One-Year Follow-Up of Collaborative Depression Care for Low-Income, Predominantly Hispanic Patients With Cancer
Abstract
Objective:
This study assessed longer-term outcomes of low-income patients with cancer (predominantly female and Hispanic) after treatment in a collaborative model of depression care or in enhanced usual care.
Methods:
The randomized controlled trial, conducted in safety-net oncology clinics, recruited 472 patients with major depression symptoms. Patients randomly assigned to a 12-month intervention (a depression care manager and psychiatrist provided problem-solving therapy, antidepressants, and symptom monitoring and relapse prevention) or enhanced usual care (control group) were interviewed at 18 and 24 months after enrollment.
Results:
At 24 months, 46% of patients in the intervention group and 32% in the control group had a ≥50% decrease in depression score over baseline (odds ratio=2.09, 95% confidence interval=1.13–3.86; p=.02); intervention patients had significantly better social (p=.03) and functional (p=.01) well-being. Treatment receipt among intervention patients declined (72%, 21%, and 18% at 12, 18, and 24 months, respectively); few control group patients reported treatment receipt (10%, 6%, and 13%, respectively). Significant differences in receipt of counseling or antidepressants disappeared at 24 months. Depression recurrence was similar between groups (intervention, 36%; control, 39%). Among patients with depression recurrence, intervention patients were more likely to receive treatment after 12 months (34% versus 10%; p=.03). At 24 months, attrition (262 patients, 56%) did not vary by group; 22% were deceased, 20% declined further participation, and 14% could not be located.
Conclusions:
Collaborative care reduced depression symptoms and enhanced quality of life; however, results call for ongoing depression symptom monitoring and treatment for low-income cancer survivors. (Psychiatric Services 62:162–170, 2011)
Depression is frequently chronic and recurrent, even after depression treatment (1). It is not surprising that many cancer patients experience clinically significant recurrence of depression symptoms over time (2–4), but they report a lack of access to follow-up psychological care (5). Recent Institute of Medicine reports underscore the need to provide depression care during acute cancer treatment, as well as depression symptom monitoring and follow-up care for cancer survivors (6,7).
Low-income patients from racial-ethnic minority groups who have cancer have high rates of depression; however, they have less access to mental health services than individuals without a cancer history (8,9) and are less likely to receive depression treatment consistent with cultural preferences (10,11). Although persons from minority populations benefit from depression care (12–14), they are also more likely to experience ongoing social and economic stress that may contribute to depression and its recurrence and impede access to depression care (15).
Few depression cancer trials assess outcomes beyond 12 months, and attrition rates in such trials range up to 56% (16). Trials for patients with cancer that provide antidepressant medication or psychotherapy have not reported sufficient data to judge the efficacy of either antidepressants or therapy alone, which underscores the need for studies that examine multiple therapies that are consistent with current guidelines (16,17). An approach that has been found effective for patients with cancer (18–21) is collaborative care that uses a care manager and structured algorithms to guide problem-solving therapy and to adjust antidepressant treatment, that monitors patient symptoms over time, and that provides patients with relapse prevention strategies and support.
We previously reported findings from a randomized clinical trial of the ADAPt-C collaborative care model (Alleviating Depression Among Patients With Cancer), which was designed to improve access to culturally adapted depression care among low-income, predominantly Hispanic women with cancer (18–22). At 12 months, patients in the intervention group had significantly higher rates of depression treatment, greater reduction in depression symptoms, and better quality of life than patients in enhanced usual care (22).
In this study, we examined 18- and 24-month outcomes for patients who participated in the trial. We hypothesized that patients in the intervention would experience more lasting improvements in depression and quality of life than patients in enhanced usual care. We also examined posttrial receipt of depression treatment, recurrence of major depression, factors associated with recurrence (such as baseline cancer stage, cancer progression, posttrial depression care, pain, and socioeconomic stress), and trial attrition.
Methods
Sample
The trial was approved by the University of Southern California Institutional Review Board and was conducted within safety-net oncology clinics. Oncology patients provided verbal consent to depression screening by bilingual study recruiters between June 2004 and December 2006; 472 eligible patients provided written informed consent and completed a baseline interview before computer-driven randomization to intervention or enhanced usual care (23). The sample included 415 Hispanic patients (88%) and 399 women (85%). The mean±SD age of the sample was 48.7±12.9.
