 | 12.8 | 412 | 11.7 | 2,428 | 12.8 | ||
| Other | 545 | 15.1 | 5,624 | 14.1 | 532 | 15.1 | 2,696 | 14.2 | ||
| Residence | .136 | .064 | ||||||||
| Rural | 311 | 8.6 | 2,934 | 7.3 | 301 | 8.5 | 1,645 | 8.7 | ||
| Urban | 3,208 | 88.6 | 35,720 | 89.4 | 3,132 | 88.7 | 16,694 | 88.2 | ||
| Unknown | 100 | 2.8 | 1,299 | 3.3 | 99 | 2.8 | 584 | 3.1 | ||
TABLE 1. Baseline characteristics of two groups of individuals with bipolar disorder, before and after coarsened exact matching (CEM)a
HDHP members experienced an absolute pre-to-post increase in out-of-pocket spending of $479 per person per year for all health care services, compared with the control group (95% confidence interval [CI]=$409–$549, and an increase in out-of-pocket spending of $77 per person per year for pharmacy services (95% CI=$56–$98).
Among HDHP members who used psychotropic medications for bipolar disorder or for any other disorder, increases in out-of-pocket spending were as follows: $8 per 30-day fill of antipsychotics (relative change, 22.7%), $4 per 30-day fill of anticonvulsants (relative change, 13.8%), and $3 per 30-day fill of psychotropics for disorders other than bipolar disorder (relative change, 11.1%) (Table 2).
| Absolute | Relative | |||||||
|---|---|---|---|---|---|---|---|---|
| HDHP | Control | change ($)b | change (%)b | |||||
| Variable | Baseline | Follow-up | Baseline | Follow-up | $ | 95% CI | % | 95% CI |
| Out-of-pocket spending (total $ per member per year) | ||||||||
| All medication classes | 749.6 | 810.0 | 755.2 | 738.6 | 76.96 | 56.1, 97.8 | 10.5 | 7.5, 13.5 |
| Bipolar disorder medications | 182.6 | 206.7 | 184.9 | 173.4 | 35.5 | 25.9, 45.2 | 20.8 | 14.9, 26.7 |
| Antipsychotics | 82.5 | 103.0 | 84.5 | 83.1 | 21.9 | 14.6, 29.3 | 27.1 | 17.4, 36.7 |
| Anticonvulsants | 85.9 | 91.3 | 87.0 | 77.6 | 14.7 | 9.4, 20.1 | 19.2 | 11.9, 26.6 |
| Lithium | 13.9 | 12.3 | 14.2 | 13.0 | −.5 | –1.6, .6 | –3.7 | –12.2, 4.8 |
| Psychotropics for disorders other than bipolar disorder | 227.2 | 233.6 | 234.0 | 223.9 | 16.2 | 7.8, 24.6 | 7.4 | 3.4, 11.5 |
| All other medications | 354.3 | 383.1 | 344.5 | 356.2 | 16.8 | 2.6, 31.0 | 4.6 | .6, 8.5 |
| Out-of-pocket spending per 30-day pharmaceutical fill among medication users | ||||||||
| All medication classes | 34.8 | 37.4 | 34.0 | 33.7 | 3.0 | 1.6, 4.4 | 8.7 | 4.6, 12.9 |
| Bipolar disorder medications | 27.4 | 32.3 | 27.0 | 26.9 | 5.0 | 3.6, 6.3 | 18.2 | 13.0, 23.5 |
| Antipsychotics | 34.9 | 44.4 | 35.4 | 36.7 | 8.2 | 5.7, 10.7 | 22.7 | 15.3, 30.0 |
| Anticonvulsants | 26.6 | 28.7 | 25.9 | 24.5 | 3.5 | 1.9, 5.0 | 13.8 | 7.3, 20.2 |
| Lithium | 12.1 | 11.7 | 12.4 | 11.9 | .0 | −.7, .8 | .4 | –5.9, 6.7 |
| Psychotropics for disorders other than bipolar disorder | 24.9 | 26.9 | 24.8 | 24.2 | 2.7 | 1.7, 3.7 | 11.1 | 6.6, 15.5 |
