Effects of FDA Advisories on the Pharmacologic Treatment of ADHD, 2004–2008
Abstract
Objective
This study assessed the effect of public health advisories issued between 2005 and 2007 by the U.S. Food and Drug Administration (FDA) on treatments of attention-deficit hyperactivity disorder (ADHD) and physician prescribing practices.
Methods
Data obtained from the IMS Health National Disease and Therapeutic Index, a nationally representative audit of ambulatory physicians, were used to examine trends in office visits by children and adolescents (under age 18) during which ADHD was treated with Adderall, other psychostimulants, or atomoxetine. Segmented time series regressions were conducted to determine changes in use associated with three advisories issued between 2005 and 2007.
Results
In 2004, before the first FDA advisory, Adderall accounted for 36% of ADHD pharmacotherapy treatment visits. Other stimulants accounted for 46%, and atomoxetine accounted for 19%. Overall pharmacotherapy treatment rates were stable over the study period, but by 2008 the treatment visits accounted for by Adderall (that is, market share) declined to 24%, and the market share for atomoxetine declined to 8%. The market share for substitute therapies—clonidine, guanfacine, and bupropion—was stable over this period, ranging from 5% to 7%. Despite the declines in the use of Adderall and atomoxetine over the study period, results from the regression models suggest that the advisories did not have a statistically significant effect on ADHD medication prescribing.
Conclusions
FDA advisories regarding potential cardiovascular and other risks of ADHD medications had little discernible incremental effect on the use of these medicines in this nationally representative ambulatory audit.
Attention-deficit hyperactivity disorder (ADHD) is common and costly in the United States (1,2). One study has estimated that the disorder affects 8.7% of children and adolescents (3). Although several nonpharmacologic therapies for ADHD exist, pharmacologic therapies are the dominant method of treatment in the United States (3,4). Rates of stimulant therapy for children and adolescents increased markedly between 1987 and 1997 (5) and have since continued to rise slowly (6,7). Stimulants are now the most commonly prescribed medication for U.S. children and adolescents (8), with approximately 3.5% of U.S. children receiving stimulants in 2008 (6). Nonstimulant pharmacologic therapies, including atomoxetine (9,10), clonidine, guanfacine and bupropion, are also now available for the treatment of ADHD (11,12).
Side effects of ADHD medications are generally mild, but conflicting data regarding risks of cardiovascular effects and adverse psychiatric effects have emerged over the past decade (13–19). The U.S. Food and Drug Administration (FDA) conducted a review of these risks, culminating in a series of risk communications to health care providers and the general public. The first occurred in February 2005 and consisted of a public health advisory (PHA) regarding the potential for serious cardiovascular events with the use of amphetamine and dextroamphetamine (Adderall and Adderall XR) (referred to below as Adderall) (20). In September 2005, the FDA issued a PHA about the potential for sudden death and suicidal ideation associated with atomoxetine, revised the manufacturer’s patient medication guide, and altered the product labeling to include a boxed warning and additional statements to alert health care providers to these risks (21). Finally, in February 2007, an advisory was issued for all ADHD medications, cautioning of “possible cardiovascular risks and risks of adverse psychiatric symptoms.” At this time, all manufacturers were advised to develop patient medication guides (22). All three advisories were posted on the FDA’s MedWatch Web site, consistent with FDA risk communication procedures (23). In 2008, the American Heart Association and the American Academy of Pediatrics both issued statements recommending assessment for heart conditions among children who require ADHD pharmacotherapy (24,25).
We examined the association between these FDA advisories and the use of ADHD medications in the United States. In addition to quantifying the association between each advisory and ADHD treatment, we were also interested in whether the advisories shifted the share of ADHD treatment visits conducted by specialists and whether the advisories may have had different effects on treatment patterns for primary care providers and for specialists. Such effects are plausible given that specialists may have greater—yet more narrow—expertise in managing the treatment of increasingly risky treatments.
