Guided Self-Help Treatment for Recurrent Binge Eating: Replication and Extension
Abstract
Objective:
The aim of this study was to replicate and extend results of a previous blended efficacy and effectiveness trial of a low-intensity, manual-based guided self-help form of cognitive-behavioral therapy (CBT-GSH) for the treatment of binge eating disorders in a large health maintenance organization (HMO) and to compare them with usual care.
Methods:
To extend previous findings, the investigators modified earlier recruitment and assessment approaches and conducted a randomized clinical trial to better reflect procedures that may be reasonably carried out in real-world practices. The intervention was delivered by master's-level interventionists to 160 female members of a health maintenance organization who met diagnostic criteria for recurrent binge eating. Data collected at baseline, immediately posttreatment, and at six- and 12-month follow-ups were used in intent-to-treat analyses.
Results:
At the 12-month follow-up, CBT-GSH resulted in greater remission from binge eating (35%, N=26) than usual care (14%, N=10) (number needed to treat=5). The CBT-GSH group also demonstrated greater improvements in dietary restraint (d=.71) and eating, shape, and weight concerns (d=1.10, 1.24, and .98, respectively) but not weight change.
Conclusions:
Replication of the pattern of previous findings suggests that CBT-GSH is a robust treatment for patients with recurrent binge eating. The magnitude of changes was significantly smaller than in the original study, however, suggesting that patients recruited and assessed with less intensive procedures may respond differently from their counterparts enrolled in trials requiring more comprehensive procedures. (Psychiatric Services 62:367–373, 2011)
Epidemiological studies suggest that clinical and subclinical eating disorders affect more than 5% of adult women (1). The most common type of eating disorder encountered by practitioners in routine clinical services is the type “not otherwise specified,” a large proportion of which are binge eating disorders that do not meet full diagnostic criteria (2,3). Individuals with binge eating disorder report clinical distress and impairment on par with the experience of persons with bulimia nervosa (4,5). Even individuals who engage in regular binge eating at a lesser frequency than required for a diagnosis of bulimia or binge eating disorder report significant clinical impairment or distress (6). Eating disorders with binge eating as a core feature are often chronic and associated with emotional distress, medical complications, and functional impairment, as well as an increased risk of future onset of obesity, depression, and medical problems (7–12). Health service use among people with eating disorders not otherwise specified has been found to be significantly elevated and similar to levels observed for those with anorexia nervosa and bulimia nervosa (13).
Research supports the efficacy of cognitive-behavioral therapy (CBT) treatments (11,13–15), including lower-intensity interventions such as CBT-based guided self-help (CBT-GSH). The latter interventions are well suited for use in everyday practice settings (16,17). Nevertheless, only a minority of individuals who might benefit from such interventions receive them (18,19) or any other mental health treatments specifically targeting eating disorders (13,20–22). In a previous trial (23) we investigated the effectiveness of a brief CBT-GSH treatment for binge eating that augmented usual care. To conduct an intervention that may best bridge the gap between clinical efficacy and real-world implementation (24,25), we had used broader inclusion criteria (diagnostic and subdiagnostic binge eating disorders), an evidence-based manualized intervention of low burden to participants (a three-month course of eight sessions, most of which were less than 30 minutes), and master's-level interventionists unfamiliar with treatment of eating disorders, and we had recruited participants who were health care system patients and had included an intervention cost analysis. Despite adopting these important elements of an effectiveness design, we realized that the assessment burden on participants was considerable, and some of them were recruited only after their initial participation in an epidemiological study (26).
For these reasons, we conducted a replication and extension trial with three important modifications. First, we adopted an assessment more feasible and likely to be undertaken in everyday practice settings. Second, we specifically targeted those who most commonly present with eating-related concerns in such settings (women aged 25–50) (1). Third, we explicitly recruited participants who wanted the opportunity to receive treatment for binge eating concerns. Although modest, these modifications were important in moving treatment research closer to real-world care, in keeping with recent national reports urging such incremental steps in clinical research (27–29). Differences aside, all other intervention and trial design elements (setting, interventionists, and supervision level) were similar to the initial trial. We compare here our findings with those from the earlier effectiveness study.
