Psychiatric and General Medical Conditions Comorbid With Schizophrenia in the National Hospital Discharge Survey
Rates of morbidity and mortality from general medical conditions among patients with schizophrenia are elevated compared with rates in the general U.S. population ( 1 , 2 , 3 , 4 , 5 , 6 , 7 ), and this gap has widened in recent decades ( 8 , 9 , 10 ). Fifty percent ( 11 ) to 74% ( 12 ) of patients with schizophrenia have been reported to have one or more comorbid psychiatric or general medical conditions that may worsen the prognosis and contribute to increased morbidity and mortality. These rates are likely underestimates, because many comorbid illnesses are misdiagnosed or undiagnosed among patients with schizophrenia as a result of a suboptimal integration of general medical and psychiatric services, barriers to care delivery, and patients' poor ability to recognize medical problems and their reluctance to seek help ( 13 , 14 ).
High rates of cigarette smoking ( 2 , 3 , 15 , 16 , 17 ), alcohol and illicit drug abuse ( 11 ), and poor diet and lack of exercise ( 18 ) in this population may contribute to increased rates of cardiopulmonary ( 3 , 11 , 12 , 19 , 20 ), metabolic ( 2 , 3 , 11 , 12 , 16 , 21 ), and gastrointestinal ( 12 , 22 ) diseases. Poor hygiene, use of injection drugs ( 23 ), and engagement in high-risk sexual behavior ( 24 , 25 ) among individuals with schizophrenia are associated with higher risk of gastrointestinal, blood-borne, and sexually transmitted infectious diseases, including hepatitis ( 2 , 3 , 26 , 27 ) and HIV ( 2 , 3 , 12 , 27 ).
Compared with the general U.S. population, individuals with schizophrenia more often face homelessness ( 28 , 29 , 30 ), substandard living conditions ( 31 ), and lack of access to medical care and are less likely to receive general medical check-ups ( 32 ). Psychotropic medications that help alleviate symptoms of schizophrenia and improve overall disease prognosis may have significant side effects, including extrapyramidal symptoms and tardive dyskinesia, weight gain, obesity, hyperglycemia and diabetes ( 33 , 34 ), and cardiovascular disease ( 34 ). The association of schizophrenia with some general medical conditions, such as metabolic syndrome ( 35 ) and type II diabetes ( 36 , 37 ), after analyses control for other factors, may indicate shared etiopathogenic pathways.
Virtually all existing studies of comorbid disorders in schizophrenia test hypotheses and focus on a single condition. To our knowledge no systematic analysis of disorders comorbid with schizophrenia has been conducted in the U.S. hospitalized population. In addition, most previous studies have relied on relatively small and nonrepresentative samples. To overcome such limitations and seek new information about poorly examined comorbid conditions, we used National Hospital Discharge Survey (NHDS) data to conduct a systematic analysis of the occurrence of more than one ICD-9 code in the discharge records of hospitalizations for schizophrenia and hospitalizations for other reasons. Demographic characteristics of hospitalized patients with schizophrenia and the proportion of total discharges of patients with schizophrenia over time were also explored.
Methods
Data source
The NHDS is conducted annually by the National Center for Health Statistics, a branch of the U.S. Centers for Disease Control and Prevention. NHDS uses a stratified, multistage probability design and covers discharges from short-stay (average stay of less than 30 days) hospitals (except federal, military, and Department of Veterans Affairs hospitals and hospital units of institutions such as prisons) located in the 50 states and the District of Colombia. The design enables users to produce U.S. national and regional estimates of characteristics of hospitalized patients, length of stay, diagnoses, and procedures in hospitals of various sizes and types of ownership ( www.cdc.gov/nchs/about/major/hdasd/nhds.htm ).
The 1979–2003 multiyear public use data and documentation files were used for this study. The multiyear file has standardized coding of variables across the data years. The data elements used in the study reported here included several demographic characteristics, date of hospitalization, length of stay, source of payment, and up to seven ICD-9 code diagnoses. ICD-9 codes are compatible with codes provided in the DSM, which is used for evaluating and coding psychiatric disorders. In the NHDS sample individuals can be included more than once if they have more than one hospitalization during the sampling period and if data for the hospitalization are captured by the sampling methods. Therefore, all estimates reported here refer to discharges and not to individuals. However, the overall likelihood of data capture during the sampling procedures is less than 1%. Thus the probability that a single individual was represented more than once in the unweighted sample is quite low. NHDS data are publicly available and contain no patient-identifying information. Thus there was no danger of unauthorized disclosure and no need for informed consent. The Walter Reed Army Institute of Research Institutional Review Board approved this study.