Patients who had received a cancer diagnosis at least 90 days before the baseline interview and who were receiving acute or follow-up care in oncology clinics were eligible for the study if they were aged 18 or older and reported one of two cardinal depression symptoms for every day or for more than half of the days over the past two weeks. Patients were also required to have a score of ≥10 on the Patient Health Questionnaire (PHQ-9), which indicates mild (score 10–14), moderate (score 15–20), or severe (score 21–27) major depression (24–26) or positive responses to two questions from the Structured Clinical Interview for DSM-IV (27) that indicate dysthymia. Patients were excluded if they reported acute suicidal ideation; had advanced cancer or another condition that limited their life expectancy to less than six months; had a score of ≥8 on the Alcohol Use Disorders Identification Test (28); reported recent use of lithium or an antipsychotic medication; had a score of ≤2 on a self-report adaptation of the Karnofsky Performance Status Scale (KPSS) (29), which represents severe functional impairment; or were unable to speak English or Spanish. [A diagram illustrating recruitment and randomization is available in an online appendix to this article at ps.psychiatryonline.org.]
Patients randomly assigned to enhanced usual care received standard oncology care from treating oncologists informed of patients' depression status. Patients were given patient and family educational pamphlets on depression and cancer and a list of clinic- or community-provided financial, psychosocial, transportation, and child care services. At the start of the trial, treating oncologists attended a didactic session on depression treatment led by the study psychiatrist. Oncologists were free to prescribe antidepressants or refer patients to mental health care; patients could also seek care in the community.
Attrition reduction strategies focused on reducing cultural and economic barriers to ongoing participation (30–32). Study staff were bilingual, and all study materials, including problem-solving therapy homework, were consistent with language preference and sensitive to idiomatic usage and literacy. Other efforts aimed to reduce attrition included, for example, telephone reminders, evening and weekend telephone interview options, flexible telephone or in-person problem-solving therapy, and gift cards (23).
Intervention
Acute care.
Adapted from the IMPACT (Improving Mood-Promoting Access to Collaborative Treatment) collaborative stepped-care model (33), ADAPt-C care included key evidence-based components. Clinical specialists in cancer-related depression (bilingual graduate social workers) provided structured problem-solving therapy and either helped participants navigate health care and community services or provided supervision to a navigator. A psychiatrist supervised the clinical specialists and prescribed antidepressant medications. Each patient had a personalized treatment plan that included the patient's preference for first-line treatment—either antidepressant or problem-solving therapy (34,35). The model is based on a stepped-care treatment algorithm with guidelines for treatment adjustment based on ongoing symptom review (33,36). Every month the depression clinical specialist contacted each participant by telephone to monitor symptoms and provide behavioral activation for prevention of depression relapse. [An online appendix to this article, available at ps.psychiatryonline.org, includes figures illustrating the protocols for problem-solving therapy and collaborative care.]
The initial visit with the clinical specialist included a structured psychiatric and psychosocial assessment; education about depression, problem-solving therapy, and antidepressant therapy; elicitation of the patient's choice for initial treatment; and navigation assistance. On subsequent visits the specialist provided either structured problem-solving therapy or guidance on antidepressant use, as well as side-effect telephone monitoring. The study consulting psychiatrist and the principal investigator provided weekly telephone supervision of the depression clinical specialist. The psychiatrist also conducted an in-person evaluation of patients who initially chose antidepressant therapy or who did not respond to problem-solving therapy and prescribed antidepressants and additional medications, such as antianxiety agents or sedative-hypnotics if clinically indicated. A secure Web site allowed tracking of clinical data and facilitated reporting patient care by the clinical specialists and the psychiatrist.
Problem-solving therapy addresses four rational problem-solving skills aimed at the discovery of adaptive coping responses in six to 12 sequenced sessions (37). Homework materials were linguistically and idiomatically adapted, and telephone or inpatient problem-solving therapy was provided when needed. Each depression clinical specialist received structured training in problem-solving therapy and in the study algorithms. An expert in problem-solving therapy provided consultation using the PST (Problem-Solving Therapy)-Adherence Competency Rating Scale to evaluate three to five audio-taped sessions.
Relapse and recurrence prevention.
Up to 12 months after acute treatment, patients participated in a treatment maintenance and relapse prevention program, which included telephone calls once a month from the depression clinical specialist to monitor symptoms; one or more problem-solving therapy “booster” sessions, if indicated, delivered in person or by telephone; and behavioral activation and motivational support for engaging in pleasant activities, for ongoing use of problem-solving skills, or for antidepressant adherence (38). At the end of the 12-month intervention, patients who were assessed as needing further treatment were referred either to a primary care physician or to a community mental health clinic and a note was placed in the oncology medical chart.