Methods
Study population and sampling strategy
This study was conducted at the Center for Health Research, a multidisciplinary research organization located within a large health maintenance organization (HMO) in the Pacific Northwest (Kaiser Permanente Northwest). The Center for Health Research has access to the Kaiser Permanente Northwest electronic medical records and administrative databases for research purposes, of which all health plan members are informed on enrollment. Research suggests that many people with clinically significant disordered eating patterns do not seek treatment (30–32); therefore, we chose not to limit recruitment to those with an eating disorder diagnosis in their electronic medical records. Instead, we mailed recruitment invitations to female health plan members aged 25–50. We excluded individuals with severe cognitive impairment or psychosis, people being treated for cancer, pregnant women or those who had given birth within four months, and approximately one hundred plan members whose records indicated an a priori opt-out from study participation. Participants were 160 female health plan members (white, N=146, 91%; Hispanic, N=7, 4%; other race or not reported, N=7, 4%) with a mean±SD age of 39.1±6.71 and mean body mass index of 31.47±5.93, where an index above 30 is considered obese. Most women (N=134, 84%) reported completing at least some college.
Recruitment and study procedures
A detailed report of our recruitment approach has been published elsewhere (26). Patients were recruited and data were collected between August 2006 and June 2008. All participants underwent a two-stage case-finding procedure; initial screening was followed by administration of a comprehensive self-report questionnaire to assess binge eating and purging. Those who met criteria completed a brief secondary telephone interview to confirm threshold frequency of binge episodes and eligibility for the randomized controlled trial. [Recruitment, assignment and dropout are shown in a CONSORT diagram included as an online supplement to this article at ps.psychiatryonline.org.] We instituted a procedure that most closely reflected the screening and treatment referral process feasible in everyday practice settings. Posters and brochures advertised the study in HMO clinics and supplemented the mailed recruitment letters. The study was approved by participating institutions' human subjects review boards and was registered online with the National Institutes of Health (clinicaltrials.gov/show/NCT00158340).
Intervention
Efron's procedure (33) for stratifying on purging status was used to randomly assign participants into usual care or CBT-GSH. Consistent with the augmentation design, participants were not prohibited from using treatment resources offered by the HMO throughout the study, and usual care involved advising participants at trial assignment of treatment options within the HMO. CBT-GSH additionally involved eight sessions of CBT-GSH implemented over three months. The first session lasted 60 minutes; each subsequent session lasted 20–25 minutes. The first four sessions were weekly; the next four, at two-week intervals. The treatment was based on Fairburn's Overcoming Binge Eating (34), a six-step self-help program to help individuals develop a regular pattern of moderate eating using self-monitoring, self-control strategies, and problem solving. We added a seventh module to address dysfunctional body shape and weight concerns (available from GTW). In this intervention, the therapist's role included explaining the CBT-GSH rationale, helping patients develop realistic outcome expectancies, and engaging patients in manual-based program adherence.
Measures
All participants were assessed at baseline and at three-month (immediately posttreatment), six-month, and 12-month follow-ups with the instruments described below. Participants who completed the screening questionnaire online received a $5 coffeehouse gift card; those who returned the completed questionnaire by prepaid envelope received no compensation. For subsequent assessments, participants were compensated $10 (if completed by telephone) or $20 (if completed by mail) for a total compensation of $40–$100, plus a onetime $20 bonus for completing all assessments.
Participants' HMO service use during the three months postrandomization was extracted from the electronic medical record and coded into four mutually exclusive categories: weight-related services, eating disorder-related services, medications for mental health problems, and “all other services.”
Screening questionnaire.