Methods
Data
We used data from the National Disease and Therapeutic Index (NDTI), a nationally representative audit of office-based physicians conducted by IMS Health. The NDTI uses a two-stage sampling procedure and collects data from 4,300 physicians randomly selected within strata defined by specialty and geographic area, generating approximately 350,000 annual contact records. Physicians participate by recording information on all clinical contacts during two randomly selected, consecutive workdays per quarter. The NDTI links each drug therapy to a specific six-digit taxonomic code capturing diagnostic information similar to the International Classification of Diseases 9th Revision (ICD-9). The NDTI does not provide information on any nonpharmacologic therapies used. We limited our analyses to the approximately 85% of contacts generated through office visits. Previous studies have compared the NDTI with the National Ambulatory Medical Care Survey, a nationally representative survey conducted by the National Center for Health Statistics; these studies have suggested that both data sources provide similar estimates of office-based medication use (26–28).
We defined ADHD using diagnostic codes “overactivity not otherwise specified” (314.001) and “attention deficit disorder” (314.004), which correspond to ICD-9 codes for attention deficit disorder with and without hyperactivity. When analyses were conducted with a broader classification (314.xx), they yielded virtually identical findings. We focused on four groups of therapies: Adderall (including Adderall and Adderall XR), non-Adderall stimulants, all stimulants, and atomoxetine. [The brand names of drugs included are listed in an online data supplement to this article.] We also assessed changes in the market share for additional substitute medications (clonidine, guanfacine, and bupropion) that were neither stimulants nor atomoxetine and that were selected on the basis of clinical expertise and confirmed by review of the most common medications prescribed for ADHD.
Our primary unit of analysis was a visit during which at least one ADHD pharmacotherapy was prescribed or continued—referred to below as a treatment visit. Because individuals may have received more than one therapy during a single visit, the total reported treatment visits may exceed 100% during a given period of observation. For analyses that used physician specialty, we defined primary care providers as those trained in pediatrics, family practice, general practice, internal medicine, or osteopathic medicine. Specialists were defined as all other provider types. The study was exempted from institutional review board review at the University of Chicago.
Advisories and analyses
We focused on three FDA advisories: the February 2005 advisory concerning Adderall use, which cautioned that “patients with underlying heart defects might be at increased risk for sudden death”; the September 2005 advisory concerning atomoxetine use, which cautioned “an increased risk of suicidal thinking in children and adolescents being treated with this drug”; and the February 2007 advisory concerning use of all FDA-approved ADHD medications, which cautioned “possible cardiovascular risks and risks of adverse psychiatric symptoms.” Thus, although one advisory focused on children and adolescents with cardiac comorbidities, subsequent advisories warned of serious psychiatric risks, including suicidality, that were not limited to those with preexisting symptoms.
We first used descriptive statistics to examine 2004–2008 trends in the pharmacologic treatments of ADHD among children and adolescents less than 18 years old. Next, we conducted multivariate regression to isolate the effect of the FDA advisories of interest. We conducted two sets of segmented linear regressions. For the first set, we defined our outcome variable as the proportion of all ADHD treatment visits accounted for by the specific treatments of interest (that is, market share), which included Adderall, non-Adderall stimulants, all psychostimulants, and atomoxetine. By examining the market share rather than the raw frequency of treatment visits for a given therapy, we reduced sampling variability and seasonal effects. Diagnostic tests and graphs revealed evidence of an autoregressive correlation structure in the market share time series. Thus we used a generalized least-squares estimator to adjust for autocorrelation of this type. We repeated these analyses after stratifying visits into those with primary care physicians or specialists in order to assess whether the advisories had a different impact on these two subpopulations of physicians. We assessed both changes in market share slope and jump changes in market share for each new period relative to the preceding period.
In a second set of segmented regressions, we defined our outcome as the total number of treatment visits for a given group of therapies, rather than the proportion of the total market. By doing so, we were able to examine whether there were changes in the absolute number of treatment visits occurring, regardless of whether the market share changed. We conducted two separate regressions examining total number of treatment visits, with one focused on stimulants and atomoxetine and the other on stimulants, atomoxetine, and substitutes alike.
We also assessed whether the advisories shifted care to specialists, because such shifts might be plausible with potentially greater risks associated with medication management. Such a shift would also be supported by evidence of similar broader secular change (7) as well as the effects for advisories in other therapeutic areas (29,30).