Measures
The PHQ-9 was used as both a screen and an outcome measure because it provides a diagnosis of major depression (score of 10–27) as well as a continuous severity score (25,39), measures a common concept of depression across racial and ethnic groups (40), has been used with cancer patients (21,41), and is believed to be practically feasible and sustainable in real-world oncology clinics. The PHQ-9 has been found to make accurate diagnoses of mild, moderate, and severe major depression, to track severity of depression over time, and to monitor significant therapeutic improvement (42–48). Systematic reviews have found that the sensitivity of the PHQ-9 is moderate (about 80%) but that specificity is high (over 90%) (45,48), suggesting that some cases of depression are missed but that there are few false positives. These studies have also noted that individuals whose PHQ-9 score is ≥10 are likely to meet criteria for major depression on a structured psychiatric interview.
Health-related quality of life was assessed with the Functional Assessment of Cancer Therapy Scale (FACT-G), a 27-item questionnaire with a Spanish translation (49,50) that has subscale scores for physical well-being (Cronbach's α=.82), functional well-being (Cronbach's α=.80), social well-being (Cronbach's α=.69), and emotional well-being (Cronbach's α=.74). The Brief Symptom Inventory (51) was used to assess anxiety (Cronbach's α=.81). The Brief Pain Inventory Short Form (BPI) assessed pain (Cronbach's α=.77–.91) (52,53). Standardized Cronbach's coefficient alphas were similar in the study population (measures assessed at baseline, six, 12, 18, and 24 months: FACT-G physical well-being, α=.80–.85; functional well-being, α=.76–.88; social well-being, α=.61–.70; and emotional well-being, α=.52–.68; anxiety, α=.81–.89; and pain, α=.78–.88). Also assessed was socioeconomic distress (for example, work, unemployment, financial problems, and marital or family conflicts). Data on cancer treatment (chemotherapy, radiation, or acute hormone therapy), emergency room use, and hospitalization were obtained from electronic medical records. A widely used definition of depression recurrence was applied in which recurrence is defined as the appearance of a new episode after a period of recovery (1,54).
Analyses
Attrition analyses were conducted. Various databases were used to classify types of attrition: was deceased, declined participation, and was not locatable [see the CONSORT diagram in the online appendix for more details.] At 24 months, 102 patients (22%) were deceased, 96 patients (20%) had declined participation, and 64 (14%) could not be located. The three types of attrition did not vary significantly between the intervention and enhanced-usual-care groups. Patients who completed the 24-month study were separately compared with each attrition group on baseline demographic variables (gender, age, and length of time living in the United States) and baseline clinical characteristics (cancer stage, cancer treatment, history of major depression, KPSS score, pain, depression, anxiety, and quality of life) (Table 1). Worsening cancer status postbaseline (new cancer, advancing stage, cancer progression, or metastasis or in palliative care) was not related to study retention (data not shown). A further analysis of variance that excluded the deceased group was conducted to examine baseline demographic and clinical characteristics for independent effects of the three types of attrition and the interaction of attrition type with treatment group (intervention or enhanced usual care). Significant differences among the three types of attrition were observed only for gender (p=.01) and FACT-G functional well-being (p=.02). No significant interaction of treatment group and attrition was found.
Intent-to-treat analysis was conducted to evaluate postintervention effects. Clinically meaningful improvement of depressive symptoms was assessed as a ≥50% decrease or a ≥5-point reduction in PHQ-9 score since baseline; remission of depressive symptoms was defined as a PHQ-9 score <5. Logistic regression models were conducted to compare depression outcomes at 24 months between patients in the intervention and enhanced usual care groups. Generalized linear mixed-effects models implemented in the SAS Proc Mixed procedure were fitted with longitudinal data from baseline to 24 months to evaluate intervention effects on functional, socioeconomic, and clinical outcomes (55,56). Unstructured covariance was specified in the mixed-effects model to account for within-patient correlations of repeated observations over time. Fixed effects of time, study group, and their interactions were examined. Both logistic regression and linear mixed-effects models were adjusted for gender, ethnicity, years in the United States (less than ten versus ten or more), dysthymia, baseline depression severity (PHQ-9 score ≥15 versus <15), anxiety, cancer stage, cancer type, and treatment status.