Study population
For descriptive and time trend analyses, all discharge records for persons aged one year and older were included (N=5,733,781). Comorbidity was examined only in the discharge records of patients between the ages of 15 and 64 (N=2,623,229), because this age group accounted for more than 85% of the discharges that listed schizophrenia as a diagnosis. Hospitalizations associated with normal childbirth ( ICD-9 code V 27) were excluded. Only discharges with a primary diagnosis of schizophrenia were defined as cases in the comorbidity analyses; records that had schizophrenia codes in any other position were excluded. Among the 2,623,229 discharges, a total of 1,963,155 (74.8%) listed more than one diagnosis. Among discharges with more than one diagnosis, 26,279 (1.3%) had a primary diagnosis of schizophrenia. The proportions of comorbid conditions in this group were compared with the proportions in the group with any other primary diagnosis (N=1,936,876). [A figure displaying the study subgroups is available as an online supplement to this article at ps.psychiatryonline.org .]
Data coding
NHDS discharge records list between one and seven diagnoses; records with at least one diagnosis of a schizophrenia disorder ( ICD-9 codes 295.0–295.9) were classified as discharges with schizophrenia (N=64,113). All other records were considered to be discharges without schizophrenia. A record was considered to list a comorbid condition if it listed more than one ICD-9 discharge diagnosis. In this report comorbid conditions are presented as three-digit ICD-9 codes, except for diabetes type II, which has to be a five-digit ICD-9 code, with 250 as the first three digits, a number from 1 through 9 as the fourth digit (indicating complications), and a fifth digit of 0 or 2. Diabetes type II was included in the analysis because of previously reported associations with schizophrenia ( 21 , 38 ).
Statistical analyses
We used the NHDS variable "weight" to provide national estimates (weighted frequencies) for demographic characteristics, region, source of payment, and length of stay for hospital discharges with schizophrenia compared with those without schizophrenia. Frequencies of comorbid conditions were analyzed for discharges with schizophrenia as the primary diagnosis and for those with any other primary diagnosis.
Because the NHDS variable "weight" is not designed to provide national estimates of the co-occurrence of diagnoses, these estimates were not used to determine the proportions of concurrent conditions and their associations with primary diagnosis. The results of weighted and unweighted analyses were essentially the same.
We counted and sorted by frequencies all concurrent conditions and calculated proportional morbidity and the proportional morbidity ratio (PMR) in order to examine the prevalence of disorders among discharges with and without a primary diagnosis of schizophrenia. The PMR was calculated for every comorbid condition that was more prevalent among the discharges with schizophrenia and that had a proportional morbidity of at least .5%. Because of the definition used, it is possible to have a cumulative prevalence (or count) of conditions up to six times greater than the count of records.
Results
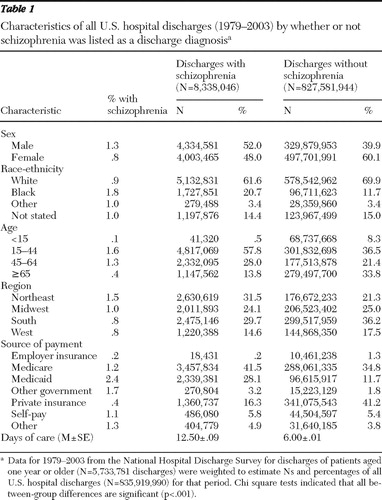
Table 1 presents demographic information from the records of patients with and without a diagnosis of schizophrenia weighted to reflect all U.S. hospital discharges. The overall proportion of discharges with schizophrenia as a diagnosis (not necessarily the primary diagnosis) was 1.0%, and the proportion varied significantly (p<.001) by demographic group. The proportion with schizophrenia was higher among males (1.3%) than females (.8%) and among blacks (1.8%) than whites (.9%) or the "other" racial-ethnic group (1.0%). The proportion was highest among the group aged 15 to 44 (1.6%), followed by the group aged 45 to 64 (1.3%). It was also highest among discharges in the Northeast region (1.5%). The proportion of discharges with schizophrenia was highest among those with Medicaid as source of payment (2.4%), followed by other government funding (1.7%), other sources of payment (1.3%), and Medicare (1.2%).