Results
Trends in ADHD diagnoses and treatment visits
Between 2004 and 2008, annual ADHD treatment visits were stable, ranging from 9.0 to 10.1 million (Table 1). The market share for all stimulants increased modestly between 2004 and 2006, from 82% to 90% of treatment visits for ADHD, before leveling off. The market shares for both atomoxetine and Adderall decreased over the study period, with atomoxetine declining from 19% in 2004 to 8% in 2008 and Adderall declining from 36% in 2004 to 24% in 2008. There were concomitant increases in the market share for non-Adderall stimulants, from 46% in 2004 to 67% in 2008. The market share for substitutes was stable over this period, ranging from 5% to 7%. Figure 1 shows trends in monthly market shares for all stimulants, Adderall, and atomoxetine between 2000 and 2008. The drop in the market share for stimulants in 2003 corresponds to the entry of atomoxetine at the end of 2002.
| Variable | 2004 | 2005 | 2006 | 2007 | 2008 |
|---|---|---|---|---|---|
| Total ADHD treatment visits (thousands) | 9,495 | 10,063 | 9,900 | 9,630 | 8,993 |
| All stimulants (%) | 82 | 86 | 90 | 90 | 91 |
| Adderall (%) | 36 | 37 | 36 | 31 | 24 |
| Atomoxetine (%) | 19 | 13 | 10 | 10 | 8 |
| Substitutes (%) | 6 | 7 | 7 | 5 | 6 |
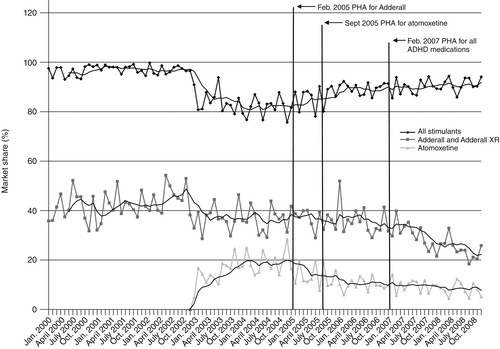
aSource: IMS Health National Disease and Therapeutic Index, 2000–2008. Market share was defined as the proportion of treatment visits accounted for by each drug. PHA, public health advisory. Adderall includes Adderall XR. Data for 2000–2003 are presented for context and were not included in statistical models.
Changes in market share associated with FDA advisories
Tables 2 and 3 summarize data on the impact of the three advisories on the use of Adderall, atomoxetine, non-Adderall stimulants, and all stimulants. Changes in the slope of the regression for each period are given relative to the preceding period. The predicted market share is given at the end of each period. The difference in market share is the difference between this value and the predicted market share for the same time point based on the regression for the new time period. Mean market shares were calculated for each time segment.
| Period and PHA | All stimulants | Adderall | Non-Adderall stimulants | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference in market shareb | Average market share | Slope per month | Change in slope per month | Predicted share at end of period | Difference in market shareb | Average market share | Slope per month | Change in slope per month | Predicted share at end of period | Difference in market shareb | Average market share | Slope per month | Change in slope per month | Predicted share at end of period | |
| Preadvisory period: Jan. 2004–Jan. 2005 | — | 81.59 | .16 | — | 82.94 | — | 36.46 | .17 | — | 37.29 | — | 45.13 | .00 | — | 45.16 |
| After PHA on Adderall: Feb.–Aug. 2005 | +1.36 | 85.59 | −.03 | −.19 | 84.28 | +2.19 | 36.97 | −1.04 | −1.21 | 33.41 | +1.16 | 48.62 | .76 | +.76 | 50.90 |
| After PHA on atomoxetine: Sept. 2005–Jan. 2007 | +4.29* | 88.90 | .07 | +.10 | 89.62 | +5.95 | 35.78 | −.30 | +.74 | 33.55 | −2.03 | 53.13 | .44 | −.33 | 56.62 |
| After PHA on all ADHD medications: Feb. 2007–Dec. 2008 | −.29 | 90.32 | .10 | +.04 | 91.59 | +.75 | 27.40 | −.61 | −.31 | 21.22 | −1.82 | 62.92 | .72 | +.28 | 70.34 |
| Period and PHA | Atomoxetine | Stimulants and atomoxetineb | Stimulants, atomoxetine, and substitutesb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference in market share (%)c | Average market share (%) | Slope per month (%) | Change in slope per month (%) | Predicted share at end of period (%) | Difference in treatment visits (N) | Average treatment visits (N) | Slope per month (N) | Change in slope per month (N) | Predicted visits at end of period (N) | Difference in treatment visits (N) | Average treatment visits (N) | Slope per month (N) | Change in slope per month (N) | Predicted visits at end of period (N) | |
