Systematic Review of Integrated General Medical and Psychiatric Self-Management Interventions for Adults With Serious Mental Illness
Abstract
Objective:
Adults with serious mental illness are disproportionately affected by general medical comorbidity, earlier onset of disease, and premature mortality. Integrated self-management interventions have been developed to address both general medical and psychiatric illnesses. This systematic review examined evidence about the effect of self-management interventions that target both general medical and psychiatric illnesses and evaluated the potential for implementation.
Methods:
Databases, including CINAHL, Cochrane Central, Ovid MEDLINE, PsycINFO, and Web of Science, were searched for articles published between 1946 and July 2015. Studies evaluating integrated general medical and psychiatric self-management interventions for adults with schizophrenia spectrum or mood disorders and general medical comorbidity were included.
Results:
Fifteen studies (nine randomized controlled trials and six pre-post designs) reported on nine interventions: automated telehealth, Health and Recovery Peer program, Helping Older People Experience Success, Integrated Illness Management and Recovery, Life Goals Collaborative Care, Living Well, Norlunga Chronic Disease Self-Management program, Paxton House, and Targeted Training in Illness Management. Most studies demonstrated feasibility, acceptability, and preliminary effectiveness; however, clinical effectiveness could not be established in most studies because of methodological limitations. Factors identified that may deter implementation included operating costs, impractical length, and workforce requirements.
Conclusions:
Integrated general medical and psychiatric illness self-management interventions appear feasible and acceptable, with high potential for clinical effectiveness. However, implementation factors were rarely considered in intervention development, which may contribute to limited uptake and reach in real-world settings.
Adults with serious mental illness are disproportionately affected by general medical comorbidity (1) and earlier onset of disease, and they die up to 32 years earlier than the general population (median=10.1 years) (2). These high rates of morbidity and early mortality are often linked to poorly managed general medical and psychiatric illnesses (3), which has prompted the development of interventions to support self-management of general medical conditions in this high-risk group (4,5). Self-management interventions usually focus on a combination of three tasks: medical management (for example, teaching people how to follow through on treatment), role management (for example, encouraging healthy behaviors), and emotional management (for example, learning how to monitor symptoms and identify early warning signs of relapse) (6).
A series of randomized controlled trials has also demonstrated the effectiveness of psychiatric self-management interventions (7–10) in improving mental health outcomes for adults with serious mental illness. However, interventions that focus on self-management of general medical or psychiatric conditions may be insufficient because of the interdependence of general medical and psychiatric symptoms and disorders (11–13). For example, psychiatric symptoms might exacerbate general medical illness and vice versa.
As a result, self-management interventions have been developed more recently that build on the existing evidence base and simultaneously address both general medical and psychiatric illnesses. However, the current state of evidence about integrated self-management interventions has not been examined. Our objective was to expand on prior reviews that focused on general medical self-management only (14) or selective reviews of illness self-management (15) and conduct a systematic review to assess the feasibility, acceptability, and potential effectiveness of integrated general medical and psychiatric self-management interventions that target adults with serious mental illnesses and chronic general medical conditions. We also examined the potential for implementation of these interventions.
Methods
Search Strategy
We searched the following databases from 1946 to July 2015 (dates reflect available high-quality electronic reference databases beginning in 1946): CINAHL, Cochrane Central, Ovid MEDLINE, PsycINFO, and Web of Science. We used the following search terms for serious mental illness: schizophrenia, schizophrenia and disorders with psychotic features, psychotic, bipolar, schizoaffective, paranoia, severe mental illness, serious mental illness, serious mental disease, serious psychotic illness, persistent mental illness, and persistent mental disease. These terms were used in combination with the following terms for self-management: illness self-management, self-management interventions, self-care, self-management, patient advocacy, self-advocacy, and empowerment.
Each term was entered as a keyword and assigned the corresponding Medical Subject Heading term. To identify articles not included in our original search, we reviewed reference lists of studies that met inclusion criteria and searched Google Scholar by using different combinations of the terms.
Study Selection Criteria
Studies were selected by the first two authors, who independently screened titles and abstracts for inclusion criteria: self-management intervention studies that addressed both general medical and psychiatric self-management, defined as interventions that target general medical management, role management, or emotional management (6,16) and that enrolled adults ages 18 and older with a diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder and a medical illness, including diabetes, heart disease, chronic obstructive pulmonary disease, or arthritis or chronic pain. We excluded preventive interventions and health promotion or lifestyle interventions targeting substance use, smoking cessation, weight loss, weight gain prevention, physical activity, or fitness. The first and second authors independently reviewed the full text of articles that met inclusion criteria. Any discrepancies were discussed and resolved by these authors.
There was no restriction on language. We included randomized controlled trials, pre-post designs, and secondary data analyses if outcomes were relevant to the effect of the self-management intervention. Research protocols, review articles, pharmacological studies, and theoretical articles were excluded.
Data Extraction
Study characteristics extracted included country of origin, study design, sample size, sociodemographic characteristics of the sample, study duration, control group, intervention duration, location of intervention, intervention description, interventionist, measures, and main outcomes.
Methodological Quality Assessment
To determine the methodological quality of the studies included, we used the Methodological Quality Rating Scale (MQRS) (17). MQRS has been used in other systematic reviews (18,19). We measured 12 methodological attributes of quality. Cumulative scores range from 0 (poor quality) to 17 (high quality). Studies that receive a cumulative score of at least 14 are considered to be high-quality studies (17). The first and second authors independently completed the MQRS for the studies that met inclusion criteria. Discrepancies in MQRS ratings were addressed and resolved by the first two authors.