In addition, landmark analyses were conducted on depression recurrence (PHQ-9 score ≥10 over 18 and 24 months) for patients who no longer had major depressive disorder at 12 months. Clinical, functional, and socioeconomic variables were examined for association with recurrence in logistic regression models that controlled for study group. All analyses were conducted using SAS, version 9.1. Hot-deck imputations as well as predictive-model-based multiple imputations (57,58) implemented in SOLAS, version 3.2, were adopted to validate the evaluation of the intervention effect. Results with imputed data were consistent with the results analyzed with all available data; thus, we present only the results for the analyses with all available data.
Results
Population
Patients (242 in the intervention group and 230 in enhanced usual care) were predominantly Latina and foreign born; most (N=249, 53%) were from Mexico, El Salvador (N=66, 14%), and Guatemala (N=43, 9%). A total of 352 patients (75%) had been in the United States for at least ten years, and 300 (64%) had not completed high school. A total of 304 patients (64%) were diagnosed as having gynecologic or breast cancer, 132 (28%) had stage 3 or 4 cancer or recurrent cancer, and 139 (29%) had moderate to severe depression (PHQ-9 score ≥15) at baseline.
Depression care
Table 2 presents data on depression care received by each group at baseline and at 12, 18, and 24 months. The number of patients in the intervention group who reported having had any depression treatment declined from 72% at 12 months to 21% at 18 months and 18% at 24 months, whereas relatively small proportions of patients in enhanced usual care reported having received treatment—10% at 12 months, 6% at 18 months, and 13% at 24 months. Significant group differences in receipt of antidepressant medication disappeared at the 18-month assessment, and significant group differences in receipt of counseling or antidepressant treatment disappeared at 24 months. Among patients who experienced recurrence of major depression—35 in the intervention group (36%) and 29 in enhanced usual care (39%)—the intervention patients were significantly more likely to have received depression treatment after 12 months (N=12, 34%, compared with N=3, 10%; p=.03) (data not shown).
Sustained depression improvement
Table 3 presents data on depression outcome measures over the 24 months for the two treatment groups. Intervention patients were more likely than those in enhanced usual care to show improvement in depression symptoms (50% reduction in PHQ-9 score) at 24 months (46% compared with 32%; odds ratio [OR]=2.09), and their PHQ-9 score was significantly more likely to have decreased by 5 points since baseline (54% compared with 37%; OR=2.07). No significant interactions between treatment group and race-ethnicity, baseline depression severity, baseline cancer site or stage, or worsening cancer status over time were found for depression improvement.
Recurrence
As shown in Table 4, 172 patients no longer met criteria at 12 months for mild, moderate, or severe major depression (PHQ-9 score <10). Of these, 64 patients (37%) (36% in the intervention group and 39% in enhanced usual care) experienced recurrence of depression (PHQ-9 score ≥10) at 18 or 24 months. Because recurrence rates did not vary significantly between the treatment groups, data for intervention and enhanced usual care patients were combined to examine clinical, functional, and socioeconomic factors that may have been associated with recurrence. At 18 or 24 months, patients who had stage 3 or 4 cancer or recurrent cancer at baseline were more likely have recurrent depression (38% had recurrent depression and 19% had nonrecurrent depression; p=.01). Twelve-month PHQ-9, BPI, KPSS, and FACT-G scores each had a highly significant association with recurrence (p=.004 for KPSS scores and p<.001 for all others), as did the number of stressors (p=.01). A greater proportion of patients with recurrent depression had an emergency room visit between 12 and 24 months (47% compared with 20% who had nonrecurrent depression; p<.001). Recurrence was not associated with age; gender; receipt of chemotherapy, radiation, or acute hormone therapy after 12 months; cancer progression or remission; use of complementary and alternative medicine; number of emergency room visits; hospitalization; or length of hospital stay.
Quality of life and patient satisfaction
Table 5 presents data on quality-of-life measures over time for the two treatment groups. Random-effects models indicated significantly greater improvement among patients in the intervention group (reflected as a significant time × study group interaction) in FACT-G social and family well-being and emotional well-being (p<.001 for each). Significant group differences in FACT-G adjusted mean scores were also found: intervention patients had significantly better functional well-being at each follow-up (adjusted mean difference ranging from 1.41 to 1.79; p values of .01 and .02), and better social and family well-being at 24 months (adjusted mean difference=1.89, p=.03). No significant interactions on quality-of-life outcomes were found between study group and ethnicity, baseline cancer site or cancer stage, history of depression, dysthymia, or depression severity.