| All other medications | 48.3 | 45.6 | 45.2 | 43.8 | –1.2 | –4.6, 2.1 | –2.7 | –9.7, 4.4 |
| Medication use (monthly N of standardized medication doses per member) | ||||||||
| Bipolar disorder medications | 19.9 | 19.6 | 20.1 | 19.9 | −.4 | −.7, .6 | −.2 | –3.4, 3.0 |
| Antipsychotics | 7.7 | 7.5 | 7.9 | 7.8 | −.2 | −.6, .3 | –2.1 | –7.5, 3.3 |
| Anticonvulsants | 9.2 | 9.2 | 9.3 | 9.2 | .1 | −.2, .5 | 1.6 | –2.5, 5.7 |
| Lithium | 3.0 | 2.9 | 3.0 | 2.9 | .0 | −.2, .1 | –1.7 | –8.1, 4.7 |
| Psychotropics for disorders other than bipolar disorder | 31.5 | 31.7 | 32.3 | 32.4 | .1 | −.8, 1.0 | 3.7 | –2.4, 3.2 |
| All other medications | 38.7 | 43.5 | 37.1 | 40.4 | 1.4 | –3.8, 6.7 | 3.4 | –9.0, 15.9 |
TABLE 2. Adjusted difference-in-differences estimates in annual out-of-pocket medication spending and use of medications among individuals with bipolar disorder in two groupsa
In addition to the analyses of 30-day fills, we examined out-of-pocket spending per person in HDHP and control groups, thus accounting for members who did and did not have fills of a given medication. Figure 1 presents interrupted-time-series plots of monthly out-of-pocket spending per person for bipolar disorder medications. In adjusted difference-in-differences analyses, the absolute annual increase in out-of-pocket spending for bipolar disorder medications was $36 per HDHP member, compared with the control group, and the relative increase was 20.8% (Table 2). Specifically, out-of-pocket spending increased for antipsychotics (relative change, 27.1%) and anticonvulsants (relative change, 19.2%), but it did not change for lithium (relative change, –3.7%). Out-of-pocket spending for psychotropics for disorders other than bipolar disorder and for all other medications also increased (7.4% and 4.6%, respectively) (Table 2).
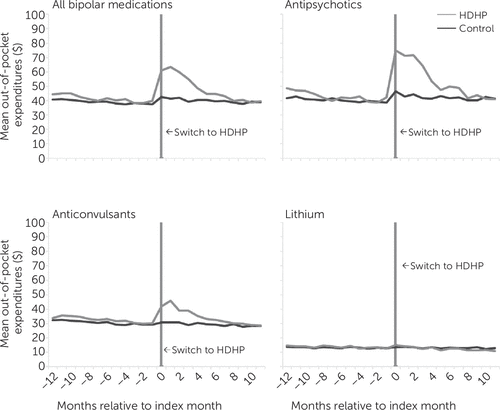
FIGURE 1. Mean monthly out-of-pocket spending for bipolar disorder medications among individuals with bipolar disorder in two groupsa
a Spending is shown before and after an employer-mandated switch to high-deductible health plans (HDHP group) and for a matched control group of individuals who remained in low-deductible plans in both years. A color version of the figure appears in the online supplement to this article.
Figure 2 presents interrupted-time-series plots of monthly SMD to assess intensity of medication use. In adjusted difference-in-differences analyses, we detected no statistically significant changes in use of bipolar disorder medications, psychotropic medications for other disorders, or all other medications (Table 2). Overall, 37.7% and 39% of HDHP members and the control group, respectively, had at least one fill for bipolar disorder medications in the baseline year; there was no significant change after the HDHP switch.