Potential for Implementation
To examine potential for implementation, we picked variables that could facilitate uptake and that could also be reported in the articles included in this review. These included intervention structure, intervention duration, setting, and interventionist.
Results
The search strategy identified 739 citations. Of these, 76 citations were duplicates. A total of 663 titles and abstracts were reviewed, and 605 did not meet inclusion criteria. The full text of the remaining 64 articles was assessed for inclusion criteria, and 50 did not meet criteria. None of the non–English language articles met inclusion criteria. Four additional articles were found by searching reference lists from the 14 articles that met inclusion criteria, and one of the four met inclusion criteria. Overall, 15 studies met our inclusion criteria and were included in this review. [A flowchart summarizing article selection is included in an online supplement to this article.]
Many of the eliminated studies reported on interventions targeting only psychosocial skills training (for example, Functional Adaptation Skills Training and Behavioral Social Skills Training [20]), general medical comorbidities (for example, the chronic disease self-management program [4]), or serious mental illness (for example, FOCUS [21]).
The 15 included studies reported on nine interventions (Table 1): automated telehealth (22), Health and Recovery Peer program (HARP) (23), Helping Older People Experience Success (HOPES) (24–26), Integrated Illness Management and Recovery (I-IMR) (27,28), Life Goals Collaborative Care (LGCC) (29–31), Living Well (32), Norlunga Chronic Disease Self-Management program (33), Paxton House (34), and Targeted Training in Illness Management (TTIM) (35,36). Interventions were studied in diverse types of settings. Two interventions, HOPES (24–26) and I-IMR (27,28), were developed for middle-aged and older adults with serious mental illness.
| Study | Design | Sample | Setting | Diagnosis | Intervention | Duration | Interventionist | Comparison | Measures | Main outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Automated telehealth | ||||||||||
| Pratt et al., 2013 (22) | Pre-post | N=70 (54 female; 1 minority); mean age, 52.7±10.6 | Remote and home based | 12 bipolar disorder; 29 major depressive disorder; 18 PTSD; 11 schizophrenia; 7 COPD; 11 chronic pain; 6 congestive heart failure; 46 diabetes mellitus | A remote device that targets self-management of multiple psychiatric and general medical illnesses | 6 months of automated self-management behavioral prompts (e.g., “Have you taken your medication today?”), prompts to input biological indicators of health (e.g., blood glucose, weight), and psychiatric and general medical psychoeducation prompts | Remote technology in the consumer’s home supported by a nurse at a mental health center | na | Feasibility based on participant adherence; SCDSES, SF-12, BPRS, self-reported service use, health measures collected by the remote technology (BP, weight, and blood glucose) | Adherence over 70%; significant (p<.05) improvements at 6-month follow-up in depression self-management (SCDSES) (ES=–.33) and decrease in diastolic BP (ES=.60). Among those with diabetes, significant (p<.05) reductions in blood glucose (ES=.43), routine primary care visits (ES=.64), and urgent care visits (ES=1.11) |
| HARP | ||||||||||
| Druss et al., 2010 (23) | RCT | N=80 (41 HARP; 39 usual care; 56 female; 66 minority); mean age, 48.1±10.1 | CMHC | 26 bipolar disorder; 21 major depressive disorder; 9 PTSD; 23 schizophrenia; 39 arthritis; 18 asthma; 18 heart disease; 50 hypertension | Targets self-management (i.e., connection between mind and body, cross-systems medication coordination, psychiatric and general medical advance directives, healthy lifestyles, pain and fatigue management, finding and interacting with a doctor) | 6 group sessions; 6 months | 2 certified mental health peer specialists | Usual care (continuation of all general medical, mental health, and peer-based services prior to study entry) | PAM, BRFSS, health service use, self-reported medication adherence, SF-36 PCS and MCS | Significant (p<.05) improvement in patient activation (PAM) and use of primary care, compared with usual care |
| HOPES | ||||||||||
| Bartels et al., 2004 (26) | Controlled pilot | N=24; mean age, 66.5±5.7 | Assisted living facility | 4 bipolar disorder; 2 major depressive disorder; 2 psychotic disorder NOS; 3 schizoaffective disorder; 13 schizophrenia; (mean medical diagnoses, 4.3±2.7) | Enhanced Skills Training and Healthcare Management (STHM), later renamed HOPES (22) | 12 months of twice weekly group meetings and weekly nurse preventive health care visits | Rehabilitation specialist and psychiatric nurse | Health care management only (N=12) | ILSS, SBS, BPRS, SANS, GDS, MMSE | STHM resulted in significant (p<.05) improvements in care-of-possessions item (ILSS), compared with the control group, but not on the mean ILSS score. No between-group differences for other measures |
