Factors in Second-Generation Antipsychotic Switching Patterns in a National Sample of Older Veterans With Schizophrenia
Abstract
Objective:
A 2004 consensus statement by the American Psychiatric Association and other groups noted that metabolic side effects of second-generation antipsychotics require monitoring. To reduce risk, prescribers may consider factors differentially associated with development of metabolic abnormalities, such as age, gender, and race-ethnicity. As part of a study of older patients with schizophrenia (50–102 years), this study evaluated factors associated with antipsychotic switches and switches that incurred a greater or lesser metabolic risk.
Methods:
Administrative data were analyzed for a national cohort of 16,103 Veterans Health Administration patients with schizophrenia receiving second-generation antipsychotics. Multinomial logistic regression predicted the likelihood of switches from 2002 to 2003 and again from 2004 to 2005.
Results:
At baseline nearly half the patients (45%) had a diagnosis of hypertension, a third (34%) had dyslipidemia, and 15% had a diagnosis of obesity. In both periods diabetes was associated with switches to lower-risk antipsychotics, and older patients were likely to experience neutral or no switches. Women were more likely to experience switches to higher-risk antipsychotics in 2004–2005.
Conclusions:
General medical conditions potentially associated with antipsychotic-related metabolic concerns were common; however, half of these patients were prescribed medication that made them liable to developing metabolic problems. Modest evidence suggests that metabolic considerations became a higher priority during the study. Future research should investigate the differential impact of antipsychotics on metabolic dysregulation for women and elderly patients. Findings underscore the need to monitor metabolic parameters of older patients taking antipsychotics. (Psychiatric Services 62:47–53, 2011)
There is a burgeoning literature on metabolic effects of antipsychotics and the need to recognize these important side effects when prescribing antipsychotics (1). Research has identified differences among antipsychotics in patients' likelihood of developing metabolic abnormalities (2,3) and has documented the worsening of metabolic control when certain second-generation antipsychotics are used by patients with type 2 diabetes mellitus (4).
Studies have also suggested that patients' demographic characteristics may influence prescribers' choice of antipsychotics in order to reduce cardiovascular risk, although results are mixed. The CATIE study (Clinical Antipsychotic Trials in Intervention Effectiveness) indicated that female gender, white race, and older age were associated with an increased risk of metabolic symptoms among CATIE-enrolled patients (5). However, nonwhite persons, especially African Americans and Hispanics, were identified in the Standards of Diabetes Care as being at higher risk of metabolic disorders in general (6,7). The role of race-ethnicity requires assessment in various patient samples. A commentary on CATIE and other studies (8) noted that switching too early during a trial of a new medication, perhaps because of side effects such as weight gain, may prevent a patient from experiencing a drug's full benefits.
The need to monitor metabolic parameters was summarized in a February 2004 consensus statement on the problem of obesity and diabetes related to the use of antipsychotic medications that was issued by the American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and North American Association for the Study of Obesity (2). The Veterans Health Administration (VA) clinical practice guidelines for psychosis, updated in March 2004, specifically mentioned the association of diabetes with second-generation antipsychotics (www.healthquality.va.gov). However, Morrato and colleagues (9) found low monitoring rates for patients taking antipsychotic agents and little evidence of response to the consensus recommendations.
Significant variation in prescription practices by location and by patients' race was reported by Owen and colleagues (10). They noted that among persons with schizophrenia, whites were twice as likely as nonwhites to receive second-generation antipsychotics at hospital discharge. Race may also be a factor in the impact of specific second-generation antipsychotics on metabolic parameters. For example, compared with patients of other racial-ethnic groups, African Americans who take second-generation antipsychotics are more likely to experience metabolic abnormalities (11,12) and more susceptible to weight gain with clozapine (13). Krakowski and colleagues (14) reported that African Americans randomly assigned to receive clozapine gained more weight than white or Hispanic patients.
Given the impact of second-generation antipsychotics on metabolic dysregulation and our interest in the health of older psychiatric patients, this study had two primary objectives. In a sample of older VA patients using second-generation antipsychotics, this study sought to evaluate patient and system factors associated with switching among antipsychotics and with switches that incurred greater or lesser metabolic risk. We hypothesized that switches to antipsychotics with better metabolic profiles would increase over the study period.