Patient satisfaction with care was surveyed at 18 and 24 months. A significantly greater proportion of patients in the intervention group than in enhanced usual care reported being satisfied or very satisfied with the care they received for their emotional problems (93%, N=138, compared with 80%, N=101; p=.001). Over 90% of patients in each study group (138–146 in the intervention group and 116–121 in enhanced usual care) were satisfied or very satisfied with their overall health care, clinic services, courtesy and respect from their doctor and nurses, and ability to take part in decisions made about care.
Discussion
Use of the ADAPt-C collaborative care model resulted in long-term improvement in depression symptoms and satisfaction with emotional care among low-income predominantly Hispanic patients with cancer. Although FACT-G scores showed some improved quality-of-life outcomes, differences were small and may reflect negative effects of socioeconomic stress (12). Notably, long-term depression improvement was found despite declining access to follow-up depression care and ongoing socioeconomic stress. Also important with respect to the needs of predominantly Hispanic cancer patients were the relatively high rates of clinically significant depression at baseline, the strong initial treatment preference for problem-solving therapy over antidepressants (receipt of which may have facilitated subsequent antidepressant acceptance), and the significant rate of depression recurrence.
Long-term benefits of collaborative care have been noted among older adults with depression (59), and improvement at 12 months was found for a subgroup of cancer patients (21). However, even though patients in the study reported here received evidence-based combined therapies that have been found to be associated with better longer-term outcomes in other studies, 24-month recurrence rates in this safety-net population were relatively high. Cancer patients frequently face ongoing cancer-related and socioeconomic stress (12), and the finding that clinical, functional, and socioeconomic factors were associated with recurrence of depression is consistent with findings of other studies (1). Moreover, the negative relationship between depression and quality of life is well documented (60–62). This underscores a critical need to develop transition pathways for the management of mental health problems as detailed in the Institute of Medicine reports (6,7) to ensure that patients with cancer receive follow-up symptom monitoring and depression care as they make the transition from acute oncology care to follow-up primary care.
Despite relatively high rates of clinically significant major depression (highest for mild depression), treatment rates were low in the control group, and treatment rates dropped significantly over time for both study groups, including among patients experiencing recurrence. Recurrence of depression across populations ranges up to 50% among those who recover from a first episode of depression, and recurrence is common among patients with comorbid general medical illness and negatively affects overall health and economic stress (1). Among cancer survivors, the risk of depression recurrence highlights a need to develop continuation models for psychological care (3–5,63,64), including tailored models for diverse racial-ethnic populations and socioeconomically disadvantaged populations (65).
Even though the study used practical and culturally sensitive strategies to maintain the sample, at 24 months, deaths, dropout (possibly attributable to declining health and ongoing socioeconomic stress), and inability to locate patients (attributable to the geographic mobility of the population) resulted in relatively high postbaseline attrition, with some effects on randomization. As is common in mental health services effectiveness trials, our study design may have biased our comparisons in favor of the enhanced usual care group. Increased antidepressant treatment among patients in enhanced usual care may be partly attributable to the fact that oncologists were informed of patients' depression and study enrollment status by the recruiters. In addition, all oncologists participated in a depression didactic and were given the stepped care algorithm, and acutely suicidal patients were referred to specialty mental health. Patients in enhanced usual care may have benefited from receiving patient and family educational pamphlets and information about community resources. These biases may contribute to an underestimation of the effectiveness of the intervention compared with enhanced usual care. Additional limitations include reliance on patient self-reports of psychotherapy receipt and the inability to determine which intervention components led to better outcomes.
Conclusions
The study findings suggest that collaborative care results in long-term modest improvement in depression among cancer survivors. However, results underscore the need for long-term symptom monitoring and treatment adjustments and for collaboration between oncologists and primary care providers in the care of cancer survivors.