 |
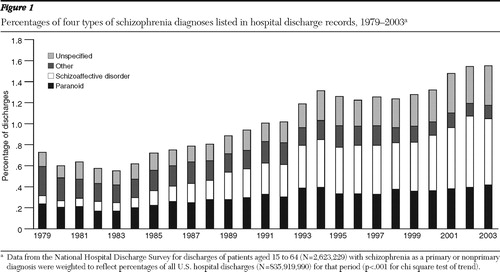
As shown in Figure 1 , the proportion of discharges with any diagnosis of schizophrenia (primary or nonprimary) increased significantly over time (p<.001). The trend in discharges with paranoid schizophrenia, schizoaffective disorder, and schizophrenia not otherwise specified was significant among both males and females (p<.001) (data not shown). Among males the trend was mostly attributable to increases in schizoaffective disorder and paranoid schizophrenia, whereas among females it was primarily attributable to an increase in schizoaffective disorder. In the early 1980s schizoaffective disorder was the least prevalent type among males and females. Starting in the late 1980s it became the most prevalent type among males. This trend was evident among females starting in early 1990s.
The mean±SE length of hospital stay for discharges with any diagnosis of schizophrenia over the study period was 12.50±.09 days, compared with 6.00±.01 days for discharges without schizophrenia. The mean length of hospital stay for all discharges with schizophrenia decreased significantly (p<.001) from 15.81±.51 days in 1979 to 9.92±.37 in 2003. There was also a significant but less substantial decline in the length of stay among discharges without schizophrenia, from 7.19±.02 days in 1979 to 4.82±.02 days in 2003 (p<.001).
The median number of comorbid conditions for discharges with and without any diagnosis of schizophrenia was two among all hospitalizations (aged 15 to 64) and three for discharges restricted to those with two or more ICD-9 codes. Among 55,292 discharge records that listed a diagnosis of schizophrenia, most (75%, N=41,587) listed it as the primary diagnosis (that is, in the first position), including 26,279 records with at least one comorbid condition. Table 2 presents data on the number of conditions and proportional morbidity for the 20 most prevalent (>2%) comorbid conditions for the two discharge groups. Psychiatric and behavior-related diagnoses accounted for 45% of comorbid diagnostic categories among schizophrenia discharges, compared with 15% among other discharges.
 |
As shown in Table 3 , the proportion of discharges with comorbid mental disorders was much higher among discharges with a primary diagnosis of schizophrenia, including mild mental retardation (PMR=19.5), personality disorders (PMR=7.6), affective psychoses (PMR=4.6), nondependent abuse of drugs (PMR=3.4), adjustment reaction (PMR=2.2), alcohol dependence (PMR=2.1), drug dependence (PMR=1.9), depressive disorder not elsewhere classified (PMR=1.8), and neurotic disorders (PMR=1.8). In addition, discharge records with schizophrenia as the primary diagnosis were significantly more likely (p<.05) to list the following nonpsychiatric comorbid conditions with a proportional morbidity of more than 2%: acquired hypothyroidism (PMR=2.9), obesity and other hyperalimentation (PMR=2.0), asthma (PMR=1.5), chronic airway obstruction not elsewhere classified (PMR=1.4), essential hypertension (PMR=1.2), and diabetes type II (PMR=1.2). Proportional morbidity of diabetes type II was 6.7% for discharges with schizophrenia and 7.3% for those without schizophrenia (PMR=1.2, 95% confidence interval [CI]=1.2–1.3, p<.001) (data not shown). Discharge records with a primary diagnosis of schizophrenia were also significantly more likely (p<.005) to list other less prevalent (.5%–2%) comorbid nonpsychiatric conditions ( Table 4 ). Other extrapyramidal symptoms and abnormal movement disorder was present among 1.8% of discharges with schizophrenia, and poisoning by a psychotropic agent was present among .5%, compared with .1% and .2%, respectively, among discharges without schizophrenia.
 |
 |
Discussion
Consistent with previous findings, the proportion of hospital discharges that included any diagnosis of schizophrenia was higher among males than among females ( 39 , 40 ), among blacks than among other racial-ethnic groups ( 41 , 42 ), and in discharges in the Northeast—the area with higher urbanicity—compared with other U.S. regions ( 39 , 43 , 44 ). The increase in the proportion of discharges with schizophrenia over the study period could be partly explained by changes in diagnostic criteria. The concept of schizophrenia was very narrow in DSM-III (1980) ( 45 , 46 , 47 ) and even narrower in DSM-III-R (1987) ( 45 , 47 , 48 ), and thus the proportion of discharges with schizophrenia declined over that period. As criteria progressively broadened in DSM-IV (1994) and DSM-IV-TR (2000), the proportion increased. However, DSM-IV criteria were introduced cautiously to avoid an increase in the epidemiological base rate of schizophrenia so that prevalence estimates would not change substantially ( 47 ). In fact, high diagnostic agreement was found between DSM-III-R and DSM-IV ( 49 ).