| Preadvisory period: Jan. 2004–Jan. 2005 | — | 19.20 | .20 | — | 20.29 | — | 807.13 | 12.76 | — | 887.44 | — | 854.77 | 12.52 | — | 933.85 |
| After PHA on Adderall: Feb.–Aug. 2005 | −6.83* | 13.28 | .01 | −.19 | 13.72 | −45.25 | 832.41 | −5.47 | −18.22 | 822.15 | −29.55 | 893.55 | −5.39 | −17.91 | 884.45 |
| After PHA on atomoxetine: Sept. 2005–Jan. 2007 | −1.79 | 10.87 | −.15 | −.16 | 9.55 | −6.26 | 823.30 | 1.46 | +6.93 | 833.83 | −9.85 | 880.80 | 1.26 | +6.65 | 889.30 |
| After PHA on all ADHD medications: Feb. 2007– Dec. 2008 | +.79 | 9.04 | −.12 | +.03 | 7.69 | −46.13 | 765.70 | −2.06 | −3.52 | 745.93 | −66.61 | 807.72 | −1.32 | −2.58 | 796.14 |
Other than a decrease in atomoxetine market share after the Adderall PHA and an increase in the market share for all stimulants after the atomoxetine PHA, there were no statistically significant differences in market share or slope after any of the advisories examined. For example, after the Adderall PHA in February 2005, the difference in predicted market share for Adderall using the regression for the prior and new time periods was small (+2.19%), and the mean market share remained stable between the periods (+.51%, from 36.46% to 36.97%). After the same advisory, the predicted market share for non-Adderall stimulants increased slightly (+1.16%) and the predicted market share for atomoxetine decreased by 6.83% (p=.001).
After the atomoxetine PHA in September 2005, there were small decreases in the predicted market shares for atomoxetine (–1.79%) and non-Adderall stimulants (–2.03%) and increases in the market shares for Adderall (+5.95%) and all stimulants (+4.29%, p=.04). Although mean market shares for atomoxetine and Adderall were stable or declined after each advisory, mean market shares for all stimulants and for non-Adderall stimulants increased in each time segment.
Effect of advisories on changes in specialist share
There were modest changes in specialist share during the period examined (Table 4). Overall, the average share of all ADHD visits accounted for by specialists ranged from 36.2% to 41.8%. The largest difference in expected specialist share between two segments occurred after the February 2005 Adderall PHA concerning potential cardiovascular risk, although none of the observed changes in specialist share and slope were statistically significant.
| Period and PHA | Difference in specialist shareb | Average specialist share | Slope per month | Change in slope per month | Predicted share at end of period |
|---|---|---|---|---|---|
| Preadvisory period: Jan. 2004–Jan. 2005 | — | 36.23 | .34 | — | 38.27 |
| After PHA on Adderall: Feb.–Aug. 2005 | +4.84 | 41.78 | −.52 | −.86 | 40.33 |
| After PHA on atomoxetine: Sept. 2005–Jan. 2007 | −1.19 | 41.00 | .26 | +.78 | 42.71 |
| After PHA on all ADHD medications: Feb. 2007–Dec. 2008 | −2.79 | 38.41 | −.18 | −.44 | 36.30 |
Changes in market share for specialist providers
When the analysis of medication market share was limited to primary care providers, findings were similar as for all providers. [A table presenting the results of this analysis is included in the online data supplement to this article.] Little discernible association was found between the advisories examined and measures of ADHD treatment utilization except for an unexpected decrease in the level of atomoxetine use by primary care providers associated with the February 2005 Adderall PHA. An additional statistically significant decrease in atomoxetine use was found after the atomoxetine advisory (–5.7%, p=.02)
Analysis limited to specialists yielded inconsistent findings. For example, no statistically significant associations were found between any of the three advisories examined and either overall stimulant use or the use of non-Adderall stimulants. By contrast, among specialists, the February 2005 Adderall PHA was associated with an increase in the predicted Adderall market share (from 35.63% to 49.85%, p=.02) yet a decrease in the slope of Adderall market share (decreased by –2.37% per month, p=.02). The slope of Adderall market share increased after the atomoxetine PHA in September 2005.