The screening questionnaire collected information on demographic characteristics, current height and weight, and eating disorder symptoms measured with a modified version of the Patient Health Questionnaire (35) eating disorder module, which, as previously reported, has high sensitivity and specificity (23). Participants who reported binge eating at least once per week during the past three months (positive screen) were invited for further assessment to verify study eligibility.
Eating pathology.
In contrast to our initial study's assessment procedure (23), in which binge eating was assessed with an abbreviated version of the Eating Disorder Examination (EDE) (36), in this study, screen-positive participants were asked to complete the 36-item self-report questionnaire version of the EDE (EDE-Q) to assess binge eating and purging. The EDE-Q (36) was administered at baseline, posttreatment, and at each follow-up assessment (six and 12 months) to determine whether participants reported cessation of binge eating (our main study outcome). Cessation was determined by two questions, one about frequency of overeating episodes and the other about loss of control during such episodes. To meet the study's abstinence criterion, participants could not report any overeating episodes with loss of control within the past month. The EDE-Q also provided a continuous measure of eating disorder symptomatology along four symptom domains (dietary restraint, eating concern, weight concern, and shape concern).
Data analyses
Comparisons between the usual care and intervention groups at baseline were conducted with chi square and t tests. Multilevel modeling with HLM, version 6.0, was used to test for differences between the usual care and intervention groups across time for the outcomes of interest. A quadratic model for time (baseline, posttreatment, and six and 12 months) was used to capture nonlinear change across time. Multilevel modeling allows the inclusion of all participants regardless of missing data across time, which is consistent with an intent-to-treat approach. Effect sizes in regard to number needed to treat were computed for the main study outcome (37), which addressed the question, “How many more patients would need to be treated with CBT-GSH in order to avoid one more failure (that is, the patient continues to binge) than would have occurred had the patient been treated as usual?” For all other outcomes, Cohen's d (38) was computed for the change from baseline to the 12-month follow-up. Finally, to preserve patient anonymity, we aggregated all reported results when there were three or fewer subjects per condition.
Results
Preliminary analyses
We verified that the usual care (N=79) and CBT-GSH (N=81) groups did not differ at baseline on the most important demographic and clinical characteristics (Table 1), suggesting that randomization created initially equivalent groups. The groups differed significantly on race-ethnicity, with a greater proportion of the usual care group being Hispanic (p<.01), and on days of binge eating, with those in the intervention group reporting modestly more binge eating (p<.05).
Intervention attendance and missing data
About one-third of the CBT-GSH group (N=28, 35%) attended all eight sessions, 44 (54%) attended at least seven sessions, and 55 (68%) attended at least six sessions. The mean number of sessions attended was 5.72±2.68, and only 12 (15%) attended two or fewer sessions. There were minimal missing data: 139 (87%) of the total sample had data on the main study outcome at all four time points (N=70, 89% usual care; N=69, 85% CBT-GSH), 154 (96%) had data at three or more time points, and 158 (99%) had data at two or more time points. At 12 months, 74 of the 79 usual care and 75 of the 81 CBT-GSH patients had data on the primary outcome.
Proportion abstinent
The main outcome, abstinence from binge eating, differed significantly between the groups: the initial improvement in abstinence from baseline was greater for the CBT-GSH group than for the group that received usual care (B=.164, df=158, p<.001), and the deceleration from the initial change from baseline was significantly greater for the CBT-GSH group than for the usual care group (B=−.003, df=158, p<.001) (Table 2). At posttreatment, four (5%) of the 76 usual care participants and 24 (33%) of the 73 CBT-GSH participants were abstinent from binge eating (χ2=17.99, df=1, p<.001, number needed to treat=4); eight (10%) of the 78 usual care participants and 29 (38%) of the 76 CBT-GSH participants were abstinent at the six-month follow-up (χ2=17.70, df=1, p<.001, number needed to treat=3), and at 12 months, ten (14%) of the 74 usual care participants and 26 (35%) of the 75 CBT-GSH participants were abstinent from binge eating (χ2=9.66, df=1, p=.002, number needed to treat=5). These group differences reflected large effects (Table 2).