Changes in criteria from DSM-III through DSM-IV-TR also likely contributed to the increase in the proportion of discharges with schizoaffective disorder ( 46 ). In addition, there has been considerable controversy about the stability and usefulness of the diagnosis of schizoaffective disorder since it was introduced in 1933 ( 50 , 51 , 52 ). In our study we used a very broad case definition that included schizophrenia disorders listed in the ICD-9 classification instead of using the DSM-IV-TR definition of schizophrenia. We did this in order to overcome uncertainties in regard to the diagnosis of schizoaffective disorder and to capture fluctuations in the population of cases that were artifacts of changes in DSM definitions of schizophrenia and schizoaffective disorder.
The high prevalence rates of comorbid psychiatric conditions, such as mild mental retardation (PMR=19.5) and personality disorders (PMR=7.6), among discharges with a primary diagnosis of schizophrenia is in accordance with the multiaxial assessment system. As noted in DSM-IV-TR, the multiaxial system is used for psychiatric assessments to facilitate comprehensive and systematic evaluation with attention to various mental disorders and general medical conditions. Use of the multiaxial system could at least partly explain the much higher prevalence of psychiatric conditions among discharges with a primary diagnosis of schizophrenia. Consistent with previous findings ( 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 ), this study found a higher proportion of alcohol dependence (PMR=2.1), drug dependence (PMR=1.9), nondependent abuse of drugs (PMR=3.4), and psychiatric comorbid conditions including depressive symptoms (PMR=4.6 and 1.8) among discharges with schizophrenia.
Of the nonpsychiatric comorbid conditions, acquired hypothyroidism was nearly three times more prevalent among discharges with a primary diagnosis of schizophrenia. This finding is in line with those of previous studies ( 2 , 6 , 61 ) and case reports ( 62 , 63 ), but reasons for this association remain unclear.
The observed increase in metabolic disorders among discharges with schizophrenia is also consistent with previous reports ( 2 , 3 , 11 , 12 , 16 , 21 , 37 , 64 ). Obesity and other hyperalimentation were observed more frequently among discharges with schizophrenia. From 1979 through 1983 the likelihood of comorbid obesity was not significantly different between discharges with and without a primary diagnosis of schizophrenia, but in 1984–1988 the PMR rose to 1.5 (CI=1.3–1.9) and in subsequent years the average PMR was 2.0 (CI=1.9–2.1). A less substantial increase was found for diabetes mellitus type II (PMR=1.2) among discharges with schizophrenia. This increase was driven only by the difference in 1998–2003. The temporal increase in obesity and diabetes type II among discharges with schizophrenia might reflect increased use of some second-generation antipsychotic medications ( 65 ).
Certain second-generation psychotropic agents can increase levels of triglycerides and low-density lipoprotein cholesterol and decrease high-density lipoprotein cholesterol ( 14 , 65 , 66 ). However, we did not find an increase in these conditions among discharges with schizophrenia, probably for at least two reasons. First, the ICD-9 codes lack specificity to capture dyslipidemia. Second, screening for and treatment of dyslipidemia are reported to be lower among psychiatric patients than among patients without psychiatric disorders ( 14 , 67 ).
Chronic obstructive pulmonary diseases were prevalent in a larger proportion of discharges with schizophrenia, specifically asthma (PMR=1.5), chronic airway obstruction not elsewhere classified (PMR=1.4), and chronic bronchitis (PMR=1.2). The prevalence of tobacco use disorder ( ICD-9 305.1) was 1.6 times higher (CI=1.5–1.7) among discharges with schizophrenia. These results are consistent with other findings of a high prevalence of smoking among persons with schizophrenia ( 7 , 56 , 64 ). When tobacco use disorder was added to the multivariable models, the PMR for chronic obstructive pulmonary diseases did not change substantially, which is consistent with recent findings ( 68 ).