Discussion
In this analysis of a nationally representative audit of office-based providers between 2004 and 2008, changes were observed in the market share for stimulant therapies and for atomoxetine. However, FDA advisories regarding the potential risks of ADHD medications had little discernible effect on their use. These results are important given how common ADHD is in the United States and how frequently it is treated with pharmacologic therapies such as those examined.
Although some FDA communications may have immediate or substantial effects on medication use (31), there are several reasons that others may have limited impact (32–34). First, given that the primary goal of regulatory communications is to convey information rather than change behavior, assessments of changes in prescription drug use or other health care use after an advisory provide a limited window through which to assess whether the advisory was successful. It is possible that the advisories were successful in communicating the uncertainties about ADHD therapies but insufficient to change the overall balance of the treatment decisions that providers and patients faced. For example, instead of reducing their prescribing, physicians may have monitored patients more closely. Second, providers’ lack of response to these advisories may reflect their doubts about the seriousness or reliability of the information upon which they were based. Indeed, a recent retrospective study that used data from four health plans suggested that ADHD drugs do not increase risks of cardiovascular problems among children and young adults (19). Additional factors that may have limited the impact of these FDA advisories include physician knowledge of drug risks that preceded the relevant warnings, changes in the advertising and promotion of these drugs to consumers and providers, and the failure of the advisories to reach their intended audiences or to communicate risk information effectively (35,36).
Furthermore, although we were unable to detect differences in ADHD medication prescribing when rates of prescribing before and after the FDA advisories were compared, it is possible that the FDA advisories nevertheless had an impact on prescribing behavior. Lag effects or interventions that are rolled out in multiple stages (for example, via a series of high-profile reports in the press) may limit the application of time series analyses (37). High-profile news reports and scientific investigations may have preceded FDA advisories, and in some cases, publicity regarding risks may have continued to build after the FDA warnings were issued (38). Even though changes in medication use were not closely timed to FDA advisories, the overall declines in use of Adderall and atomoxetine that we observed and the concomitant increase in the use of other stimulants may reflect shifting assessments of safety.
We also found paradoxical effects of some advisories, such as the association between the February 2005 Adderall PHA and increases in Adderall market share among specialists. These inconsistencies may reflect the fact that other influences on prescribing, such as therapies entering and leaving the marketplace or industry marketing and promotion, may have undermined any effect that the FDA communications might have had. In the case of declines in atomoxetine use that occurred after the Adderall advisories, it is possible that these declines represented “spillover” effects or were due to the influence of scientific or media reports about atomoxetine that occurred during the same period (39).
Although we did not find a statistically significant effect of the advisories on use of specialty care, the modest increase in overall rates of specialty care that we identified is consistent with other reports regarding care patterns for ADHD (7,40). Shifts toward greater specialization of labor associated with increasing drug risks are consistent with theory and also have been evident in other settings, as in the case of antidepressants, when FDA advisories regarding the risk of suicidality among children were accompanied by shifts from primary care physicians to specialists (29,30). Increases in Adderall market share among specialists after the Adderall PHA may in part reflect the referral of patients to specialist care or an increase in follow-up visits among those already on Adderall.