Methods
Patients were part of a larger study of patterns of health care utilization among older VA patients, which examined outcomes of care for patients with schizophrenia who were 50 years or older. This age cutoff was chosen to capture data from later life for a patient population that may experience death ten to 25 years earlier than persons without schizophrenia (15,16). Institutional review board approval was granted before study initiation.
To be included in the study reported here patients had to be over 50 years old as of October 1, 2001; eligible for care in the VA; be diagnosed as having schizophrenia (ICD-9 codes 295.xx, excluding 295.5) for two or more outpatient visits in fiscal year 2002; be alive at the end of the four-year study period; receive second-generation antipsychotic monotherapy at baseline; and have at least two outpatient care visits in each study year (2002–2005). This resulted in a cohort of 16,103 veterans with schizophrenia who remained in VA care over the four years.
Administrative data were analyzed. These data sets contain nationwide extracts from the VA's all-electronic medical record, which are transmitted nightly from VA facilities to a central data repository and updated semimonthly in a uniform format. Although the medical record is a dynamic system that changes daily, administrative extracts are finalized in October of each year for the preceding 12-month fiscal year.
Measures included age as of October 1, 2001, race (white, African American, and other or unknown), Hispanic ethnicity, gender, marital status, and VA priority status. In VA databases, race is sometimes self-reported and sometimes based on observation by clinical staff; for this study, multiple values were distilled into the most frequently recorded race. Priority status is associated with physical and mental health status and correlates with socioeconomic status and severity of illness (17–19). Priority 1 patients had 50%–100% service-connected disability and no copayments for VA care. Priority 2 through 6 patients met various eligibility criteria (including catastrophically disabled, low income, Purple Heart, former prisoner of war, or Gulf War syndrome) and had pharmacy but not medical care copayments. Priority 7 and 8 patients had no service-connected disability and were subject to copayments for both pharmacy and medical care.
Clinical measures included diagnosis of hypertension (ICD-9 codes 401–405) (20), dyslipidemia (ICD-9 code 272), obesity (ICD-9 code 278), and diabetes (ICD-9 code 250). The analyses also included a modified Selim physical comorbidity index (21) as a case-mix adjuster, with omission of assessments of diabetes and hypertension, whose effects were modeled independently. The Selim physical comorbidity index counts 30 medical conditions. The index was developed with self-report data and has been operationalized and validated in VA administrative data (19).
Metabolic risk related to switching among second-generation antipsychotics was operationalized as follows. First, the second-generation antipsychotics were categorized on the basis of the consensus statement as strongly associated with metabolic risk (clozapine and olanzapine), moderately associated with metabolic risk (quetiapine and risperidone), or weakly associated with metabolic risk (aripiprazole and ziprasidone) (2). On the basis of all second-generation antipsychotics prescribed, the highest antipsychotic-associated metabolic risk level was determined for each patient for each year.
Switching was categorized as a change from one metabolic risk level to another or as a risk-neutral switch. For example, if a patient was prescribed risperidone in 2002 but both risperidone and olanzapine in 2003, the patient was categorized as experiencing a switch to a higher risk level. If the patient was prescribed risperidone and olanzapine in 2004 but aripiprazole in 2005, the patient was categorized as experiencing a switch to a lower risk level. Thus four outcomes were analyzed. Switching to an antipsychotic with a lower metabolic risk (decreased-risk switch) was compared with switching from a lower to a higher level of risk (increased-risk switch), remaining at the same level of risk (neutral switch), and experiencing no switch in medication. Switching was assessed from 2002 to 2003 (before the consensus statement) and again with the same cohort from 2004 to 2005 (in the wake of the consensus statement).
Facility-level measures included region of the country: Northeast, South, Midwest, West, and Puerto Rico and the U.S. Virgin Islands. Regions reflect the U.S. Census regions—plus Puerto Rico and the U.S. Virgin Islands, locations outside Census regions—where the VA maintains health care systems. Measures identified facilities that served a relatively high proportion of Hispanic patients (10% or higher) and a relatively high proportion of African-American patients (20% or higher). This was done because it was expected that providers treating a large proportion of high-risk patients (6) would be more sensitized to their risk.
