Outcome Assessment of an Antipsychotic Drug Algorithm: Effects of the Mississippi State Hospital Algorithm Project
Abstract
Objective:
This study evaluated a state psychiatric hospital's algorithm for prescribing antipsychotic drugs for inpatients with schizophrenia to determine whether its emphasis on cost efficiency is compatible with quality of care.
Methods:
Outcomes were compared for patients who received medication that was algorithm adherent or nonadherent. Risperidone and ziprasidone were first-step oral antipsychotics. Documentation of clinical rationale was acceptable for nonpreferred drug use. Outcomes of interest were length of hospitalization and “much improved” or “very much improved” status on the Clinical Global Impression severity scale (CGI-S).
Results:
Of 401 patients, 70% were male. The CGI-S modal rating of severity was “markedly ill.” Duration of illness was longer for patients given algorithm-nonadherent (17.6±9.7 years) versus -adherent (14.9±11.6 years, p=.013) medication. No statistically significant between-group differences were observed for mean length of stay (51.4±35.5 days versus 43.8±27.4 days, adjusted difference p=.18) or median improvement time (adherent, 41 days; nonadherent, 42 days; CI=34–48 days for both group medians).
Conclusions:
Prescription algorithm adherence was not associated with significantly increased length of inpatient stay or delayed time to improvement. (Psychiatric Services 62:963–965, 2011)
Various algorithms for prescribing antipsychotic drugs have been developed and implemented to improve treatment for patients with schizophrenia (1). Multiple factors influence the adoption of these treatment algorithms, but data indicate that they can be successfully incorporated into usual practice (2,3) and that adherence to them is associated with generally improved clinical outcomes (4,5).
Mississippi State Hospital (MSH) developed an antipsychotic drug use algorithm that specifies preferred first-choice drugs (risperidone and ziprasidone), followed by sequenced trials of alternate agents. In this algorithm, combination antipsychotic drug use is discouraged, requiring multiple antipsychotic monotherapy trials before proceeding to maintenance combination therapy. Efficacy was a consideration, but because no consistent evidence in comparative trials indicates a difference in antipsychotic efficacy, choice of preferred drugs was based more on cost, flexibility of use (dosage forms), and safety, especially with regard to metabolic risks. The algorithm is a guideline that is intended to improve cost efficiency without harming quality of care. The purpose of this study was to evaluate the effect of adherence versus nonadherence to the treatment algorithm on length of hospital stay and time to improvement.
Methods
This was a naturalistic study involving a cohort of adult patients (18 or more years of age) admitted to MSH between March 1, 2005, and November 30, 2005, who had a diagnosis of schizophrenia (N=236), schizoaffective disorder (N=154), or schizophreniform disorder (N=11).
MSH is a publicly funded psychiatric hospital and nursing home facility of approximately 1,000 beds operated under the direction of the Mississippi Department of Mental Health. Approximately 180 beds are acute psychiatric admission beds for adult patients and were the source of patients for this study.
The algorithm was developed in 2004 and designated risperidone and ziprasidone as preferred drugs in step 1. At the time, risperidone was the most commonly used antipsychotic drug, was available in a variety of formulations, was considered moderate among second-generation antipsychotics in likelihood to cause metabolic adverse effects, and had the lowest acquisition cost. Choice of a second preferred agent took into account the high prevalence of metabolic disease in the population served by the hospital, particularly obesity and diabetes. Ziprasidone was considered less likely than certain other second-generation agents to cause metabolic adverse effects and was second lowest in average acquisition cost among these drugs. First-generation antipsychotics were not included as step 1 preferred agents, reflecting common drug use patterns and guidelines at the time of algorithm development. Use of nonpreferred drugs, augmentation drugs, and combination antipsychotic drug therapy was specified in a stepwise approach. [An appendix showing the algorithm for antipsychotic usage is included as an online supplement to this report at ps.psychiatryonline.org.]