| Bartels et al., 2014 (24); Mueser et al., 2010 (25) | RCT | N=183 (90 HOPES; 93 treatment as usual); mean age, 60.2 | Mental health center or local senior center | 26 bipolar disorder; 44 major depressive disorder; 52 schizoaffective disorder; 51 schizophrenia; 25 asthma; 23 heart disease; 42 COPD; 50 diabetes; 80 hypertension; 32 hypothyroidism | Targets psychosocial rehabilitation and psychiatric and general medical self-management (i.e., social skills, illness self-management, medication management, communication with health care providers) | 12 months of weekly group meetings, two community trips per month to practice skills, and monthly preventive health care visits; during maintenance phase are monthly group skills training, community trips, and meetings with nurse | Groups co-led by a nurse and bachelor’s-level case manager; nurse preventive health care visits | Treatment as usual (usual mental health services, including pharmacotherapy, case management or outreach by nonnurses, individual therapy, and rehabilitation services, such as groups or psychoeducation | UPSA, MCAS, SBS, ILSS, RSES, SANS, cognitive functioning (D-KEFS, CVLT-II, card sorting test, Tower of London task, proverbs test, word context test, 20 questions test), BPRS, SF-36, CESD, CCI | Retention was high (80%). Compared with treatment as usual at 2-year follow-up, HOPES resulted in significant (p<.05) improvements in performance skills (UPSA), psychosocial and community functioning (MCAS), leisure and recreation (ILSS), negative symptoms (SANS), and self-efficacy (RSES). Results at 3-year follow-up showed significant (p<.05) improvements in community living skills (ILSS and MCAS), performance skills (UPSA), self-efficacy (RSES), and psychiatric symptoms (BPRS). At 3-year follow-up, significantly (p<.05) greater use of preventive health care, screening, and care planning with advance directives |
| I-IMR | ||||||||||
| Mueser, et al., 2012 (27) | Pre-post | N=7 (3 female); mean age, 59 | CMHC | 3 schizophrenia; 1 bipolar disorder; 3 major depressive disorder; 7 hypertension; 4 hyperlipidemia; 3 COPD; 2 osteoarthritis; 1 diabetes | Targets self-management of multiple psychiatric and general medical illnesses (i.e., individualized goal development, recovery strategies, connection between mind and body, psychoeducation, healthy lifestyles, managing stress, managing psychiatric and general medical health, medication management, and relapse prevention plans). | 8 months of weekly individual sessions | Master’s-level social worker supported by a nurse care manager | na | BPRS, IIMR scales, SRAHP | High participation in I-IMR (71% attended ≥10 sessions); some improvements among individual participants but no aggregate results reported |
| Bartels et al., 2014 (28) | RCT | N=71 (36 I-IMR; 35 usual care; 39 female; 2 minority); mean age, 60.3±6.5 | CMHC | 13 bipolar disorder; 31 major depressive disorder; 27 schizophrenia spectrum; 16 COPD; 34 diabetes; 33 hyperlipidemia; 29 hypertension; 46 osteoarthritis | See above (27) | 8 months of weekly individual sessions | Master’s level social worker supported by a nurse care manager | Usual care (included pharmacotherapy, case management or outreach by nonnurse clinicians, individual therapy, and access to psychosocial support and rehabilitation services) | I-IMR scales, SCDSES (SCDSES-adapted measures of diabetes, COPD, hypertension, hyperlipidemia, arthritis self-management), BPRS, MCAS, SPCS, API, self-reported hospitalizations and emergency visits | Compared with usual care, I-IMR resulted in significant improvements (I-IMR scales) in psychiatric (ES=.46) and general medical (ES=.29) illness self-management and significant improvements (SCDSES) in diabetes self-management skills (ES=.15). During primary care visits, there was an increase (API subscale) in preference for comprehensive diagnosis and treatment information (ES=.88) and decreases in self-reported psychiatric or medical hospitalizations (p=.037). |
| LGCC | ||||||||||
| Kilbourne et al., 2008 (29) | RCT | N=58 (all veterans; 27 BCM; 31 usual care; 5 female; 6 minority); mean age, 55.3±8.4 | Veterans Affairs outpatient mental health facility | 44 bipolar disorder I; 4 bipolar disorder II; 10 bipolar disorder NOS; 19 diabetes; 52 obesity; 47 hypertension; 44 hyperlipidemia; 11 coronary artery disease; 10 COPD; 4 chronic hepatitis; 19 osteoarthritis | BCM, later renamed LGCC (30) | 4 2-hour group sessions followed by 6 months of monthly telephone care management services | Care management by a nurse; groups led by a nurse manager | Usual care | SF-12, WHO-DAS, ISS, LSMECD, CAS, current medication use, number of outpatient general medical visits | Compared with usual care, BCM resulted in significant (p<.05) improvements in physical health–related quality of life (SF-12 PCS). |
| Kilbourne et al., 2012 (30) | RCT | N=65 (32 LGCC; 33 usual care; 39 female; 12 minority); mean age, 45.3±12.8 | Community-based mental health outpatient clinic | 65 bipolar disorder; 35.2±7.3 BMI; 45.0±6.0 inches waist circumference; 133.9±1.7 mmHg systolic BP; 85.1±11.1 mmHg diastolic BP | Targets self-management of bipolar spectrum disorder and risk factors for cardiovascular disease (i.e., individualized goal development, healthy behavioral change, and action plans to cope with symptoms); care management, and self-management practice guidelines are given to providers | 4 2-hour group sessions followed by 6 months of telephone care management services | Master’s-level social worker | Enhanced treatment as usual (included monthly receipt of mailings on wellness topics over the 6-month LGCC intervention period, in addition to available mental health care and referral to primary care services off site | Cardiometabolic risk (BMI, systolic/diastolic BP), SF-12; WHO-DAS, ISS | Only for consumers with BMI ≥30, compared with usual care, LGCC resulted in a significant decrease in impaired functioning (WHO-DAS) (β=–2.2) and depressive symptoms (ISS) (β=–2.0). Among those with systolic BP ≥140, LGCC resulted in a significant decrease in impaired functioning (WHO-DAS) (β=–3.8) and depressive symptoms (ISS) (β=–3.5). |
| Kilbourne al., 2013 (31) | RCT | N=118 (all veterans; 60 LGCC; 58 usual care; 20 female; 6 minority; mean age, 52.8±9.9 | Outpatient mental health or primary care clinic | 44 bipolar disorder I; 26 bipolar disorder II; 45 bipolar disorder; 3 schizoaffective bipolar disorder subtype; 30 diabetes mellitus; 22 heart disease; 98 hyperlipidemia; 81 hypertension; 58 obesity | See above (30) | 4 2-hour group sessions followed by 6 months of telephone care management services | Master’s-level health specialist | Enhanced usual care (regular mailings on wellness topics, in addition to standard mental health and general medical treatment; standard treatment included outpatient case management and group psychotherapy sessions specifically focused on mental health treatment and provided by a mental health team, as well as access to a primary care provider) | Systolic/diastolic BP, cholesterol, SF-12, WHO-DAS, ISS | Compared with usual care, LGCC resulted in a significant (p<.05) reduction in systolic (β=–3.1) and diastolic BP (β=–2.1) and fewer manic symptoms (ISS) (β=–23.9). |