Data were characterized with descriptive statistics and analyzed in multinomial logistic regression models of the four-level outcomes. Multinomial regression is an expansion of logistic regression to assess factors in nominal, nonordered outcomes that have more than two categories. The multinomial regression effects are reported as relative risk ratios (RRRs) with their 95% confidence intervals (CIs). RRRs are similar to the more familiar odds ratios produced by logistic regression on dichotomous outcomes but apply to polytomous outcomes. RRRs greater than 1 indicate a positive association, and RRRs between 0 and 1 (fractional values) indicate a negative association. Effect sizes are also similar to those of odds ratios, with large effects denoted by RRRs ≥2.0 for positive effects and by RRRs ≤.5 for negative effects (22). Multivariable models adjusted for age (in decades); sex; race and ethnicity (African American versus other race and Hispanic versus non-Hispanic ethnicity); marital status (married versus other); VA priority status (priority 1 versus other); comorbid diagnosis of obesity, diabetes, hyperlipidemia, or hypertension; modified Selim physical comorbidity index; region; and facility characteristics regarding the proportion of patients served who were from minority groups. Because clustering of VA patients within sites may affect some outcomes, multivariable models also adjusted for this effect (24). Analyses were completed with SAS, version 9.1, and Stata/SE, version 9.2.
Results
As shown in Table 1, the mean age of the 16,103 patients in the sample was 58.2 (range 50–102). Consistent with historical trends in U.S. military recruitment, only 4% of the patients were women. In addition, 56% were white, 23% were African American, and 10% were Hispanic. Less than a third were married (29%). At baseline patients had a mean of 2.0 general medical conditions (range 0–12). Comorbid diagnoses in the baseline year included obesity (15% of the sample), diabetes (24%), dyslipidemia (34%), and hypertension (45%). More than a third of the study cohort (36%) lived in the South, and about half (53%) were VA priority 1 patients.
The most commonly used antipsychotics in the baseline year 2002 were olanzapine, used by 44% of the sample (N=7,156), and risperidone, prescribed to 41% (N=6,557); 3% of patients (N=482) were taking clozapine at baseline, 1% (N=156) were taking ziprasidone, and 11% (N=1,752) were taking quetiapine. Olanzapine and risperidone were prescribed for 81% of patients (N=13,013) in 2003, 75% of patients in 2004 (N=12,075), and 70% of patients in 2005 (N=11,218). The newest antipsychotics, ziprasidone and aripiprazole, were least prescribed, although they present the lowest metabolic risk. Use of these two antipsychotics increased from 1% initially (N=156) to 10% of patients in 2005 (N=1,590). Despite an increase in prescriptions in the final years, only 2% of patients (N=331) used these two lower-risk antipsychotics exclusively. Each year after baseline a small percentage of patients were prescribed three or more second-generation antipsychotics (<2%), first-generation antipsychotics, or both first- and second-generation antipsychotics (4%–6%).
Antipsychotic switches were more common after the consensus statement than before—13% in 2002–2003 and 25% in 2004–2005 (Table 1). In 2002–2003, 1.5% of patients (N=233) switched to lower-risk antipsychotics, 9.3% (N=1,493) had neutral switches, 86.7% (N=13,963) did not switch, and 2.6% (N=414) switched to higher-risk antipsychotics. During 2004–2005, however, a greater proportion of patients switched to lower-risk antipsychotics (5.6%, N=897) than to higher-risk antipsychotics (1.2%, N=195), whereas 18.5% (N=2,975) had neutral switches and 74.7% (N=12,036) did not switch.
Results of the multivariable analyses of changes in antipsychotic metabolic risk level for 2002–2003, which controlled for clustering of patients within facilities, are shown in Table 2. Significant factors in switches included age, gender, Hispanic ethnicity, dyslipidemia, obesity, diabetes, Selim comorbidity score, and region but not race. The referent outcome was no change in second-generation antipsychotic from 2002 to 2003. Decreased-risk switches were negatively associated with age (RRR=.74 per decade), dyslipidemia (RRR=.59), obesity (RRR=.56), and living in the Northeast (RRR=.54) or Puerto Rico or the Virgin Islands (RRR=.51) and relatively more likely among patients with diabetes (RRR=1.50). That is, having any of these characteristics except diabetes made a decreased-risk switch less likely, whereas diabetes made such a switch more likely. Neutral-risk switches were associated with age, gender, Hispanic ethnicity, Selim comorbidity score, and region. Increased-risk switches were negatively associated with age (RRR=.87 per decade), dyslipidemia (RRR=.65), obesity (RRR=.64), and living in Puerto Rico or the U.S. Virgin Islands (RRR=.50). In short, two risk factors—obesity and dyslipidemia—were modestly associated with switches that were in the appropriate direction for those risk factors. Older patients were less likely to experience switches of any kind.