1 : Risk for recurrence in depression. Clinical Psychology Review 27:959–985, 2007 Crossref, Medline, Google Scholar
2 : Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Research and Treatment 110:9–17, 2008 Crossref, Medline, Google Scholar
3 : Major depression after breast cancer: a review of epidemiology and treatment. General Hospital Psychiatry 30:112–126, 2008 Crossref, Medline, Google Scholar
4 : Risk for hospitalization with depression after a cancer diagnosis: a nationwide, population-based study of cancer patients in Denmark from 1973–2003. Journal of Clinical Oncology 27:1440–1445, 2009 Crossref, Medline, Google Scholar
5 : Patients' supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. Journal of Clinical Oncology 27:6172–6179, 2009 Crossref, Medline, Google Scholar
6
7
8 : Mental health service use among adult cancer survivors: analyses of the National Health Interview Study. Journal of Clinical Oncology 20:4581–4590, 2002 Crossref, Medline, Google Scholar
9 : Depression, receipt of depression care, and correlates of depression among low-income women with breast or gynecological cancer. Journal of Clinical Oncology 23:3052–3060, 2005 Crossref, Medline, Google Scholar
10 : Identification of patient attitudes and preferences regarding treatment of depression. Journal of General Internal Medicine 12:431–438, 1997 Crossref, Medline, Google Scholar
11 : Treatment preferences among depressed primary care patients. Journal of General Internal Medicine 15:527–534, 2000 Crossref, Medline, Google Scholar
12 : Economic stress among low-income patients with cancer: effects on quality of life. Cancer 112:616–625, 2008 Crossref, Medline, Google Scholar
13 : Improving care for minorities: can quality improvement intervention improve care and outcomes for depressed minorities? Health Services Research 38:613–630, 2003 Crossref, Medline, Google Scholar
14 : Treating depression in predominantly low-income young minority women: a randomized controlled trial. JAMA 290:57–65, 2003 Crossref, Medline, Google Scholar
15 : Service use and outcomes among elderly persons with low incomes being treated for depression. Psychiatric Services 58:1057–1064, 2007 Link, Google Scholar
16 : The effectiveness of treatment for depression/depressive symptoms in adults with cancer: a systematic review. British Journal of Cancer 94:372–390, 2006 Crossref, Medline, Google Scholar
17 : Treatment of depression in cancer patients. Current Oncology 14:180–188, 2007 Crossref, Medline, Google Scholar
18 : Can collaborative care address the needs of low-income Latinas with comorbid depression and cancer? Results from a randomized pilot study. Psychosomatics 46:224–232, 2005 Crossref, Medline, Google Scholar
19 : Management of depression for people with cancer (SMaRT Oncology 1): a randomized trial. Lancet 372:40–48, 2008 Crossref, Medline, Google Scholar
20 : Depression care for people with cancer: a collaborative care intervention. General Hospital Psychiatry 31:436–441, 2009 Crossref, Medline, Google Scholar
21 : Improving primary care for older adults with cancer and depression. Journal of General Internal Medicine 24(suppl 2):S417–S424, 2009 Crossref, Medline, Google Scholar
22 : Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. Journal of Clinical Oncology 26:4488–4496, 2008 Crossref, Medline, Google Scholar
23 : Improving treatment of depression among low-income patients with cancer: the design of the ADAPt-C study. General Hospital Psychiatry 29:223–231, 2007 Crossref, Medline, Google Scholar