| Living Well | ||||||||||
| Goldberg et al., 2013 (32) | RCT | N=63 (32 Living Well; 31 usual care; 33 female; 42 minority); mean age, 49.5±9.1 | Outpatient clinic and psychiatric rehabilitation day program | All bipolar disorder with psychotic features or schizophrenia spectrum disorder; 27 arthritis; 17 cardiovascular disease; 31 diabetes; 25 respiratory disease | Targets psychiatric and general medical illness self-management (i.e., healthy lifestyles, medication management, addictive behaviors, and cross-system coordination of services); adapted from the CDSMP and focuses on confidence building to develop self-management skills, with additional material on how serious mental illness affects general medical status and vice versa | 13 weekly group sessions and weekly follow-up telephone calls | Groups co-led by a mental health peer and a mental health provider or by 2 mental health peers who also have a chronic general medical condition; follow-up telephone calls by peers | Usual care | SF-12, SMSES, PAS, MHLC, RAS-SF, IMSM, MMAS | Compared with usual care, between baseline and 13 weeks, Living Well resulted in significant (p<.05) improvements as follows: in physical functioning (ES=.55), emotional well-being (ES=.66), and general health functioning (ES=.68) (SF–12 MCS and PCS); in self-management self-efficacy (ES=.65) (SMSES); in patient activation (ES=.55) and approach to health care (ES=.61) (PAS); and in self-management behaviors (ES=.57) and use of health care (ES=.81) (IMSM). Only improvement in use of health care remained significant at 2-month follow-up (ES=.54) (IMSM). At 2-month follow-up, significant improvements in internal locus of control (ES=.66) (MHLC) and in physical activity (ES=.56) and healthy eating (ES=.64) (IMSM) |
| Norlunga program | ||||||||||
| Lawn et al., 2007 (33) | Pre-post | N=38 (35 Flinders care planning; 17 Stanford groups; 3 Stanford groups only; 21 female); males’mean age, 39; females’ mean age, 46 | Primary care | 4 anxiety disorder; 5 bipolar disorder; 8 major depressive disorder; 1 personality disorder; 4 schizoaffective disorder; 16 schizophrenia; 22 with ≥2 conditions; most common conditions: obesity, asthma and other respiratory conditions, heart disease, and diabetes | Targets psychiatric and general medical self-management through education and peer support | 12 months of a hybrid individual and group approach | Peer educators | na | PIH, P&G, WSAS, SF-12 | Norlunga program resulted in significant (p<.05) improvements at 3 and 6 months in self-management (PIH) and mental functioning (SF-12 MCS), in social leisure activities (WSAS), and in reduced problem impact (P&G). Qualitative feedback was supportive of the intervention. |
| Paxton House | ||||||||||
| Teachout et al., 2011 (34) | Pre-post | N=13 (4 female; 9 minority); mean age, 45.0±6.9 | Supported housing residence | 2 major depressive disorder; 1 psychotic disorder NOS; 6 schizophrenia; 4 schizoaffective disorder; all with type II diabetes | Offers psychosocial rehabilitation and diabetes self-management services (i.e., diabetes education and nutritional programs), psychiatric support, intensive case management, and residential support | 6 months of individual and group sessions | Advanced-practice nurses and clinical staff | na | Weekly weight; daily blood glucose, satisfaction survey, DES-SF | At 6-month follow-up, Paxton House resulted in weight loss for all participants (mean=20.35 pounds), improved fasting glucose for 40%, and overall satisfaction with the program. |
| TTIM | ||||||||||
| Blixen et al, 2011 (35); Sajatovic et al., 2011 (36) | Pre-post | N=12; age range, 33–62; median age, 49.5 | Primary care | Bipolar disorder, major depressive disorder, schizophrenia, or schizoaffective disorder; all with diabetes | Targets psychiatric and diabetes self-management | 12 weekly 60- to 90-minute group sessions and 4-week maintenance period consisting of weekly telephone sessions | Groups co-led by peer educator with serious mental illness and diabetes and nurse educator | na | BPRS, MADRS, CGI, GAF, SDS, HbA1c, BMI; SF-12, SDSCA | TTIM qualitative findings suggest increased illness knowledge, self-confidence, and motivation. Quantitative findings suggest increased self-management of serious mental illness and diabetes, such as reduction in psychiatric symptom severity (BPRS) and improvement in perceived mental and physical health status (SF-12 MCS and PCS). For 8 participants, improvement in HbA1c was noted. Significant (p<.05) improvement in dietary behaviors for diabetes self-management (SDSCA) |
TABLE 1. Integrated general medical and psychiatric self-management interventions for adults with serious mental illnessa
Evidence of Intervention Feasibility, Acceptability, and Effectiveness
Six interventions (automated telehealth, HARP, HOPES, I-IMR, Living Well, and Norlunga Chronic Disease Self-Management program) targeted a heterogeneous set of serious mental illnesses and general medical illnesses that require ongoing treatment (congestive heart failure, hypertension, diabetes, chronic obstructive pulmonary disease, hypothyroidism, asthma, and heart disease) (22,24–28). One intervention (LGCC) specifically addressed bipolar disorder and general medical illnesses that require ongoing treatment (hypertension, hyperlipidemia, diabetes mellitus, obesity, and heart disease) (29–31). Two interventions (Paxton House and TTIM) addressed a heterogeneous set of serious mental illnesses and one general medical illness that requires ongoing treatment (diabetes) (34–36).