Our analysis has several limitations and raises additional questions for future research. First, segmented regression analysis depends upon a sufficient number of time points and may be sensitive to how the segments are defined. In light of this, we conducted sensitivity analysis that adjusted the length of the time segments (for example, before and after the Adderall PHA in February 2005), and these yielded substantively similar findings. Second, shifts in care on the basis of patients’ sex, socioeconomic status, disease severity, and comorbidities may not have been captured by our methodology. These advisories may also have had spillover effects in the treatment of adult populations that were not captured by our analysis. Third, the advisories may have shifted the rate at which families fill written prescriptions, but these changes were not captured in the NDTI. Fourth, our focus was on office-based diagnosis and pharmacotherapy of children and adolescents, and thus we do not have data regarding the variety of nonpharmacologic treatments that play an important role in ADHD treatment, such as diet, alternative medications, cognitive and behavioral therapies, or social and educational interventions. Future studies might also examine whether FDA advisories had a larger effect among patients with relevant comorbid conditions, such as cardiovascular disease or other mood disorders, that might increase risks. Finally, this analysis was limited to one audit of office-based encounters, and our findings should be corroborated via other office-based audits, such as the National Ambulatory Medical Care Survey.
Conclusions
Overall rates of ADHD pharmacologic treatment of children and adolescents were stable between 2004 and 2008, despite a series of FDA advisories concerning the risks of common ADHD medications. Although the market shares for Adderall and atomoxetine declined over this period, we did not identify any consistent association between FDA advisories and the use of these drugs. Continued assessments of consumer and provider responses to FDA advisories may help identify factors associated with patient and physician responsiveness and will be important for informing future communications about medication risks.
1 : Use and costs of medical care for children and adolescents with and without attention-deficit/hyperactivity disorder. JAMA 285:60–66, 2001Crossref, Medline, Google Scholar
2 : The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. Ambulatory Pediatrics 7(suppl):121–131, 2007Crossref, Medline, Google Scholar
3 : Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Archives of Pediatrics and Adolescent Medicine 161:857–864, 2007Crossref, Medline, Google Scholar
4 Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children—United States, 2003 and 2007. Morbidity and Mortality Weekly Report 59:1439–1443, 2010Medline, Google Scholar
5 : National trends in the treatment of attention deficit hyperactivity disorder. American Journal of Psychiatry 160:1071–1077, 2003Link, Google Scholar
6 : Stimulant medication use in children: a 12-year perspective. American Journal of Psychiatry 169:160–166, 2012Link, Google Scholar
7 : Trends in attention deficit hyperactivity disorder ambulatory diagnosis and medical treatment in the United States, 2000–2010. Academic Pediatrics 12:110–116, 2012Crossref, Medline, Google Scholar
8 : Psychotropic practice patterns for youth: a 10-year perspective. Archives of Pediatrics and Adolescent Medicine 157:17–25, 2003Crossref, Medline, Google Scholar
9 : Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. American Journal of Psychiatry 159:1896–1901, 2002Link, Google Scholar
10 : Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. Journal of the American Academy of Child and Adolescent Psychiatry 41:776–784, 2002Crossref, Medline, Google Scholar
11
12 : ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011Crossref, Medline, Google Scholar
13 : Blood pressure changes associated with medication treatment of adults with attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry 66:253–259, 2005Crossref, Medline, Google Scholar
14 : Suicidal ideation among children taking atomoxetine (Strattera). Canadian Medical Association Journal 173:1447, 2005Crossref, Google Scholar
15 : Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Safety 26:729–740, 2003Crossref, Medline, Google Scholar
16 : ADHD drugs and cardiovascular risk. New England Journal of Medicine 354:1445–1448, 2006Crossref, Medline, Google Scholar