Other study outcomes
As Table 2 shows, the two groups differed significantly in the pattern of restraint (B =−.065, df=158, p<.001, d=.71), eating concern (B=−.092, df=158, p<.001, d=1.10), shape concern (B=−.087, df=158, p<.001, d=1.24), and weight concern (B=−.072, df=158, p<.001, d=.98), with the CBT-GSH group showing more improvement than usual care over time for each of these EDE-Q subscales. The two groups did not differ significantly on change in body mass index over time.
Health services and medication use
Table 3 illustrates the proportion of participants using health services or medication in the year before study enrollment and during the three and 12 months after random assignment and, among those utilizing services or medication, the amount of service use. There were almost no recorded visits in which services focused on weight concerns or eating disorder concerns across the time points measured. During the course of treatment (the three-month course), a higher proportion of CBT-GSH participants received psychotropic medication and used significantly more health services overall. However, in the 12 months after randomization, we saw no differences in the proportion receiving medications or overall health services or the amounts of such services received.
Discussion
This study was a replication and extension trial of our original study examining the effectiveness and cost-effectiveness of a brief guided self-help treatment for binge eating disorders in a nonspecialty health care (HMO) setting (23,39). Despite several changes to recruitment, assessment, and target population to reflect how such a program might be utilized in everyday practice conditions, the results pattern was similar to what we found in the earlier study. Further, the magnitude of the effect, as reflected in the number needed to treat and the effect sizes, exceeded that of the earlier trial. This finding suggests that the CBT-GSH intervention effects are robust and replicable. Significantly more participants in the CBT-GSH intervention abstained from binge eating after treatment, an effect maintained throughout the year after trial enrollment. This treatment also was associated with a significantly larger decrease in related eating pathology compared with usual care, although not all of these effects were sustained through the year-long follow-up. EDE-Q scores greater than 4 are considered clinically significant disturbances, suggesting that treatment-related reductions in concerns about shape and weight were of clinical significance.
Our analyses of treatment utilization suggested a modest increase in the proportion of CBT-GSH participants using psychiatric medication or other health services during the treatment window compared with those in the usual care condition. Because we anticipated that in-person sessions increased CBT-GSH participants' access to adjoining pharmacies and other clinic services, such modest differences may have been related to easier access. When we examined amount of service use, medication, and other overall health service use through the year after randomization, no significant differences were found between participants in the two conditions; these results suggest that the intervention had a negligible effect on the use of other health services. Notably, our data show that most participants in both conditions utilized health services during the three months after random assignment and thus had opportunity to request specific services related to eating disorders.
Similar to our earlier trial, the relatively large numbers of participants receiving psychotropic medications who had concomitant diagnosed mood disorders at study entry suggests a considerable level of distress or comorbid psychopathology in this sample. Although the sample size precluded further analysis of the potential moderating effects of such variables in this study, examining the potential impact of such mood disorders and concomitant pharmacological treatment on CBT-GSH response would be important for future research. Finally, consistent with other studies suggesting the infrequent use of care targeted to eating disorders (13,22,40), there was little indication that participants were treated specifically for an eating disorder outside the context of the intervention. When asked directly, participants in our earlier study cited receipt of some ancillary services associated with concerns about weight and eating disorders, which we then verified through targeted review of electronic medical records (23,39). This information suggests the importance of communicating directly with participants to accurately and comprehensively describe service utilization.