The studies reported findings on an array of clinical outcomes (Table 1). Clinical outcomes examined in more than one study included self-management skills and behaviors, self-management attitudes, biological outcomes, service use, and functional status. More than 70 different outcome measures were used in these studies, ranging from self-report to biological measures. Self-management skills and behaviors significantly increased in seven studies (22–25,28,32,33). Self-management attitudes significantly increased in four studies (25,26,28,32), and one study reported qualitative evidence of increased self-management attitudes (35). Biological outcomes related to risk factors for premature mortality (for example, blood pressure and weight) significantly improved among consumers receiving integrated self-management interventions in four studies (22,31,34,36) (Figure 1). Use of acute services significantly decreased in two studies (22,28). In seven studies, functional status showed significant positive changes (25,28–33).
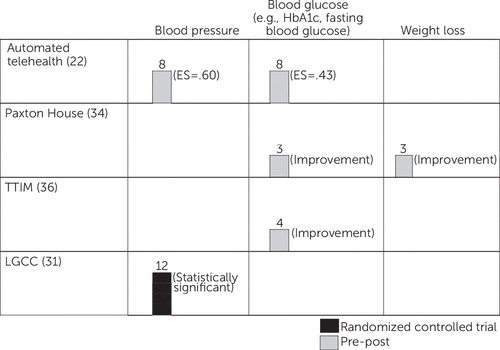
FIGURE 1. Harvest plots of the effectiveness of integrated general medical and psychiatric illness self-management interventions on risk factors for early mortality riska
aTTIM, Targeted Training in Illness Management; LGCC, Life Goals Collaborative Care. Each bar represents a study categorized by intervention. The height of the bar shows the relative sample size, and the number is the Methodological Quality Rating Scale score. Possible scores range from 0, poor quality, to 17, high quality.
Methodological Quality Assessment
Using the MQRS, we evaluated the methodological quality of the studies. The MQRS scores ranged from 3 to 14, with a mean±SD score of 8.69±3.88 and a median score of 9; two studies had a score over 14, indicating a high-quality study (Table 2). Factors that contributed to lower scores included lack of objective measurement of outcomes (N=8, 62%) and not enumerating dropouts (N=8, 62%). Strengths included use of a manualized intervention design (N=9, 69%), provision of sufficient information for replication (N=11, 85%), and inclusion of baseline characteristics (N=10, 77%).
| Attribute (range of possible scores) | Sajatovic et al. (36); Blixen et al. (35) | Druss et al. (23) | Bartels et al. (26) | Goldberg et al. (32) | Mueser et al. (25); Bartels et al. (24) | Kilbourne et al. (29) | Kilbourne et al. (30) | Kilbourne et al. (31) | Lawn et al. (33) | Teachout et al. (34) | Mueser et al. (27) | Bartels et al. (28) | Pratt al. (22) et al. (22) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design (0–4) | 1 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 1 | 3 | 1 |
| Replicability (0–1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Baseline (0–1) | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Quality control (0–1) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| Follow-up length (0–2) | 0 | 1 | 2 | 0 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 1 |
| Follow-up rate (0–2) | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 1 | 2 |
| Objective measurement of outcomes (0–1) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| Dropouts (0–1) | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Independent (0–1) | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Analyses (0–1) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| Study site (0–1) | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| Collateral (0–1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total score | 4 | 10 | 6 | 10 | 14 | 12 | 13 | 12 | 4 | 3 | 5 | 12 | 8 |
TABLE 2. Methodological quality of studies included in the review, by attributea
Potential for Implementation
To assess for implementation potential, we examined intervention structure, duration of the intervention, setting, and interventionist. Most of these interventions were designed primarily for groups, including HARP (23), HOPES (24,25), LGCC (29–31), and Living Well (32) (Table 2). The average group intervention duration was 18.75 group sessions, ranging from four to 52 group sessions. Telephone care management, community trips, and monthly preventive health care supported these interventions (23–25,29–32).