It is of interest that epilepsy was twice as prevalent among discharges with schizophrenia. This association has no clear pathogenic mechanism and has been reported in only a few previous studies ( 6 , 64 , 69 ).
Among discharges with a primary diagnosis of schizophrenia, we also found PMRs of 6.5 for dermatophytosis, 3.3 for diseases of sebaceous glands, and 2.9 for contact dermatitis and other eczema. Although the associations between these conditions and schizophrenia are largely unexplored, some supportive findings exist in the literature ( 2 , 70 , 71 ). Also consistent with previous findings ( 6 , 26 , 27 ), the prevalence of viral hepatitis was higher (PMR=1.4) among discharges with schizophrenia.
Higher rates of general medical conditions among persons with schizophrenia may be attributable to unhealthy behaviors, low socioeconomic status, and side effects of psychotropic medications. Poor socioeconomic status in itself can reduce access to medical care and increase exposure to unhealthy behaviors and lifestyles. In addition, schizophrenia may share etiopathogenic pathways with some other medical conditions.
This study may have underestimated PMRs for a few reasons. Records selected for comorbidity analyses had schizophrenia as a primary diagnosis, and the patients were presumably attended by psychiatrists. Mental health was most likely the major focus of diagnostic and treatment procedures for these hospitalizations. Therefore, the likelihood that these individuals were routinely screened for a variety of non-life-threatening medical conditions is probably lower than for those who were admitted with a primary diagnosis of a general medical condition. Consistent with this assumption is our finding of a higher prevalence of a few poorly specified conditions and disorders among discharges with schizophrenia. In addition, patients with schizophrenia are much less likely to be aware of their general medical problems and to communicate with health care providers about them ( 58 ), which makes it more likely that these problems were not evaluated during primarily psychiatric hospitalizations.
Our study has several strengths. We have described comorbidity among hospital discharge records in a large nationally representative database. This unique and robust data source has been relatively neglected in previous psychiatric research. We systematically determined the most prevalent comorbid conditions for hospitalizations with a primary diagnosis of schizophrenia and for those with any other primary diagnosis and examined temporal trends in the diagnostic coding of schizophrenia types and length of hospitalization over a 25-year period. The multiyear data set was used to standardize variables across years and decrease year-to-year variability. The analysis of proportional morbidity was adjusted for sex, race, age, and geographic region and selectively adjusted for tobacco use disorder.
However, the study has some limitations. First, the observations in these data are hospitalizations, and it is possible that an individual was represented more than once. This is an important confound warranting investigation. If individuals with schizophrenia are more likely to be hospitalized than those without schizophrenia, then the proportional morbidity of schizophrenia among hospital discharges could be overestimated. Also, if individuals with schizophrenia and a comorbid condition have a higher rate of rehospitalization than those with other primary diagnoses and the same comorbid condition, the PMRs for this condition would be biased away from the null. However, because the probability of the same person's being represented in the unweighted data set more than once is low, any such bias would be unlikely to materially change our results or conclusions.
In addition, changes in diagnostic criteria during the study years—particularly the increasing emphasis on listing "comorbid conditions" as part of the diagnosis rather than considering the additional symptoms to be an expression of the primary disorder—could contribute to observations of increasing comorbidity. However, the increase in comorbidity in later study years somewhat compensates for the underestimation of comorbidity in earlier study years, resulting in a realistic median number of comorbid conditions for the overall study population.
Also, our data are cross-sectional. No directionality can be assumed in associations between conditions, and associations do not suggest cause-and-effect relationships. Finally, a database of hospital discharges may disproportionately capture some schizophrenia subpopulations that may have higher rates of hospitalization—for example, individuals with more severe illnesses, those who receive less outpatient care, and those who lack insurance. Thus we cannot extrapolate our findings to the population of nonhospitalized patients with schizophrenia who receive outpatient care in the community, which is presumably much larger but which comprises patients with less severe symptoms.
Conclusions
Knowledge of the risks of comorbid psychiatric and general medical conditions is critical for both clinicians and patients with schizophrenia. Closer attention to prevention, early diagnosis, and treatment of comorbid conditions may decrease associated morbidity and mortality and improve prognosis among patients with schizophrenia.
Acknowledgments and disclosures
This work was funded by the Stanley Medical Research Institute and the Department of the Army. The authors thank E. Fuller Torrey, M.D., and Robert Yolken, M.D., for careful review of the manuscript. The views expressed are those of the authors and should not be construed to represent the positions of the Department of the Army or Department of Defense.