Adherence was determined on the basis of drug choice or documentation for deviation from preferred drugs. Choice of a nonpreferred drug with documented rationale, such as previous failure to respond to or tolerate a preferred drug, was considered acceptable and not a deviation from the algorithm. Prescribing according to steps of the algorithm was assessed from time of hospital admission until discharge criteria were met. The algorithm was provided as a guideline, not a mandated prescribing pathway. All prescribers were trained regarding the algorithm, and implementation was approved by a vote from the hospital's medical staff. Prescriber adherence to the algorithm was assessed on the basis of patient record review by a research nurse. Assessment of outcomes was limited to patients admitted to the acute service of the hospital. Patients from long-term units and the child and adolescent service were not included.
The primary outcome measure was length of hospital stay, which was calculated as the difference in number of days from hospital admission date to the date patients no longer met admission criteria, based on resource utilization management criteria documented in patient records. These include judgments of dangerousness to self or others and of inability to manage basic needs. As a result, time awaiting community placement was specifically not included in the study's measure of length of hospital stay. The secondary outcome measure was time to achievement of “much improved” or “very much improved” ratings, defined by the Clinical Global Impression of Improvement (CGI-I). Patient records were reviewed to determine algorithm adherence and nonadherence.
Baseline measures of age, gender, racial-ethnic group, duration of illness, number of hospitalizations, extrapyramidal symptoms, and illness severity on the CGI severity scale (CGI-S) were recorded. During the course of the patient's hospitalization, the CGI-I was used to record changes in status at one-week intervals and at discharge. Assessments were based on documentation in patient records. Baseline data analysis used chi squaretests for categorical measures and t tests for continuous measures. Linear regression was used to compare length of stay between treatment algorithm cohorts, and survival analysis (Kaplan-Meyer life table method and Cox proportional hazards regression) was used to compare time to improvement, as measured by the CGI-I.
This study was approved by the MSH Institutional Review Board. Data were collected only from patient records, study inclusion did not affect treatment, and patients were not at risk; therefore, the requirement for obtaining informed consent was waived.
Results
A total of 401 patients were included in the study (263 in the algorithm-adherent group and 138 in the algorithm-nonadherent group). Table 1 lists baseline characteristics for each group. The two groups did not differ significantly on age, race-ethnicity, diagnosis, and number of past hospitalizations. However, patients in the algorithm-nonadherent group were more often male (76% versus 67%, χ2=3.92, df=1, p=.048) and had been ill significantly longer (17.6 years versus 14.9 years, t=2.49, dfunequal variance=324.6, p=.013). Illness severity, as measured by the CGI-S and the presence of extrapyramidal symptoms, did not differ significantly between the groups, although the algorithm-adherent group had more patients at the worse extremes of these two measures. Overall, the modal level of severity was “markedly ill.” Most patients exhibited no extrapyramidal symptoms.
The unadjusted mean length of stay in the algorithm-adherent group was 51.4±35.5 days, versus 43.8±27.4 days in the algorithm-nonadherent group (t=2.36, dfunequal variance=345, p=.019), an unadjusted difference of 7.5 days. We ran a general linear model comparing length of stay by algorithm adherence group, including gender, age, baseline CGI-S score, duration of illness, number of past hospitalizations, and extrapyramidal symptoms as covariates. Of these covariates, only gender and baseline CGI-S score were significant predictors of length of stay. In a reduced model that included algorithm adherence group, gender, and baseline CGI-S score as predictors, the adjusted difference between algorithm adherence groups was reduced (difference=3.9 days; 95% confidence interval [CI]=−1.8–9.7; F=1.80, df=1 and 394, p=.18).
On the secondary outcome of time to improvement on the CGI-I to a rating of much improved or very much improved, there was no significant difference between groups (Wilcoxon statistic=.002, df=1, p=.97). The median time to improvement was similar between groups: 41 days in the algorithm-adherent group and 42 days in the algorithm-nonadherent group (CI=34–48 days for both group medians). Cox proportional hazards regression that controlled for gender, baseline CGI-S score, and number of past hospitalizations, all of which were significant univariate predictors of time to improvement, did not result in any change in the effect of algorithm adherence on time to improvement. The hazard ratio for the algorithm-adherent group was 1.10 (CI=.84–1.43).