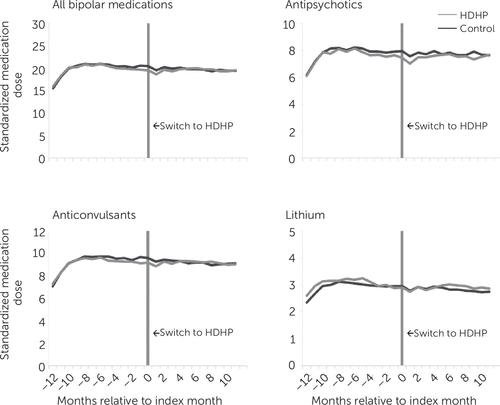
FIGURE 2. Mean monthly number of standardized medication doses for bipolar disorder medications among individuals with bipolar disorder in two groupsa
a Standardized doses are shown before and after an employer-mandated switch to high-deductible health plans (HDHP group) and for a matched control group of individuals who remained in low-deductible plans in both years. A color version of the figure appears in the online supplement to this article.
The annual proportion of HDHP members who received appropriate laboratory monitoring ranged from 44.3% for valproic acid to 28.9% for lithium in the baseline year; the control group had rates ranging from 40.6% for valproic acid to 30.1% for lithium (Table 3) (see monitoring by test in the online supplement). Appropriate laboratory monitoring for specific medications did not change after the HDHP switch. Based on our summary measure, 14.1% of the HDHP group and 13.2% of the control group received all appropriate laboratory monitoring in the baseline year, and we did not detect significant changes after the HDHP switch (relative change, –9.4%).
| Received monitoring (%) | Absolute | Relative | ||||||
|---|---|---|---|---|---|---|---|---|
| Appropriate lab | HDHP | Control | change (%)b | change (%)b | ||||
| monitoring | Baseline | Follow-up | Baseline | Follow-up | % | 95% CI | % | 95% CI |
| Summary measurec | 14.1 | 12.3 | 13.2 | 12.7 | −.01 | −.04, .02 | –9.4 | –29.1, 10.4 |
| For lithium usersd | 28.9 | 23.6 | 30.1 | 26.3 | −.02 | −.08, .05 | –6.8 | –31.9, 18.3 |
| For antipsychotic userse | 33.8 | 33.2 | 32.1 | 30.8 | .01 | −.04, .06 | 2.3 | –13.3, 18.0 |
| For carbamazepine usersf | 36.7 | 47.8 | 40.6 | 39.7 | .12 | −.08, .32 | 33.1 | –32.1, 98.3 |
| For valproic acid usersg | 44.3 | 37.7 | 40.6 | 40.2 | −.06 | −.17, .05 | –14.0 | –37.4, –9.4 |
TABLE 3. Adjusted difference-in-differences estimates in annual percentage of medication users receiving appropriate laboratory monitoring among individuals with bipolar disorder in two groupsa
Discussion
This study found that HDHP members with bipolar disorder maintained stable medication use despite 19%−27% increases in out-of-pocket spending for antipsychotic and anticonvulsant medications. The switch to HDHPs was not associated with detectable differences in appropriate laboratory monitoring of medications.
Previous studies identified that higher cost-sharing among Medicaid and commercially insured enrollees with severe mental illness was associated with lower medication use (18–23). However, we found no significant changes in use of psychotropic medications for bipolar disorder and for other disorders among HDHP members with bipolar disorder. Our findings might be explained by a variety of factors. The annual increase in out-of-pocket spending of $36 per HDHP member (or a $5 increase for a 30-day fill) for bipolar disorder medications—in the context of an annual increase of $479 per person in out-of-pocket spending for all health care services—was perhaps relatively small to affect average utilization among a commercially insured population. Funds in health savings accounts or health reimbursement arrangements, available to approximately 30% of enrollees (11), might have played a role in maintaining medication use. In addition, this cohort of individuals with a chronic illness might be reasonably familiar with the nuances of commercial health insurance designs despite being switched to an HDHP. Because they might anticipate exceeding their deductibles each year, and subsequently have minimal cost-sharing above the deductible, HDHP members might have little incentive to cut back on medications.