The authors report no competing interests.
1. Brown S, Inskip H, Barraclough B: Causes of the excess mortality of schizophrenia. British Journal of Psychiatry 177:212–217, 2000Google Scholar
2. Carney CP, Jones L, Woolson RF: Medical comorbidity in women and men with schizophrenia: a population-based controlled study. Journal of General Internal Medicine 21:1133–1137, 2006Google Scholar
3. Goff DC, Cather C, Evins AE, et al: Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. Journal of Clinical Psychiatry 66:183–194, 2005Google Scholar
4. Newman SC, Bland RC: Mortality in a cohort of patients with schizophrenia: a record linkage study. Canadian Journal of Psychiatry 36:239–245, 1991Google Scholar
5. Capasso RM, Lineberry TW, Bostwick JM, et al: Mortality in schizophrenia and schizoaffective disorder: an Olmsted County, Minnesota cohort: 1950–2005. Schizophrenia Research, 2007Google Scholar
6. Leucht S, Burkard T, Henderson J, et al: Physical illness and schizophrenia: a review of the literature. Acta Psychiatrica Scandinavica 116:317–333, 2007Google Scholar
7. Tandon R, Keshavan MS, Nasrallah HA: Schizophrenia, "just the facts": what we know in 2008: part 1. overview. Schizophrenia Research 100:4–19, 2008Google Scholar
8. Heila H, Haukka J, Suvisaari J, et al: Mortality among patients with schizophrenia and reduced psychiatric hospital care. Psychological Medicine 35:725–732, 2005Google Scholar
9. Saha S, Chant D, McGrath J: A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of General Psychiatry 64: 1123–1131, 2007Google Scholar
10. Seeman MV: An outcome measure in schizophrenia: mortality. Canadian Journal of Psychiatry 52:55–60, 2007Google Scholar
11. Green AI, Canuso CM, Brenner MJ, et al: Detection and management of comorbidity in patients with schizophrenia. Psychiatric Clinics of North America 26:115–139, 2003Google Scholar
12. Jones DR, Macias C, Barreira PJ, et al: Prevalence, severity, and co-occurrence of chronic physical health problems of persons with serious mental illness. Psychiatric Services 55:1250–1257, 2004Google Scholar
13. Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, et al: Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. Journal of Clinical Psychiatry 69:514–519, 2008Google Scholar
14. Nasrallah HA, Meyer JM, Goff DC, et al: Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophrenia Research 86:15–22, 2006Google Scholar
15. Kelly C, McCreadie RG: Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. American Journal of Psychiatry 156:1751–1757, 1999Google Scholar
16. Daumit GL, Pratt LA, Crum RM, et al: Characteristics of primary care visits for individuals with severe mental illness in a national sample. General Hospital Psychiatry 24:391–395, 2002Google Scholar
17. Chuang HT, Mansell C, Patten SB: Lifestyle characteristics of psychiatric outpatients. Canadian Journal of Psychiatry 53:260–266, 2008Google Scholar
18. Brown S, Birtwistle J, Roe L, et al: The unhealthy lifestyle of people with schizophrenia. Psychological Medicine 29:697–701, 1999Google Scholar
19. Curkendall SM, Mo J, Glasser DB, et al: Cardiovascular disease in patients with schizophrenia in Saskatchewan, Canada. Journal of Clinical Psychiatry 65:715–720, 2004Google Scholar
20. Newcomer JW, Hennekens CH: Severe mental illness and risk of cardiovascular disease. JAMA 298:1794–1796, 2007Google Scholar
21. Cohen D, Stolk RP, Grobbee DE, et al: Hyperglycemia and diabetes in patients with schizophrenia or schizoaffective disorders. Diabetes Care 29:786–791, 2006Google Scholar
22. Garakani A, Win T, Virk S, et al: Comorbidity of irritable bowel syndrome in psychiatric patients: a review. American Journal of Therapeutics 10:61–67, 2003Google Scholar
23. Horwath E, Cournos F, McKinnon K, et al: Illicit-drug injection among psychiatric patients without a primary substance use disorder. Psychiatric Services 47:181–185, 1996Google Scholar
24. Coverdale JH, Turbott SH, Roberts H: Family planning needs and STD risk behaviours of female psychiatric out-patients. British Journal of Psychiatry 171:69–72, 1997Google Scholar