Results of the multivariable analyses for 2004–2005 switches are shown in Table 3. Diabetes was positively associated with decreased-risk switches (RRR=1.38), as was hypertension (RRR=1.22). Older age (RRR=.67) and receiving care in the Northeast (RRR=.76) were negatively associated. Factors in neutral switches were age, dyslipidemia, hypertension, diabetes, and Selim score. Older age was negatively associated with increased-risk switches (RRR=.76), and female gender was positively associated (RRR=2.56). No race factors were significant in any switching outcome.
Discussion
In this sample the proportion of older persons with schizophrenia who experienced switching among second-generation antipsychotics increased modestly over the study period—2002 through 2005. During this period, one additional second-generation antipsychotic (aripiprazole) became available, which increased the choices of clinicians and patients seeking improved response to or tolerability of antipsychotic medication. Aripiprazole was used by 2.4% of these patients in 2004 and by 6.1% in 2005. Other second-generation antipsychotics were available before the study began: clozapine (1989), risperidone (1993), olanzapine (1996), quetiapine (1997), and ziprasidone (2001).
Comorbid conditions indicative of metabolic risk were common, with high rates of diagnosed obesity, diabetes, hypertension, and hyperlipidemia. Only one-third of the sample had none of these diagnoses. Undiagnosed obesity is common and may have affected many more patients. These issues are now understood to be important considerations in selecting an antipsychotic agent, because unintended weight gain or glucose abnormalities result from many second-generation antipsychotics (24).
Before the February 2004 consensus statement on the hazards of second-generation antipsychotics (2), changes in second-generation antipsychotic prescriptions appeared to be mixed in sensitivity to specific metabolic risk factors, with some risk factors associated with beneficial switches at the same time that other risk factors were negatively associated. Our multivariable model assessed second-generation antipsychotic switches in terms of their metabolic risk level from 2002 to 2003 and found significant negative associations with age, dyslipidemia, and obesity for changes to either higher- or lower-risk second-generation antipsychotics—that is, there was unclear evidence that the changes took the risk factors of obesity and hyperlipidemia into account. However, diabetes was associated with decreased-risk switches.
After the consensus statement, on the other hand, decreased-risk switches were no longer less likely for patients with dyslipidemia or obesity and remained positively associated with a diabetes diagnosis. Time could have been a factor in this finding because ziprasidone and aripiprazole became available only in 2001 and late 2002, respectively. These two second-generation antipsychotics may be less likely to be associated with metabolic dysregulation, yet their adoption for use in this sample of older patients with schizophrenia was limited. It is conceivable that as these newer antipsychotics became available, psychiatrists were inclined to use them because of an increased awareness of the need to treat psychiatric symptoms in balance with physical disease.
Other indicators of risk were not salient factors in switching. Although it was conjectured that African-American and Hispanic patients, who are known to be at higher risk of metabolic disorders, would be more likely to experience beneficial second-generation antipsychotic switches, no evidence of this was found. Contrary to our expectation that prescribers in facilities that served a larger proportion of patients from minority groups would be more sensitive to their metabolic risk factors, no evidence was found of risk tailoring in antipsychotic switches for patients treated in facilities serving a larger proportion of minority patients.
Older age was uniformly associated with risk-neutral switches or no changes in second-generation antipsychotics over the four years, an interesting finding given the association of older age with metabolic disorders (5). Presumably the oldest patients were most stable, and a change, even if it held promise of improved metabolic status, was rarely pursued. It remains to be shown whether this course of action optimizes quality of life.
Limitations of the study include reliance on archival patient care data. The reasons for provider prescription choices and patient (or family) preferences for antipsychotic medications cannot be determined from these data. The study design does not permit drawing causal inferences. Associations were interpreted as a proxy for prescribers' decision-making process. In addition, medication switches were defined as prescription of an antipsychotic with a different metabolic risk level than in the prior year; whether the patient was in the process of switching again or was on concurrent medications was not captured. Only patients at least 50 years old were studied; results should not be generalized to younger patients. Finally, the study design identified a cohort of patients with persistent use of the VA health care system, because use of outpatient care in each of four consecutive years was an eligibility criterion. An alternative study design that examined a new cohort defined in fiscal year 2004 may have yielded different results and should be considered in future research.