Other interventions included hybrid, individual, and group models. The Paxton House intervention includes a six-month hybrid individual and group model (34), TTIM includes 12 weekly 60–90 minute group sessions and a four-week maintenance period consisting of weekly telephone sessions (35,36), and Norlunga Chronic Disease Self-Management includes 12 months of a group-hybrid model (33). The remaining interventions were automated telehealth, which provides daily self-management prompts by using a device in a person’s home for six months (24 weeks) (22), and I-IMR, which requires weekly individual sessions for eight months (32 weekly sessions) (27,28).
Interventions were studied in a range of settings, including remote (that is, within a person’s home) (22), community mental health center (23,27,28), assisted living facility (26), mental health center or local senior center (24,25), Veterans Affairs outpatient mental health facility (29,30), Veterans Affairs primary care (31), primary care (33,35,36), outpatient clinic and psychiatric rehabilitation programs (32), and a supported housing residence (34).
Interventionists included a rehabilitation specialist and psychiatric nurse (26), nurse and bachelor’s-level case manager (24,25), social worker supported by a nurse care manager (27,28), nurse (29), master’s-level social worker or health specialist (30,31), advanced-practice nurse and clinical staff (34), peers (23,32,33), and peers and nurse educators (35,36). In one study, the interventionists monitored progress via technology and intervened only when clinically necessary (22).
Discussion
There is growing evidence that integrated general medical and psychiatric interventions can improve the lives of adults with serious mental illness. This systematic review identified 15 studies that reported on nine integrated self-management interventions. Most of the studies established support for the feasibility, acceptability, and preliminary clinical effectiveness of the intervention in regard to enhancing participants' knowledge of self-management skills, promoting behavioral and attitudinal changes toward managing illnesses, reducing psychiatric symptoms, stimulating changes in biological indicators of general medical illnesses, and reducing use of acute services.
Although these studies had positive findings, outcome measures varied greatly. More than 70 different measures were used to collect data, which indicates the complexity involved in simultaneously measuring multiple general medical and psychiatric outcomes. Composite measures that aggregate data on biological indicators of various diseases and their severity may more efficiently measure outcomes for multiple morbidities.
However, composite measures, such as the Framingham Risk Score, may not be optimal for adults with serious mental illness. A systematic review found that the Framingham Risk Score was not associated with any changes among adults with serious mental illness who were participating in multicomponent intervention models (37). One explanation for differences in findings between the general population and adults with serious mental illness may be that the algorithms used to establish the Framingham Heart Study risk scores were determined with a sample that excluded adults with serious mental illness (38). Adults with serious mental illness experience unique biobehavioral and environmental risk exposures, such as to antipsychotic medication (39), trauma (40), and chronic stress (41), that could result in variations in risk scores on biometric indices of cardiovascular disease, compared with the general population. Research has shown that risk prediction models that included biobehavioral variables, such as social deprivation, psychiatric diagnosis, prescriptions for antidepressants and antipsychotics, and alcohol use, were better than the Framingham Risk Score for predicting risk among adults with serious mental illness (42). Future development of risk assessments for morbidity and mortality might be improved by inclusion of unique biobehavioral and environmental risk exposures of adults with serious mental illnesses.
The current evidence highlights the feasibility and acceptability of addressing cardiovascular disease and risk factors for early mortality (for example, high blood pressure and obesity) among individuals with serious mental illness. However, the clinical effectiveness of these interventions could not be established because of methodological limitations. Therefore, we do not know the extent to which integrated general medical and psychiatric self-management interventions have a direct impact on the general medical health of individuals with serious mental illness. More rigorous evaluations of these interventions are warranted.
The evidence base for integrated self-management interventions is predominantly built on single-site trials that included small samples and varied in follow-up length, which greatly limited the external validity of these interventions in real-world settings. One intervention, HOPES (24,25), received a score of 14 on the MQRS, indicating a high-quality study. However, despite the effectiveness of HOPES and sustained long-term outcomes (24,25), the required effort and associated costs of professional staff to conduct a weekly group intervention for 12 months may deter broad implementation.
A strength of this systematic review was its examination of the potential for implementation of these interventions. Although the identified interventions have demonstrated feasibility, acceptability, and preliminary clinical effectiveness, their impact may be limited because of the costly efforts of professional staff and the intensity and duration of these interventions. Thus the potential to widely implement and disseminate effective integrated self-management interventions for adults with serious mental illness is limited because of workforce needs, length and intensity, and associated costs of implementation.
Some promising intervention characteristics may increase the potential for implementation—such as limiting the physical resources required to implement an intervention, using technology, and hiring and training peers to deliver services. Meeting as a group can also shorten the amount of time a provider spends with consumers delivering the intervention; however, meeting as a group may have an impact on intervention effect. Future research is needed to examine active intervention components and mechanisms of behavioral change. This research has the potential to reduce the duration of the intervention.
In one study, automated telehealth was used to deliver an integrated self-management intervention (22). Results of this study suggest that a remote technology–based integrated general medical and psychiatric self-management intervention is feasible and acceptable and may be effective with participants who have heterogeneous general medical and psychiatric conditions. Although remote technology–based interventions are promising, it is not clear whether this type of technology provides benefits similar to those of in-person integrated self-management interventions that promote community participation (24,25) and practice of self-management skills in the community (for example, grocery shopping and cooking). Remote technology–based interventions may be best suited for adults with serious mental illness who have mobility limitations or transportation difficulties or who live in rural areas. An emerging research literature documents that adults with serious mental illness are using mobile health and electronic health technologies, including online, wearable, or remote devices, to engage in behavioral health interventions (43). However, it is not known whether such technologies can facilitate the implementation and delivery of effective integrated self-management interventions outside remote locations.