25. Raja M, Azzoni A: Sexual behavior and sexual problems among patients with severe chronic psychoses. European Psychiatry 18:70–76, 2003Google Scholar
26. Osher FC, Goldberg RW, McNary SW, et al: Substance abuse and the transmission of hepatitis C among persons with severe mental illness. Psychiatric Services 54:842–847, 2003Google Scholar
27. Rosenberg SD, Goodman LA, Osher FC, et al: Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. American Journal of Public Health 91:31–37, 2001Google Scholar
28. Martens WH: A review of physical and mental health in homeless persons. Public Health Reviews 29:13–33, 2001Google Scholar
29. Teesson M, Hodder T, Buhrich N: Psychiatric disorders in homeless men and women in inner Sydney. Australian and New Zealand Journal of Psychiatry 38:162–168, 2004Google Scholar
30. Torchalla I, Albrecht F, Buchkremer G, et al: Homeless women with psychiatric disorders: a field study [in German]. Psychiatrische Praxis 31:228–235, 2004Google Scholar
31. Newman SJ: The housing and neighborhood conditions of persons with severe mental illness. Hospital and Community Psychiatry 45:338–343, 1994Google Scholar
32. Roberts L, Roalfe A, Wilson S, et al: Physical health care of patients with schizophrenia in primary care: a comparative study. Family Practice 24:34–40, 2007Google Scholar
33. Citrome L, Blonde L, Damatarca C: Metabolic issues in patients with severe mental illness. Southern Medical Journal 98:714–720, 2005Google Scholar
34. Henderson DC, Nguyen DD, Copeland PM, et al: Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. Journal of Clinical Psychiatry 66:1116–1121, 2005Google Scholar
35. Peet M: The metabolic syndrome, omega-3 fatty acids and inflammatory processes in relation to schizophrenia. Prostaglandins, Leukotrienes, and Essential Fatty Acids 75:323–327, 2006Google Scholar
36. Mukherjee S, Schnur DB, Reddy R: Family history of type 2 diabetes in schizophrenic patients. Lancet 1(8636):495, 1989Google Scholar
37. Gough SC, O'Donovan MC: Clustering of metabolic comorbidity in schizophrenia: a genetic contribution? Journal of Psychopharmacology 19:47–55, 2005Google Scholar
38. Suvisaari J, Perala J, Saarni SI, et al: Type 2 diabetes among persons with schizophrenia and other psychotic disorders in a general population survey. European Archives of Psychiatry and Clinical Neuroscience 258:129–136, 2007Google Scholar
39. Tandon R, Keshavan MS, Nasrallah HA: Schizophrenia, "just the facts": what we know in 2008: 2. epidemiology and etiology. Schizophrenia Research 102:1–18, 2008Google Scholar
40. Aleman A, Kahn RS, Selten JP: Sex differences in the risk of schizophrenia: evidence from meta-analysis. Archives of General Psychiatry 60:565–571, 2003Google Scholar
41. Bresnahan M, Begg MD, Brown A, et al: Race and risk of schizophrenia in a US birth cohort: another example of health disparity? International Journal of Epidemiology 36:751–758, 2007Google Scholar
42. Cooper C, Morgan C, Byrne M, et al: Perceptions of disadvantage, ethnicity and psychosis. British Journal of Psychiatry 192:185–190, 2008Google Scholar
43. Torrey EF, Bowler A: Geographical distribution of insanity in America: evidence for an urban factor. Schizophrenia Bulletin 16:591–604, 1990Google Scholar
44. Pedersen CB, Mortensen PB: Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Archives of General Psychiatry 58:1039–1046, 2001Google Scholar
45. Andreasen NC, Flaum M: Schizophrenia: the characteristic symptoms. Schizophrenia Bulletin 17:27–49, 1991Google Scholar
46. Flaum M, Andreasen NC: Diagnostic criteria for schizophrenia and related disorders: options for DSM-IV. Schizophrenia Bulletin 17:133–156, 1991Google Scholar
47. Andreasen NC, Carpenter WT Jr: Diagnosis and classification of schizophrenia. Schizophrenia Bulletin 19:199–214, 1993Google Scholar
48. Fenton WS, McGlashan TH, Heinssen RK: A comparison of DSM-III and DSM-III-R schizophrenia. American Journal of Psychiatry 145:1446–1449, 1988Google Scholar