17 : Attention deficit/hyperactivity disorder and methylphenidate: a review of height/weight, cardiovascular, and somatic complaint side effects. Clinical Psychology Review 22:1107–1131, 2002Crossref, Medline, Google Scholar
18 : Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. Journal of Pediatrics 147:348–354, 2005Crossref, Medline, Google Scholar
19 : ADHD drugs and serious cardiovascular events in children and young adults. New England Journal of Medicine 365:1896–1904, 2011Crossref, Medline, Google Scholar
20 Public Health Advisory for Adderall and Adderall XR. Silver Spring, Md, US Food and Drug Administration, Feb 9, 2005. Available at www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051672.htmGoogle Scholar
21 Public Health Advisory: Suicidal Thinking in Children and Adolescents Being Treated With Strattera (Atomoxetine). Silver Spring, Md, US Food and Drug Administration, Sept 29, 2005. Available at www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/UCM051733Google Scholar
22 FDA Directs ADHD Drug Manufacturers to Notify Patients About Cardiovascular Adverse Events and Psychiatric Adverse Events. Silver Spring, Md, US Food and Drug Administration, Feb 21, 2007. Available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108849.htmGoogle Scholar
23 Manual of Policies and Procedures: NDAs: “Dear Health Care Professional” Letters. Silver Spring, Md, US Food and Drug Administration, Center for Drug Evaluation and Research, July 7, 2003. Available at www.fda.gov/downloads/AboutFDA/CentersOffices/CDER/ManualofPoliciesProcedures/ucm082012.pdfGoogle Scholar
24 American Academy of Pediatrics/American Heart Association Clarification of Statement on Cardiovascular Evaluation and Monitoring of Children and Adolescents With Heart Disease Receiving Medications for ADHD. Elk Grove Village, Ill, American Academy of Pediatrics, May 16, 2008. Available at www.aap.org/pressroom/aap-ahastatement.htm.Google Scholar
25 : Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation 117:2407–2423, 2008. Available at circ.ahajournals.org/cgi/content/full/117/18/2407.Crossref, Medline, Google Scholar
26 Zell ER, McCaig LF, Kupronis BA, et al: A comparison of the National Disease and Therapeutic Index and the National Ambulatory Medical Care Survey to evaluate antibiotic usage; in Proceedings of the Survey Research Methods Section, American Statistical Association. Alexandria, Va, American Statistical Association, 2000. Available at www.amstat.org/sections/srms/Proceedings/papers/2000_143.pdfGoogle Scholar
27 : The underutilization of cardiac medications of proven benefit, 1990 to 2002. Journal of the American College of Cardiology 41:56–61, 2003Crossref, Medline, Google Scholar
28 : National trends in ambulatory asthma treatment, 1997–2009. Journal of General Internal Medicine 26:1465–1470, 2011Crossref, Medline, Google Scholar
29 : Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. American Journal of Psychiatry 164:884–891, 2007Link, Google Scholar
30 : Impact of publicity concerning pediatric suicidality data on physician practice patterns in the United States. Archives of General Psychiatry 64:466–472, 2007Crossref, Medline, Google Scholar
31 : Promotion and prescribing of hormone therapy after report of harm by the Women’s Health Initiative. JAMA 292:1983–1988, 2004Crossref, Medline, Google Scholar
32 : Liver enzyme monitoring in patients treated with troglitazone. JAMA 286:831–833, 2001Crossref, Medline, Google Scholar
33 : Understanding responses to contradictory information about products. Marketing Science 30:1098–1114, 2011Crossref, Google Scholar
34 : A study of compliance with FDA recommendations for pemoline (Cylert). Journal of the American Academy of Child and Adolescent Psychiatry 41:785–790, 2002Crossref, Medline, Google Scholar
35 : Impact of FDA drug risk communications on health care utilization and health behaviors: a systematic review. Medical Care 50:466–478, 2012Crossref, Medline, Google Scholar
36 : Do pharmaceutical sales respond to scientific evidence? Journal of Economics and Management Strategy 11:551–594, 2002Crossref, Google Scholar
37 : Segmented regression analysis of interrupted time series studies in medication use research. Journal of Clinical Pharmacy and Therapeutics 27:299–309, 2002Crossref, Medline, Google Scholar
38 : Information, learning, and drug diffusion: the case of Cox-2 inhibitors. Quantitative Marketing and Economics 7:399–443, 2009Crossref, Google Scholar
39 Szabo L: New warnings due for ADHD drugs. USA Today, June 30, 2005, p 1AGoogle Scholar
40 : Trends in the use of psychotropic medications among adolescents, 1994 to 2001. Psychiatric Services 57:63–69, 2006Link, Google Scholar