Despite the robustness of the results, in this replication trial the proportion of individuals in the CBT-GSH group who reported abstaining from binge eating was about half that found in the earlier trial. Several factors might explain this difference. Different reporting formats may result in different thresholds for the report of objective binge episodes. Unlike an interview, the self-report format used in this study does not allow ruling out self-reported binges too small to meet objective binge episode criteria and, accordingly, might produce more reports of binge eating. Another possible reason for the lower remission rate in this study is that participants in the earlier trial may have been more motivated than participants in this trial to change their eating patterns. Because participation in the earlier trial was more burdensome from the outset, less motivated individuals may have dropped out before the randomization process, as was suggested by our comparison of baseline assessment completion rates in an earlier report comparing the studies (26). Further, the better adherence in the earlier study (6.75±2.39 sessions completed in the earlier study versus 5.72±2.68 for the trial reported here) may have contributed to a higher proportion of participants reporting abstinence from binge eating in the earlier trial. Finally, those in the usual care condition in the earlier trial reported improvements paralleling the intervention group's rates in this trial. It may be that the time spent being interviewed by study staff in the earlier study (an average of 245 minutes for the assessments versus 235 minutes for the intervention) was therapeutic in its own right, hence accounting for the improvement rates reported by those in the usual care condition in the earlier trial.
Wishing to move the study further along the effectiveness spectrum informed our decision to substantially limit the assessment battery length and comprehensiveness. This served to reduce patient and staff burden but incurred several trade-offs. Although measuring eating pathology by questionnaire is acceptable and used in contexts where in-depth assessment is deemed impractical (41,42), the EDE-Q is less reliable than the EDE interview (43). Moreover, although our screening and assessment in this study were more feasible for everyday care, they limited our ability to examine broader participant characteristics as well as potentially nonspecific predictors, moderators, and mediators of outcomes. Finally, modifications to bring the study closer to real-world treatment moved only modestly beyond the effectiveness elements adopted in our earlier trial. To carry out a true effectiveness study, it would be important to use practice setting interventionists with more limited training and less intensive supervision, include a more ethnically and socioeconomically diverse patient population, and utilize a recruitment process that most closely replicates current real-world methods of identifying patients for treatment. Finally, it would be important to ascertain whether results could be replicated by another investigative group.
Conclusions
In summary, this study replicated and extended the findings from our earlier study (23), which demonstrated that a CBT-GSH intervention could significantly improve clinical outcomes for binge eating in a highly cost-effective manner (39). The study reported here provides further evidence that effective care for binge eating is possible in real-world settings. It is unusual to find larger effect sizes on replication, as we did in this study, which suggests the robustness of this low-intensity GSH intervention for binge eating. Other study strengths include the HMO setting, good patient retention through follow-up, and a broader sample of patients with clinically significant binge eating disorders than the more narrowly defined binge eating disorder or bulimia nervosa samples from previous studies of CBT-GSH. As such, we have extended the findings from our earlier study for the generalizability of evidence-based CBT-GSH.