24 : Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study: Primary Care Evaluation of Mental Disorders: Patient Health Questionnaire. JAMA 282:1737–1744, 1999 Crossref, Medline, Google Scholar
25 : The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 16:606–613, 2001 Crossref, Medline, Google Scholar
26 : The PHQ-9 as a brief assessment of lifetime major depression. Psychological Assessment 19:247–251, 2007 Crossref, Medline, Google Scholar
27 : Structured Clinical Interview for DSM-IV Axis 1 Disorders (SCID). Washington, DC, American Psychiatric Press, Inc, 1996 Google Scholar
28 : Comfortably engaging: which approach to alcohol screening should we use? Annals of Family Medicine 2:398–404, 2004 Crossref, Medline, Google Scholar
29 : The Karnofsky Performance Status Scale: an examination of its reliability and validity in a research setting. Cancer 53:2002–2007, 1984 Crossref, Medline, Google Scholar
30 : Attrition and retention in clinical trials by ethnic origin. Contemporary Clinical Trials 30:499–503, 2009 Crossref, Medline, Google Scholar
31 : Factors associated with loss to follow-up in a large tuberculosis treatment trial (TBTC Study 22). Contemporary Clinical Trials 28:288–294, 2007 Crossref, Medline, Google Scholar
32 : Early participant attrition from clinical trials: role of trial design and logistics. Clinical Trials 5:328–335, 2008 Crossref, Medline, Google Scholar
33 : Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 288:2836–2845, 2002 Crossref, Medline, Google Scholar
34 : Cancer and psychological distress: two investigations regarding the role of social problem-solving. Journal of Psychosocial Oncology 16:27–40, 1999 Crossref, Google Scholar
35 : A Problem-Solving Approach: Helping Cancer Patients Cope. Washington, DC, American Psychological Association, 1998 Crossref, Google Scholar
36
37 : A problem-solving approach to stress reduction among younger women with breast carcinoma: a randomized controlled trial. Cancer 94:3089–3100, 2002 Crossref, Medline, Google Scholar
38 : A randomized trial of relapse prevention of depression in primary care. Archives of General Psychiatry 58:241–247, 2001 Crossref, Medline, Google Scholar
39 : Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians' diagnoses. Journal of Affective Disorders 78:131–140, 2004 Crossref, Medline, Google Scholar
40 : Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. Journal of General Internal Medicine 21:547–552, 2006 Crossref, Medline, Google Scholar
41 : Prevalence of mood disorders and utility of the Prime-MD in patients undergoing radiation therapy. International Journal of Radiation Oncology, Biology, Physics 42:1105–1112, 1998 Crossref, Medline, Google Scholar
42 : Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9). Journal of Affective Disorders 81:61–66, 2004 Crossref, Medline, Google Scholar
43 : Diagnosing ICD-10 depressive episodes: superior criterion validity of the Patient Health Questionnaire. Psychotherapy and Psychosomatics 73:386–390, 2005 Crossref, Google Scholar
44 : Monitoring depression treatment outcomes with the PHQ-9. Medical Care 42:1194–1201, 2004 Crossref, Medline, Google Scholar
45 : Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. General Hospital Psychiatry 29:388–395, 2007 Crossref, Medline, Google Scholar
46 : Concordance between the PHQ-9 and the HSCL-20 in depressed primary care patients. Journal of Affective Disorders 99:139–145, 2007 Crossref, Medline, Google Scholar
47 : Responsiveness of the PHQ-9 to psychopharmacological depression treatment. Psychosomatics 47:62–67, 2006 Crossref, Medline, Google Scholar
48 : Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. Journal of General Internal Medicine 22:1596–1602, 2007 Crossref, Medline, Google Scholar