Annual medication use patterns among HDHP members with bipolar disorder (Figure 1) likely suggest sensitivity to cost-sharing among some HDHP members. The medications of members who are eligible for health savings accounts are subject to the annual deductible, and some exceed their annual deductible amount during the course of the benefit year, reducing their subsequent cost-sharing. This phenomenon accounts for the Figure 1 pattern, wherein lower out-of-pocket obligations later in the benefit year create less incentive to reduce medication use.
Our findings add to the HDHP literature by demonstrating that commercially insured members with a serious mental illness might preserve certain types of mental health care despite increases in out-of-pocket expenses. Our previous study found that HDHP members with bipolar disorder experienced a moderate decline in nonpsychiatrist mental health outpatient visits but maintained psychiatrist visits despite increases in out-of-pocket costs, indicating that they may try to preserve the visits needed for medication refills or adjustments (26). Our interview study suggested that individuals with bipolar disorder recognized that medications are essential for their well-being and daily functioning and that they preserved medication use despite higher out-of-pocket costs (37). Increases in patient cost-sharing create a financial strain for some people, and they make tradeoffs to pay for their medications (e.g., cutting back in other essential spending areas). Future analyses should examine HDHP impacts among vulnerable subgroups, such as those with low income, those from racial-ethnic minority groups, those with multiple morbidities, and those covered by health savings accounts–eligible HDHPs that subject medications to the deductible.
We also found that the switch to HDHPs was associated with greater increases in out-of-pocket spending for bipolar disorder medications than for other medications, which warrants future study to determine reasons. This study also found concerningly low treatment rates in both study groups. Less than 40% of individuals had any fills for bipolar disorder medications in a year. People with mental illness often have difficulty following a medication regimen; medication adherence among those with bipolar disorder has been reported to be as low as 35% (38). People with bipolar disorder require consistent use of medications as maintenance therapies to prevent future exacerbations and maximize functioning and clinical stability. Suboptimal medication use can result in debilitating episodes of mania or depression (8–10).
We found no effect of HDHPs on appropriate laboratory monitoring, but both study groups had low rates. Fewer than half of members received recommended laboratory tests for mood stabilizers or antipsychotics. These findings are consistent with prior literature (39, 40). Our summary measure indicated that about 85% of members did not receive all appropriate laboratory monitoring. Our summary measure was conservative because it required that each of the monitoring tests for a given medication be conducted only once in a year, even though some tests should be done more frequently. The low rates of essential laboratory monitoring represent an important gap in quality of care for all individuals with bipolar disorder.
Our study had several limitations. First, our analysis was based on claims data. The gold standard for establishing the diagnostic validity of a cohort is structured clinical interviews or chart reviews. However, prior research by Unützer et al. (41, 42) established the feasibility of using administrative claims data to establish bipolar disorder cohorts; we used similar methods to establish our study population. Any misspecification of bipolar disorder diagnosis would be balanced between the HDHP and control groups and would be unlikely to bias our results regarding the association between HDHPs and the study outcomes. Second, we were able to determine exact deductible levels for small employers but not for large employers. However, our algorithm imputing deductible levels for large employers was highly sensitive and specific, and our analyses showed that at the population level, the HDHP group experienced marked relative increases in overall out-of-pocket spending, as expected.