Discussion
This was a naturalistic study of clinical outcomes in relation to adherence to an algorithm for prescribing antipsychotic drugs. The objective was to determine whether emphasis on cost efficiency can be readily compatible with quality of care. In addition to drug acquisition cost, factors in choice of the preferred drugs (risperidone and ziprasidone) included safety, flexibility in dosage formulation, and metabolic safety. The most commonly prescribed drugs in the algorithm-nonadherent group were haloperidol (39%), quetiapine (18%), olanzapine (14%), aripiprazole (12%), and fluphenazine (12%). Algorithm-nonadherent prescribing also included antipsychotic polytherapy, which sometimes involved the two preferred drugs. Although drug costs can be substantial, they constitute a relatively small percentage of overall treatment costs in an institutional setting. Even significant savings on drug costs would be heavily offset if treatment outcomes were compromised and hospital stay were prolonged. The main finding of this study, that adherence to the algorithm was not associated with increased hospital stay or time to improvement, is perhaps not surprising in light of substantial evidence showing no consistent efficacy superiority of one second-generation antipsychotic drug over others (6). Study results do not establish noninferiority, however, because a noninferiority margin was not prespecified and a noninferiority analysis was not performed.
Study limitations include a relatively small sample obtained from a single center. It is possible that algorithm implementation in larger, multiple- hospital systems or a statewide system may yield different results. Voluntary adherence to the medication algorithm and nonrandomization of patients to prescribers may also have influenced the main study findings. It is likely that some prescribers were more adherent to the algorithm than others, but we cannot say what prescriber characteristics—gender, age, time in practice, and so on—were predictive. Prescribers selected first-line agents according to clinical judgment, and algorithms with restrictions or prescribing incentives may yield different results. Further, ratings of algorithm adherence and severity (such as with the CGI) were made by a single, unblinded rater, and we cannot exclude the possibility of bias. Although some multivariate analyses were performed, it was not possible to control for all potential confounders such as rater bias or other group differences. Finally, assessments of illness severity and improvement were based on patient record review instead of standardized clinical interviews, which may have caused some inconsistency in documentation.
Conclusions
On the basis of this naturalistic study, we conclude that implementation of a stepwise algorithm for prescribing antipsychotic drugs achieved efficiencies in drug acquisition costs and was not associated with a statistically significant effect on hospital stay or time to improvement.
1 : The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. Journal of Clinical Psychiatry 68:1751–1762, 2007 Crossref, Medline, Google Scholar
2 : Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Quarterly 82:581–629, 2004 Crossref, Medline, Google Scholar
3 : The Texas Medication Algorithm Project: clinical results for schizophrenia. Schizophrenia Bulletin 30:627–647, 2004 Crossref, Medline, Google Scholar
4 : Prescriber fidelity to a medication management evidence-based practice in the treatment of schizophrenia. Psychiatric Services 60:929–935, 2009 Link, Google Scholar
5 : Implementation of computerized medication prescribing algorithms in a community mental health system. Psychiatric Services 60:1010–1012, 2009 Link, Google Scholar
6 : Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353:1209–1223, 2005 Crossref, Medline, Google Scholar
Figures and Tables
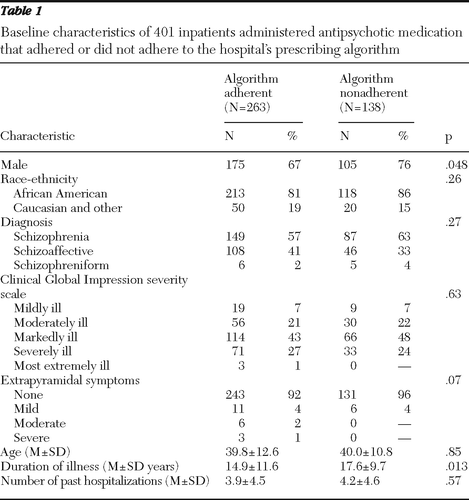
Table 1 Baseline characteristics of 401 inpatients administered antipsychotic medication that adhered or did not adhere to the hospital's prescribing algorithm