49 : Spanish language translation and initial validation of the Functional Assessment of Cancer Therapy Quality-of-Life Instrument. Medical Care 36:1407–1411, 1998 Crossref, Medline, Google Scholar
50 : The Functional Assessment of Cancer Therapy Scale: development and validation of the general measure. Journal of Clinical Oncology 11:570–579, 1993 Crossref, Medline, Google Scholar
51 : The Brief Symptom Inventory: an introductory report. Psychological Medicine 13:595–605, 1983 Crossref, Medline, Google Scholar
52 : Measurement of pain by subjective report; in Issues in Pain Mean Measurement. Edited by Chapman CRLoeser JD. New York, Raven, 1989 Google Scholar
53 : Pain assessment in cancer; in Effect of Cancer on Quality of Life. Edited by Osaba D. Boca, Raton, Fla, CRC Press, 1991 Google Scholar
54 : Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Archives of General Psychiatry 48:851–855, 1991 Crossref, Medline, Google Scholar
55 : SAS System for Mixed Models. Cary, NC, SAS Institute, 1996 Google Scholar
56 : Applied Longitudinal Analysis. Hoboken, NJ, Wiley, 2004 Google Scholar
57 : Regression with missing X's: a review. Journal of the American Statistical Association 87:1227–1237, 1992 Google Scholar
58 : Multiple Imputation for Nonresponse in Surveys. New York, Wiley, 1987 Crossref, Google Scholar
59 : Long term outcomes from the IMPACT randomized trial for depressed elderly patients in primary care. British Medical Journal 332:259–263, 2006 Crossref, Medline, Google Scholar
60 : Depression and anxiety in women with early breast cancer: five year observational cohort study. British Medical Journal 330:1–4, 2005 Crossref, Medline, Google Scholar
61 : Health-related quality of life in head and neck cancer survivors: impact of pretreatment depressive symptoms. Health Psychology 29:65–71, 2010 Crossref, Medline, Google Scholar
62 : Quality of life, social support, and uncertainty among Latina and Caucasian breast cancer survivors: a comparative study. Oncology Nursing Forum 37:93–99, 2010 Crossref, Medline, Google Scholar
63 : Perspectives on post-treatment cancer care: qualitative research with survivors, nurses, and physicians. Journal of Clinical Oncology 25:2270–2273, 2007 Crossref, Medline, Google Scholar
64 : Patient and general practitioner preferences for the treatment of depression in patients with cancer: how, who, and where? Journal of Psychiatric Research 67:399–402, 2009 Google Scholar
65 : Establishing a general medical outpatient clinic for cancer survivors in a public city hospital setting. Journal of General Internal Medicine 24:451–455, 2009 Crossref, Google Scholar
Figures and Tables
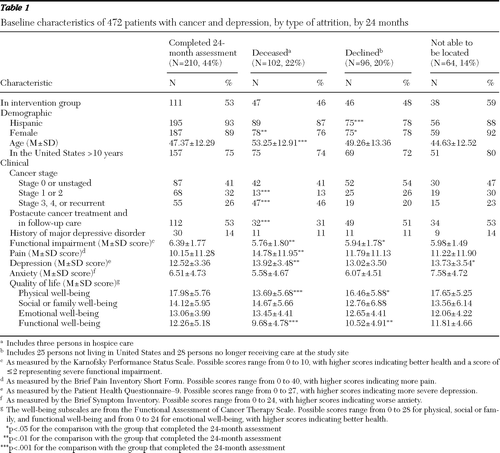
Table 1 Baseline characteristics of 472 patients with cancer and depression, by type of attrition, by 24 months
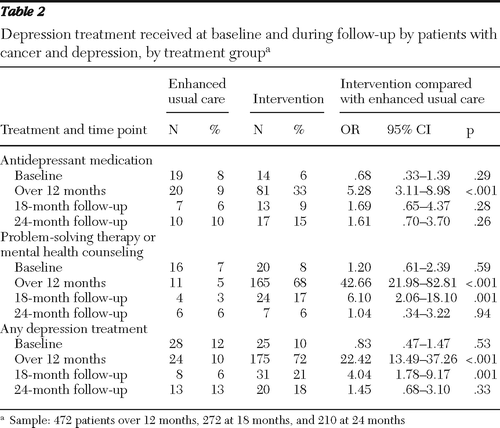
Table 2 Depression treatment received at baseline and during follow-up by patients with cancer and depression, by treatment group
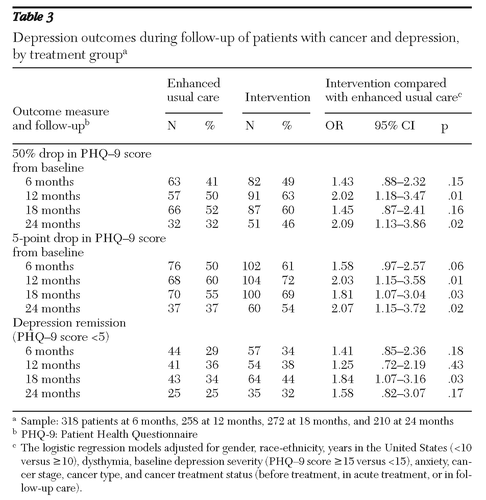
Table 3 Depression outcomes during follow-up of patients with cancer and depression, by treatment group
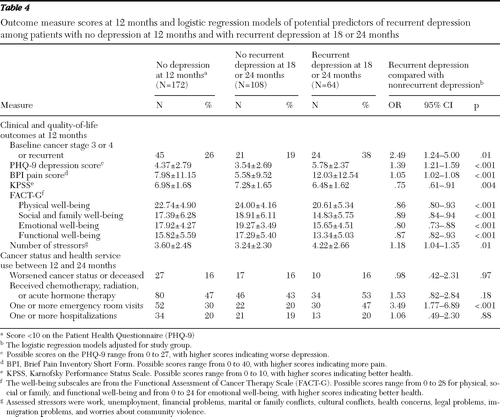
Table 4 Outcome measure scores at 12 months and logistic regression models of potential predictors of recurrent depression among patients with no depression at 12 months and with recurrent depression at 18 or 24 months
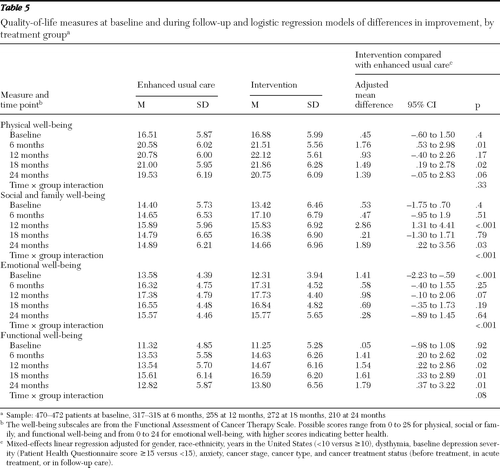
Table 5 Quality-of-life measures at baseline and during follow-up and logistic regression models of differences in improvement, by treatment group