The high prevalence of diabetes, hypertension, and dyslipidemia among patients who received high-risk antipsychotics (olanzapine and clozapine) points to the continuing need to encourage providers to monitor patients' metabolic parameters and, when appropriate, to consider switching to antipsychotic agents with lower risk profiles. We could postulate that providers prioritized comorbid conditions in terms of the risks they pose for patients, paying more attention to diagnosed diabetes than to other conditions. Alternatively, continuing education regarding the metabolic risks of second-generation antipsychotics may have focused on diabetes.
The regional differences found in prescribing patterns are in accord with a previous report of large interfacility variation in the VA system (10) and could point to potential influences of organizational, provider, and patient factors. Elucidating the impact of these factors is relevant given increasing financial pressures generated by the need to provide comprehensive care for a large cohort of aging veterans with mental and general medical disorders. Implementation of antipsychotic prescription guidelines to address metabolic sequelae may have a differential impact on some facilities in the nationwide VA system, such as facilities that serve patients with higher rates of risk factors or those with higher rates of high-risk antipsychotic prescription. Policy makers should deploy resources accordingly to effect equitable improvements in practice.
The association between gender and type of switch requires further exploration, particularly because previous reports have indicated that women were more likely to develop symptoms of metabolic dysregulation (5).
Conclusions
Incorporation of evidence-based practices in the use of second-generation antipsychotics appeared to increase between 2002 and 2005, suggesting that providers were modestly responsive to the growing data on the differential impact of second-generation antipsychotics on metabolic parameters. The preponderance of evidence points to the need to clearly recognize differences among second-generation antipsychotics and the potential benefits of switching when appropriate (8). The role of any behavioral factors, such as obesity counseling or weight management (25), should be assessed in future work. Intervention studies are needed to establish the value of incorporating medical and behavioral factors, as well as psychiatric and preference factors, into the selection of antipsychotic medications and to assess the impact of these factors on clinical outcomes.
1 : Physical health monitoring of patients with schizophrenia. American Journal of Psychiatry 161:1334–1349, 2004 Link, Google Scholar
2
3 : Atypical antipsychotics and metabolic dysregulation: evaluating the risk/benefit equation and improving the standard of care. Journal of Clinical Psychopharmacology 24:S7–S14, 2004 Crossref, Medline, Google Scholar
4 : Antipsychotic drugs may worsen metabolic control in type 2 diabetes mellitus. Journal of Clinical Psychiatry 65:674–678, 2004 Crossref, Medline, Google Scholar
5 : Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophrenia Research 80:19–32, 2005 Crossref, Medline, Google Scholar
6
7 Total Prevalence of Diabetes and Pre-Diabetes. Alexandria, Va, American Diabetes Association, 2010. Available at www.diabetes.org/diabetes-basics/diabetes-statistics Google Scholar
8 : Strategies for dosing and switching antipsychotics for optimal clinical management. Journal of Clinical Psychiatry 69(suppl 1):4–17, 2008 Crossref, Google Scholar