We also did not have information on non–cost-related reasons for medication discontinuation, such as tolerance or preference. However, these were unlikely to have differed by study group and thus should not have influenced our findings. Finally, we studied enrollees with employer-sponsored health insurance, who may be at a lower risk of increased cost-sharing, compared with those with less generous self-purchased commercial plans. However, internal and external validity for those with employer-sponsored health insurance should be robust; the distribution of bipolar I, II, and other bipolar type was similar in the HDHP and control groups and appears consistent with distributions in prior research (41, 43, 44). Furthermore, our HDHP and control groups had similar utilization of inpatient care—a commonly used proxy for disease severity (0.4 emergency room visits and 0.1 hospital visits per person per year; data not shown), and these rates were comparable with those in previous research among commercially insured individuals with bipolar disorder (45, 46). Nevertheless, our findings may not be representative of the most vulnerable subsets of HDHP members with bipolar disorder, such as members with very high deductibles, low incomes, more severe bipolar disorder, or multiple morbidities. Increased cost-sharing in such populations might cause more pronounced reductions in important care.
Conclusions
HDHP members with bipolar disorder who faced increases in cost-sharing preserved use of psychotropic medications for bipolar disorder and for other disorders. Additionally, the switch to HDHPs was not associated with changes in the already low rate of appropriate laboratory monitoring. Further research should examine effects among vulnerable subgroups, such as those with low income and those with health savings account–qualified HDHPs that require full medication cost-sharing.
1. : Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 2007; 64:543–552Crossref, Medline, Google Scholar
2. : A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003; 60:261–269Crossref, Medline, Google Scholar
3. : The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 2002; 59:530–537Crossref, Medline, Google Scholar
4. : The epidemiology of bipolar disorder: sociodemographic, disability and service utilization data from the Australian National Study of Low Prevalence (Psychotic) Disorders. Bipolar Disord 2005; 7:326–337Crossref, Medline, Google Scholar
5. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002; 159(suppl 4):1–50Google Scholar
6. : The Texas Implementation of Medication Algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry 2005; 66:870–886Crossref, Medline, Google Scholar
7. : Clinical practice guidelines for bipolar disorder from the Department of Veterans Affairs. J Clin Psychiatry 1999; 60:9–21Crossref, Medline, Google Scholar
8. : Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry 2004; 161:217–222Link, Google Scholar
9. : Long-term treatment in bipolar disorder. J Clin Psychiatry 2005; 66(suppl 1):7–12Medline, Google Scholar
10. : Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry 2005; 162:1281–1290Link, Google Scholar
11. : 2019 Employer Health Benefits Survey: Section 1: Cost of Health Insurance. San Francisco, Kaiser Family Foundation, 2019. https://www.kff.org/report-section/ehbs-2019-section-1-cost-of-health-insuranceGoogle Scholar
12. : High-deductible health insurance plans: efforts to sharpen a blunt instrument. Health Aff 2009; 28:1145–1154Crossref, Google Scholar
13. : Free for All? Lessons From the RAND Health Insurance Experiment. Cambridge, MA, Harvard University Press, 1993Google Scholar
14. : Emergency department use and subsequent hospitalizations among members of a high-deductible health plan. JAMA 2007; 297:1093–1102Crossref, Medline, Google Scholar
15. : Effect of a copayment on use of the emergency department in a health maintenance organization. N Engl J Med 1996; 334:635–641Crossref, Medline, Google Scholar
16. : Effect of high-deductible insurance on high-acuity outcomes in diabetes: a Natural Experiment for Translation in Diabetes (NEXT-D) Study. Diabetes Care 2018; 41:940–948Crossref, Medline, Google Scholar
17. : Diabetes outpatient care and acute complications before and after high-deductible insurance enrollment: a Natural Experiment for Translation in Diabetes (NEXT-D) study. JAMA Intern Med 2017; 177:358–368Crossref, Medline, Google Scholar