1 : The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry 61:348–358, 2007 Crossref, Medline, Google Scholar
2 : The severity and status of eating disorder NOS: implications for DSM-V. Behaviour Research and Therapy 45:1705–1715, 2007 Crossref, Medline, Google Scholar
3 : Eating disorder NOS (EDNOS): an example of the troublesome “not otherwise specified” (NOS) category in DSM-IV. Behaviour Research and Therapy 43:691–701, 2005 Crossref, Medline, Google Scholar
4 : The severity and status of eating disorder NOS: implications for DSM-V. Behaviour Research and Therapy 45:1705–1715, 2007 Crossref, Medline, Google Scholar
5 : The validity and clinical utility of binge eating disorder. International Journal of Eating Disorders 42:687–705, 2009 Crossref, Medline, Google Scholar
6 : Frequency of binge eating episodes in bulimia nervosa and binge eating disorder: diagnostic considerations. International Journal of Eating Disorders 42:603–610, 2009 Crossref, Medline, Google Scholar
7 : DSM-IV threshold versus subthreshold bulimia nervosa. International Journal of Eating Disorders 39:462–467, 2006 Crossref, Medline, Google Scholar
8 : Epidemiology and natural course of eating disorders in young women from adolescence to young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry 39:1284–1292, 2000 Crossref, Medline, Google Scholar
9 : The Burden of Disease and Injury in Australia. AIHW no PHE17. Canberra, Australian Institute of Health and Welfare, 1999 Google Scholar
10 : Functional impairment associated with bulimic behaviors in a community sample of men and women. International Journal of Eating Disorders 40:391–398, 2007 Crossref, Medline, Google Scholar
11 : Comparing the health burden of eating-disordered behavior and overweight in women. Journal of Women's Health 18:1081–1089, 2009 Crossref, Medline, Google Scholar
12 : Psychosocial adjustment in young adulthood of women who experienced an eating disorder during adolescence. Journal of the American Academy of Child and Adolescent Psychiatry 42:587–593, 2003 Crossref, Medline, Google Scholar
13 : Health services use in eating disorders. Psychological Medicine 38:1465–1474, 2008 Crossref, Medline, Google Scholar
14 : Binge eating disorder treatment: a systematic review of randomized controlled trials. International Journal of Eating Disorders 40:337–348, 2007 Crossref, Medline, Google Scholar
15 : Psychological treatment of eating disorders. American Psychologist 62:199–216, 2007 Crossref, Medline, Google Scholar
16 : A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behaviour Research and Therapy 43:1509–1525, 2005 Crossref, Medline, Google Scholar
17 : Psychological treatments of binge eating disorder. Archives of General Psychiatry 67:94–101, 2010 Crossref, Medline, Google Scholar
18 : The use of guidelines for dissemination of “best practice” in primary care of patients with eating disorders. International Journal of Eating Disorders 40:476–479, 2007 Crossref, Medline, Google Scholar
19 : Utilization of empirically supported psychotherapy treatments for individuals with eating disorders: a survey of psychologists. International Journal of Eating Disorders 27:230–237, 2000 Crossref, Medline, Google Scholar
20 : Overvaluation of shape and weight in binge eating disorder and overweight controls: refinement of a diagnostic construct. Journal of Abnormal Psychology 117:414–419, 2008 Crossref, Medline, Google Scholar
21 : Health service utilization for eating disorders: findings from a community-based study. International Journal of Eating Disorders 40:399–408, 2007 Crossref, Medline, Google Scholar
22 : One-year use and cost of inpatient and outpatient services among female and male patients with an eating disorder: evidence from a national database of health insurance claims. International Journal of Eating Disorders 27:381–389, 2000 Crossref, Medline, Google Scholar