9 : Metabolic screening after the American Diabetes Association's consensus statement on antipsychotic drugs and diabetes. Diabetes Care 32:1037–1042, 2009 Crossref, Medline, Google Scholar
10 : Variations in prescribing practices for novel antipsychotic medications among Veterans Affairs hospitals. Psychiatric Services 52:1523–1525, 2001 Link, Google Scholar
11 : Diabetes mellitus among outpatients receiving clozapine: prevalence and clinical-demographic correlates. Journal of Clinical Psychiatry 66:900–906, 2005 Crossref, Medline, Google Scholar
12 : Prevalence of obesity, glucose homeostasis disorders and metabolic syndrome in psychiatric patients taking typical or atypical antipsychotic drugs: a cross-sectional study. Diabetologia 48:215–221, 2005 Crossref, Medline, Google Scholar
13 : Weight gain during a double-blind multidosage clozapine study. Journal of Clinical Psychopharmacology 27:22–27, 2007 Crossref, Medline, Google Scholar
14 : Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double-blind clozapine, olanzapine or haloperidol. Schizophrenia Research 110:95–102, 2009 Crossref, Medline, Google Scholar
15 : Excess mortality of schizophrenia: a meta-analysis. British Journal of Psychiatry 171:502–508, 1997 Crossref, Medline, Google Scholar
16 : Morbidity and Mortality in People With Serious Mental Illness. Technical report 13. Alexandria Va, National Association of State Mental Health Program Directors Medical Directors Council, 2006 Google Scholar
17 : Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Archives of Internal Medicine 158:626–632, 1998 Crossref, Medline, Google Scholar
18 : VHA enrollees' health care coverage and use of care. Medical Care Research and Review 60:253–267, 2003 Crossref, Medline, Google Scholar
19 : The impact of epilepsy on health status among younger and older adults. Epilepsia 46:1820–1827, 2005 Crossref, Medline, Google Scholar
20 International Classification of Diseases, 9th Revision, Clinical Modification, 6th ed. Geneva, World Health Organization, 2008 Google Scholar
21 : Comorbidity assessments based on patient report: results from the Veterans Health Study. Journal of Ambulatory Care Management 27:281–295, 2004 Crossref, Medline, Google Scholar
22 : Multiway Contingency Tables Analysis for the Social Sciences. Hillsdale, NJ, Erlbaum, 1989 Google Scholar
23 : Whom should we profile? Examining diabetes care practice variation among primary care providers, provider groups, and health care facilities. Health Services Research 37:1159–1180, 2002 Crossref, Medline, Google Scholar
24 : New-onset type-2 diabetes associated with atypical antipsychotic medications. Progress in Neuro-psychopharmacology and Biological Psychiatry 30:919–923, 2006 Crossref, Medline, Google Scholar
25 : Nutritional intervention to prevent weight gain in patients commenced on olanzapine: a randomized controlled trial. Australian and New Zealand Journal of Psychiatry 39:479–486, 2005 Medline, Google Scholar
Figures and Tables
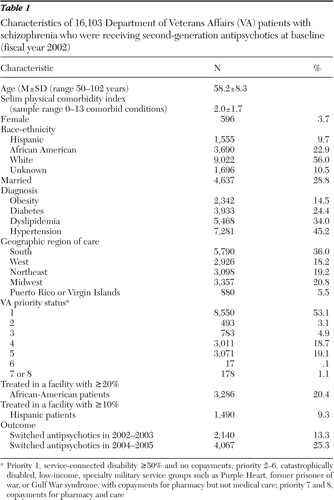
Table 1 Characteristics of 16,103 Department of Veterans Affairs (VA) patients with schizophrenia who were receiving second-generation antipsychotics at baseline (fiscal year 2002)
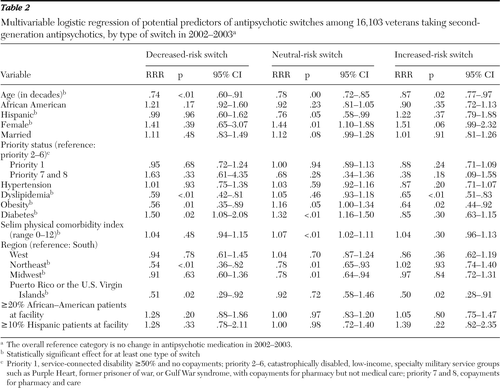
Table 2 Multivariable logistic regression of potential predictors of antipsychotic switches among 16,103 veterans taking second-generation antipsychotics, by type of switch in 2002–2003
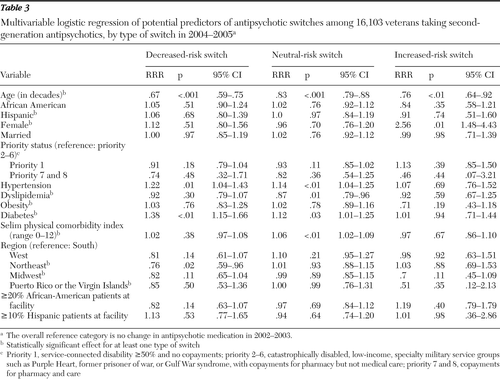
Table 3 Multivariable logistic regression of potential predictors of antipsychotic switches among 16,103 veterans taking second-generation antipsychotics, by type of switch in 2004–2005