Peer-delivered, integrated self-management interventions are another potentially viable option. Studies have shown that involving peers in interventions for adults with serious mental illness may solve problems related to workforce shortages. Interventions that include peers, including HARP (23), Living Well (32), Norlunga Chronic Disease Self-Management program (33), and TTIM (35,36), may produce costs savings. A systematic review found that peers were as effective as professional staff (44). In some settings, peers have a sustainable financial infrastructure (Medicaid reimbursement for licensed mental health peer specialist services) (45), which can help “scale up” peer-delivered integrated self-management interventions. Incorporating integrated illness self-management training in peer specialists’ state licensure requirements could create a sustainable, low-cost, national workforce of integrated illness self-management providers.
To our knowledge, this is the first systematic review of integrated general medical and psychiatric illness self-management interventions for adults with serious mental illness. However, we found that several characteristics of the existing literature limited our capacity to draw definitive conclusions on the aggregate effectiveness and implementation readiness of these interventions. First, the identified studies used more than 70 different instruments to collect data, and the variability of measurement made it impossible to conduct a meta-analysis to examine the effectiveness of these interventions. Second, we examined variables that are likely to enhance the likelihood of an intervention’s being implemented; however, these studies were limited to feasibility and effectiveness trials and did not report whether these interventions have been implemented in real-world settings.
Conclusions
This review identified five additional studies and three additional interventions not identified in previous systematic reviews (14,15). Our review expands on earlier reviews that focused on general medical self-management only (14) and psychiatric self-management only (15) by focusing on integrated interventions and identifying potential mechanisms to facilitate implementation. Evaluations of integrated self-management interventions have established strong support for their feasibility, acceptability, and potential clinical effectiveness. However, the likelihood for widespread dissemination and uptake of these interventions in their current state is limited by their designs and service delivery strategies.
Because integrated illness self-management is likely to be an important approach for addressing multiple morbidities among adults with serious mental illness, future research is needed to address several issues. We should evaluate whether such interventions can address the early mortality gap affecting adults with serious mental illness by using composite measures that consider specific biobehavioral and environmental risk exposures that affect this group. Furthermore, new and existing self-management interventions should consider alternative service delivery strategies to “scale up” interventions, including group-based interventions, mobile health or electronic health technologies, and peer-delivered services. Future research could consider expanding on efforts to modify and deliver programs that are widely available to the general public to meet the needs of high-risk groups, such as individuals with serious mental illness and co-occurring chronic general medical conditions. For example, the widely used chronic disease self-management program, which has been adapted for individuals with serious mental illness to manage general medical conditions (4), could also include psychiatric self-management. Additional efforts are needed to further explore the potential of using emerging technologies to facilitate implementation and delivery of integrated psychiatric and general medical illness self-management programs across the usual clinical settings that serve this population.
1 : Physical illness in patients with severe mental disorders: I. prevalence, impact of medications and disparities in health care. World Psychiatry 10:52–77, 2011Crossref, Medline, Google Scholar
2 : Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 72:334–341, 2015Crossref, Medline, Google Scholar
3 Parks J, Svendsen D, Singer P (eds): Morbidity and Mortality in People With Serious Mental Illness. Alexandria, Va, National Association of State Mental Health Program Directors Medical Directors Council, 2006Google Scholar
4 : Effectiveness of the chronic disease self-management program for persons with a serious mental illness: a translation study. Community Mental Health Journal 50:96–103, 2014Crossref, Medline, Google Scholar
5 : Clinical trial of wellness training: health promotion for severely mentally ill adults. Journal of Nervous and Mental Disease 196:475–483, 2008Crossref, Medline, Google Scholar
6 : Unending Work and Care: Managing Chronic Illness at Home. San Francisco, Jossey-Bass, 1988Google Scholar
7 : A randomized controlled trial of the effectiveness of the Illness Management and Recovery program. Psychiatric Services 58:1461–1466, 2007Link, Google Scholar
8 : Randomized controlled trial of Illness Management and Recovery in multiple-unit supportive housing. Psychiatric Services 60:1629–1636, 2009Link, Google Scholar
9 : A randomized controlled trial of the Illness Management and Recovery program for persons with schizophrenia. Psychiatric Services 62:606–612, 2011Link, Google Scholar
10 : The Illness Management and Recovery program: rationale, development, and preliminary findings. Schizophrenia Bulletin 32(suppl 1):S32–S43, 2006Crossref, Medline, Google Scholar
11 : Caring for the whole person: integrated health care for older adults with severe mental illness and medical comorbidity. Journal of the American Geriatrics Society 52(suppl):S249–S257, 2004Crossref, Medline, Google Scholar
12 : Mood disorders in the medically ill: scientific review and recommendations. Biological Psychiatry 58:175–189, 2005Crossref, Medline, Google Scholar