23 : Cognitive behavioral guided self-help for the treatment of recurrent binge eating. Journal of Consulting and Clinical Psychology 78:312–321, 2010 Crossref, Medline, Google Scholar
24 : Improving the transition from basic efficacy research to effectiveness studies: methodological issues and procedures. Journal of Consulting and Clinical Psychology 63:718–725, 1995 Crossref, Medline, Google Scholar
25 : How can we increase translation of research into practice? Types of evidence needed. Annual Review of Public Health 28:413–433, 2007 Crossref, Medline, Google Scholar
26 : Recruitment for a guided self-help binge eating trial: potential lessons for implementing programs in everyday practice settings. Contemporary Clinical Trials 30:326–333, 2009 Crossref, Medline, Google Scholar
27 : Moving treatment research from clinical trials to the real world. Psychiatric Services 54:327–332, 2003 Link, Google Scholar
28 : The meaning of translational research and why it matters. JAMA 299:211–213, 2008 Crossref, Medline, Google Scholar
29 : Translational and clinical science: time for a new vision. New England Journal of Medicine 353:1621–1623, 2005 Crossref, Medline, Google Scholar
30 : Barriers to treatment for eating disorders among ethnically diverse women. International Journal of Eating Disorders 30:269–278, 2001 Crossref, Medline, Google Scholar
31 : Beliefs of the public concerning the helpfulness of interventions for bulimia nervosa. International Journal of Eating Disorders 36:62–68, 2004 Crossref, Medline, Google Scholar
32 : Public perceptions of binge eating and its treatment. International Journal of Eating Disorders 41:419–426, 2008 Crossref, Medline, Google Scholar
33 : Forcing a sequential experiment to be balanced. Biometrika 58:403–417, 1971 Crossref, Google Scholar
34 : Overcoming Binge Eating. New York, Guilford, 1995 Google Scholar
35 : Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study—Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 282:1737–1744, 1999 Crossref, Medline, Google Scholar
36 : Eating Disorder Examination Questionnaire; in Cognitive Behavior Therapy and Eating Disorders. Edited by Fairburn CG. New York, Guilford, 2008 Google Scholar
37 : Size of treatment effects and their importance to clinical research and practice. Biological Psychiatry 59:990–996, 2006 Crossref, Medline, Google Scholar
38 : Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, Erlbaum, 1988 Google Scholar
39 : Cost-effectiveness of guided self-help treatment for recurrent binge eating. Journal of Consulting and Clinical Psychology 78:322–333, 2010 Crossref, Medline, Google Scholar
40 : Toward an understanding of health services use in women with binge eating disorder. Obesity Research 12:799–806, 2004 Crossref, Medline, Google Scholar
41 : Comparing definitions of purging disorder on point prevalence and associations with external validators. International Journal of Eating Disorders 43:433–439, 2010 Medline, Google Scholar
42 : Binge eating, purging, or both: eating disorder psychopathology findings from an Internet community survey. International Journal of Eating Disorders 43:724–731, 2010 Crossref, Medline, Google Scholar
43 : Assessment of eating disorders: interview or self-report questionnaire? International Journal of Eating Disorders 16:363–370, 1994 Medline, Google Scholar
Figures and Tables
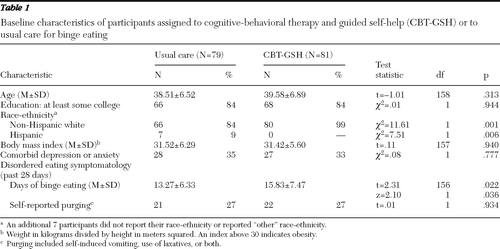
Table 1 Baseline characteristics of participants assigned to cognitive-behavioral therapy and guided self-help (CBT-GSH) or to usual care for binge eating
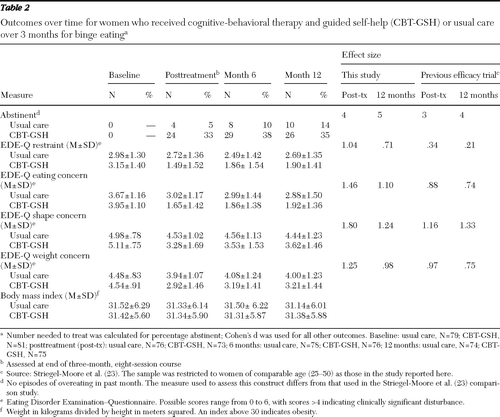
Table 2 Outcomes over time for women who received cognitive-behavioral therapy and guided self-help (CBT-GSH) or usual care over 3 months for binge eating
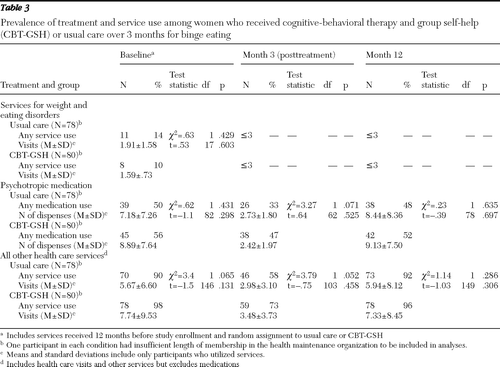
Table 3 Prevalence of treatment and service use among women who received cognitive-behavioral therapy and group self-help (CBT-GSH) or usual care over 3 months for binge eating