49. Modestin J, Huber A, Satirli E, et al: Long-term course of schizophrenic illness: Bleuler's study reconsidered. American Journal of Psychiatry 160:2202–2208, 2003Google Scholar
50. Averill PM, Reas DL, Shack A, et al: Is schizoaffective disorder a stable diagnostic category? A retrospective examination. Psychiatric Quarterly 75:215–227, 2004Google Scholar
51. Lapierre YD: Schizophrenia and manic-depression: separate illnesses or a continuum? Canadian Journal of Psychiatry 39(suppl 2): S59–S64, 1994Google Scholar
52. Chen YR, Swann AC, Johnson BA: Stability of diagnosis in bipolar disorder. Journal of Nervous and Mental Disease 186:17–23, 1998Google Scholar
53. Baigent M, Holme G, Hafner RJ: Self reports of the interaction between substance abuse and schizophrenia. Australian and New Zealand Journal of Psychiatry 29:69–74, 1995Google Scholar
54. Drake RE, Osher FC, Wallach MA: Alcohol use and abuse in schizophrenia: a prospective community study. Journal of Nervous and Mental Disease 177:408–414, 1989Google Scholar
55. Mueser KT, Bellack AS, Blanchard JJ: Comorbidity of schizophrenia and substance abuse: implications for treatment. Journal of Consulting and Clinical Psychology 60:845–856, 1992Google Scholar
56. Regier DA, Farmer ME, Rae DS, et al: Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) study. JAMA 264:2511–2518, 1990Google Scholar
57. Swartz MS, Wagner HR, Swanson JW, et al: Substance use and psychosocial functioning in schizophrenia among new enrollees in the NIMH CATIE study. Psychiatric Services 57:1110–1116, 2006Google Scholar
58. Jeste DV, Gladsjo JA, Lindamer LA, et al: Medical comorbidity in schizophrenia. Schizophrenia Bulletin 22:413–430, 1996Google Scholar
59. Mauri MC, Bravin S, Fabiano L, et al: Depressive symptoms and schizophrenia: a psychopharmacological approach. L'Encéphale 21:555–558, 1995Google Scholar
60. Siris SG: Suicide and schizophrenia. Journal of Psychopharmacology 15:127–135, 2001Google Scholar
61. Kelly DL, Conley RR: Thyroid function in treatment-resistant schizophrenia patients treated with quetiapine, risperidone, or fluphenazine. Journal of Clinical Psychiatry 66:80–84, 2005Google Scholar
62. Mendhekar DN, Duggal HS: Clozapine-induced tardive dyskinesia and hypothyroidism. Journal of Neuropsychiatry and Clinical Neurosciences 18:245–246, 2006Google Scholar
63. Taskapan C, Sahin I, Taskapan H, et al: Possible malignant neuroleptic syndrome associated with hypothyroidism. Progress in Neuro-Psychopharmacology and Biological Psychiatry 29:745–748, 2005Google Scholar
64. Makikyro T, Karvonen JT, Hakko H, et al: Comorbidity of hospital-treated psychiatric and physical disorders with special reference to schizophrenia: a 28 year follow-up of the 1966 northern Finland general population birth cohort. Public Health 112:221–228, 1998Google Scholar
65. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care 27:596–601, 2004Google Scholar
66. Birkenaes AB, Birkeland KI, Engh JA, et al: Dyslipidemia independent of body mass in antipsychotic-treated patients under real-life conditions. Journal of Clinical Psychopharmacology 28:132–137, 2008Google Scholar
67. Jennex A, Gardner DM: Monitoring and management of metabolic risk factors in outpatients taking antipsychotic drugs: a controlled study. Canadian Journal of Psychiatry 53:34–42, 2008Google Scholar
68. Copeland LA, Mortensen EM, Zeber JE, et al: Pulmonary disease among inpatient decedents: impact of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry 31:720–726, 2007Google Scholar
69. Gaitatzis A, Trimble MR, Sander JW: The psychiatric comorbidity of epilepsy. Acta Neurologica Scandinavica 110:207–220, 2004Google Scholar
70. Fogl T, Margolese HC: Fungal dermatitis with olanzapine in schizophrenia. Canadian Journal of Psychiatry 48:643–644, 2003Google Scholar
71. Rashid J, Wang R, Ramer SL: Atypical antipsychotics and seborrheic dermatitis: three case reports. Pharmacopsychiatry 40:103–106, 2007Google Scholar