18. : Effects of a limiting Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. N Engl J Med 1994; 331:650–655Crossref, Medline, Google Scholar
19. : Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med 1991; 325:1072–1077Crossref, Medline, Google Scholar
20. : Payment restrictions for prescription drugs under Medicaid: effects on therapy, cost, and equity. N Engl J Med 1987; 317:550–556Crossref, Medline, Google Scholar
21. : Association between prior authorization for medications and health service use by Medicaid patients with bipolar disorder. Psychiatr Serv 2011; 62:186–193Link, Google Scholar
22. : Unintended impacts of a Medicaid prior authorization policy on access to medications for bipolar illness. Med Care 2010; 48:4–9Crossref, Medline, Google Scholar
23. : Cost-sharing effects on adherence and persistence for second-generation antipsychotics in commercially insured patients. Manag Care 2010; 19:40–47Medline, Google Scholar
24. : Vulnerable and less vulnerable women in high-deductible health plans experienced delayed breast cancer care. Health Aff 2019; 38:408–415Crossref, Google Scholar
25. : High-deductible insurance and delay in care for the macrovascular complications of diabetes. Ann Intern Med 2018; 169:845–854Crossref, Medline, Google Scholar
26. : Effect of high-deductible insurance on health care use in bipolar disorder. Am J Manag Care 2020; 26:248–255Crossref, Medline, Google Scholar
27. : Multivariate matching methods that are monotonic imbalance bounding. J Am Stat Assoc 2011; 106:345–361Crossref, Google Scholar
28. : CEM: Coarsened Exact Matching Software. Cambridge, MA, Harvard University, 2012. https://gking.harvard.edu/cemGoogle Scholar
29. : Causal inference without balance checking: coarsened exact matching. Polit Anal 2012; 20:1–24Crossref, Google Scholar
30. : Comparative Effectiveness of Matching Methods for Causal Inference. Cambridge, MA, Harvard University, 2011Google Scholar
31. : The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord 2009; 11:559–595Crossref, Medline, Google Scholar
32. : A unified approach to measuring the effect size between two groups using SAS; in Proceedings SAS Global Forum 2012. Orlando, FL, April 22–25, 2012. http://www.lerner.ccf.org/qhs/software/lib/stddiff.pdfGoogle Scholar
33. : Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Boston, MA, Houghton Mifflin, 2001Google Scholar
34. : Pretest measures of the study outcome and the elimination of selection bias: evidence from three within study comparisons. Prev Sci 2018; 19:274–283Crossref, Medline, Google Scholar
35. : Examining the internal validity and statistical precision of the comparative interrupted time series design by comparison with a randomized experiment. Am J Eval 2014; 35:311–327Crossref, Google Scholar
36. : Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986; 42:121–130Crossref, Medline, Google Scholar
37. : Healthcare cost-sharing experiences of people with bipolar disorder in employer plans. J Affective Disorders 2020; 281:41–50Crossref, Medline, Google Scholar
38. : Adherence to medication. N Engl J Med 2005; 353:487–497Crossref, Medline, Google Scholar
39. : Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord 2007; 102:145–151Crossref, Medline, Google Scholar
40. : Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry 1999; 156:1014–1018Abstract, Google Scholar
41. : The treated prevalence of bipolar disorder in a large staff-model HMO. Psychiatr Serv 1998; 49:1072–1078Link, Google Scholar
42. : The use of administrative data to assess quality of care for bipolar disorder in a large staff model HMO. Gen Hosp Psychiatry 2000; 22:1–10Crossref, Medline, Google Scholar
43. : Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2004; 55:875–881Crossref, Medline, Google Scholar
44. : Diagnostic certainty of a voluntary bipolar disorder case registry. J Affect Disord 1999; 52:93–99Crossref, Medline, Google Scholar
45. : Health care resource utilization and costs in a commercially insured population of patients with bipolar disorder type I and frequent psychiatric interventions. Clin Ther 2011; 33:1381–1390Crossref, Medline, Google Scholar
46. : The relationship between antipsychotic medication adherence and patient outcomes among individuals diagnosed with bipolar disorder: a retrospective study. Ann Gen Psychiatry 2009; 8:7Crossref, Medline, Google Scholar