13 : Physical illness in patients with severe mental disorders: II. barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry 10:138–151, 2011Crossref, Medline, Google Scholar
14 : Chronic disease self-management interventions for adults with serious mental illness: a systematic review of the literature. General Hospital Psychiatry 36:233–244, 2014Crossref, Medline, Google Scholar
15 : Reviewing illness self-management programs: a selection guide for consumers, practitioners, and administrators. Psychiatric Services 66:1180–1193, 2015Link, Google Scholar
16 : Self-management education: history, definition, outcomes, and mechanisms. Annals of Behavioral Medicine 26:1–7, 2003Crossref, Medline, Google Scholar
17 : Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction 97:265–277, 2002Crossref, Medline, Google Scholar
18 : Lifestyle interventions for adults with serious mental illness: a systematic literature review. Psychiatric Services 61:774–782, 2010Link, Google Scholar
19 : Integrated psychosocial and opioid-antagonist treatment for alcohol dependence: a systematic review of controlled evaluations. Social Work Research 28:41–53, 2004Crossref, Google Scholar
20 : Functional Adaptation Skills Training (FAST): a randomized trial of a psychosocial intervention for middle-aged and older patients with chronic psychotic disorders. Schizophrenia Research 86:291–299, 2006Crossref, Medline, Google Scholar
21 : Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophrenia Bulletin 40:1244–1253, 2014Crossref, Medline, Google Scholar
22 : Feasibility and effectiveness of an automated telehealth intervention to improve illness self-management in people with serious psychiatric and medical disorders. Psychiatric Rehabilitation Journal 36:297–305, 2013Crossref, Medline, Google Scholar
23 : The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophrenia Research 118:264–270, 2010Crossref, Medline, Google Scholar
24 : Long-term outcomes of a randomized trial of integrated skills training and preventive healthcare for older adults with serious mental illness. American Journal of Geriatric Psychiatry 22:1251–1261, 2014Crossref, Medline, Google Scholar
25 : Randomized trial of social rehabilitation and integrated health care for older people with severe mental illness. Journal of Consulting and Clinical Psychology 78:561–573, 2010Crossref, Medline, Google Scholar
26 : Enhanced skills training and health care management for older persons with severe mental illness. Community Mental Health Journal 40:75–90, 2004Crossref, Medline, Google Scholar
27 : Integrated Illness Management and Recovery: a program for integrating physical and psychiatric illness self-management in older persons with severe mental illness. American Journal of Psychiatric Rehabilitation 15:131–156, 2012Crossref, Google Scholar
28 : Integrated IMR for psychiatric and general medical illness for adults aged 50 or older with serious mental illness. Psychiatric Services 65:330–337, 2014Link, Google Scholar
29 : Improving medical and psychiatric outcomes among individuals with bipolar disorder: a randomized controlled trial. Psychiatric Services 59:760–768, 2008Link, Google Scholar
30 : Life Goals Collaborative Care for patients with bipolar disorder and cardiovascular disease risk. Psychiatric Services 63:1234–1238, 2012Link, Google Scholar
31 : Randomized controlled trial to assess reduction of cardiovascular disease risk in patients with bipolar disorder: the Self-Management Addressing Heart Risk Trial (SMAHRT). Annals of Clinical Psychiatry 74:e655–e662, 2013Crossref, Google Scholar
32 : Living Well: an intervention to improve self-management of medical illness for individuals with serious mental illness. Psychiatric Services 64:51–57, 2013Link, Google Scholar
33 : The mental health expert patient: findings from a pilot study of a generic chronic condition self-management programme for people with mental illness. International Journal of Social Psychiatry 53:63–74, 2007Crossref, Medline, Google Scholar
34 : Paxton House: integrating mental health and diabetes care for people with serious mental illnesses in a residential setting. Psychiatric Rehabilitation Journal 34:324–327, 2011Crossref, Medline, Google Scholar
35 : Treating severe mental illnesses and comorbid medical conditions in the primary care setting: an idea whose time has come. Cutting Edge Psychiatry in Practice 1:106–110, 2011Google Scholar
36 : Optimizing care for people with serious mental illness and comorbid diabetes. Psychiatric Services 62:1001–1003, 2011Link, Google Scholar
37 Interventions to Improve Cardiovascular Risk Factors in People With Serious Mental Illness. Rockville, Md, Agency for Healthcare Research and Quality, 2013. effectivehealthcare.ahrq.gov/ehc/products/377/1464/mental-illness-cardio-risk-executive-130422.pdfGoogle Scholar
38 : General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117:743–753, 2008Crossref, Medline, Google Scholar
39 : Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database. Archives of General Psychiatry 64:242–249, 2007Crossref, Medline, Google Scholar
40 : Trauma and psychosis: an analysis of the national comorbidity survey. American Journal of Psychiatry 164:166–169, 2007Link, Google Scholar
41 : The Principles and Practice of Psychiatric Rehabilitation: An Empirical Approach. New York, Guilford, 2008Google Scholar
42 : Cardiovascular risk prediction models for people with severe mental illness: results from the prediction and management of cardiovascular risk in people with severe mental illnesses (PRIMROSE) research program. JAMA Psychiatry 72:143–151, 2015Crossref, Medline, Google Scholar
43 : Emerging mHealth and eHealth interventions for serious mental illness: a review of the literature. Journal of Mental Health 24:321–332, 2015Crossref, Medline, Google Scholar
44 : Peer support services for individuals with serious mental illnesses: assessing the evidence. Psychiatric Services 65:429–441, 2014Link, Google Scholar
45 State Medicaid Directors Letter 07-11. Baltimore, Centers for Medicare and Medicaid Services, 2007. http://downloads. cms.gov/cmsgov/archived-downloads/SMDL/+ downloads/SMD081507A.pdfGoogle Scholar




