Medicaid Prescription Drug Policies and Medication Access and Continuity: Findings From Ten States
Medicaid provides the largest source of funding for treatment of mental illness in the United States, with $26.4 billion in expenditures in 2003 ( 1 , 2 ). Medicaid is also a major purchaser of prescription drugs ( 3 , 4 ), with antipsychotics, anticonvulsants, and antidepressants accounting for three of the top five therapeutic classes for total Medicaid pharmacy payments ( 4 ). Consequently, Medicaid programs are increasingly utilizing prescription drug prior authorization and other utilization management strategies to contain Medicaid costs ( 5 ). Because many states are facing significant budget deficits, states will likely continue to seek ways to contain prescription drug utilization.
In general medicine, research has suggested that requiring prior authorization policies for prescription drugs and use of other utilization management policies for select therapeutic medication classes can result in significant cost savings on prescription drugs, with little evidence of unintended utilization or cost increases in other health care sectors ( 6 , 7 , 8 ). In psychiatry there has been scant research on these policies. One review indicated that some prescription drug utilization management strategies that Medicaid uses to constrain access to essential drug classes, including psychopharmacologic medications, can reduce appropriate care, adversely affect patient health, and increase costs of care ( 9 ). A recent study of Maine's Medicaid prior authorization and step therapy policy for second-generation antipsychotics indicated that this policy was associated with a 29% greater risk of treatment discontinuity but no associated cost savings for patients with schizophrenia ( 10 ).
We previously reported findings from a national study of psychiatric patients dually eligible for Medicare and Medicaid. We examined cases in which these beneficiaries switched from state Medicaid programs to Medicare Part D prescription drug plans and found that medication access problems affected 53.4% of patients. Most of the medication access problems studied, including switching clinically stable patients' medications and discontinuing medication because of issues concerning prescription drug coverage or utilization management, were associated with increased adverse events, including emergency visits ( 11 ).
Considerable research has examined the effects of cost sharing with patients, including copayments for prescription drugs. A recent review ( 12 ) concluded that increased cost sharing among patients is associated with reduced rates of drug treatment and adherence and increased medication discontinuations. For some chronic conditions, including schizophrenia, higher cost sharing was associated with increased utilization of medical services ( 13 ). Prescription drug cost sharing in poor and elderly populations has been shown to be associated with reduced use of essential drugs and higher rates of serious adverse events and emergency visits ( 14 ).
This study examined the clinical impact of commonly used prescription drug utilization management policies in ten state Medicaid programs of policy interest. Primary aims of this study included comparing physician-reported rates of psychopharmacologic medication access and continuity problems, assessing whether significant adverse clinical events are associated with medication access problems, and identifying whether specific prescription drug policies or management features are associated with medication access problems and adverse events.
Methods
Five hundred psychiatrists were randomly sampled from the American Medical Association's Physician Masterfile in ten states: California, Florida, Georgia, Massachusetts, Michigan, New York, Ohio, Pennsylvania, Tennessee (only 366 psychiatrists available), and Texas, for a total of 4,866 psychiatrists. Psychiatry residents, those with undeliverable addresses, and those without direct patient care as their practice were excluded (N=584). Each psychiatrist was randomly assigned one of 21 start days and times during their last typical work week to report on their next two Medicaid patients. Responses were obtained from 62% of the sample (N=2,671); 32% (N=857) met the study eligibility criterion of having treated Medicaid-only patients in their last typical work week, thus resulting in clinically detailed data for 1,625 Medicaid patients.
Data were collected by mail from September to December 2006 with practice-based survey research methods. Data were collected on patient characteristics, prescription drug utilization management practices, medication access problems, and adverse events experienced since January 1, 2006. The number of observations used in the analyses was 1,625, but all estimates were weighted on the basis of clinicians' Medicaid caseloads and the total number of psychiatrists in each state treating Medicaid patients, reflecting a weighted sample of over 43,000 Medicaid patients. Patients had a mean of 10.0 months (95% confidence interval [CI]=9.9–10.0) in which to experience medication access problems or adverse events since January 1, 2006. Participating psychiatrists received $75 as an incentive to respond and thus increase response rates. All study procedures were approved by the institutional review board of the American Psychiatric Institute for Research and Education (APIRE).
Rates of medication access problems and prescription drug utilization management features were examined across the patient subgroups and the ten states. Predictive probabilities of experiencing medication access problems and adverse events were calculated for each state. We adjusted for differences in patient case mix (including patients' age, gender, race, treatment setting, psychiatric diagnosis, and severity of psychotic, depressive, anxiety, alcohol or other substance use, and manic symptoms as well as sleep disturbances to control for patient sociodemographic and clinical confounders. Rates and odds of experiencing adverse events (adjusted for patient case mix) were examined among patients experiencing specific medication access problems and prescription drug utilization management features. In assessing the likelihood that adverse events were associated with the utilization management features, we also included two covariates that were measures of polypharmacy (prescription of three or more medications and coprescription of two or more antipsychotics) because patients with a polypharmacy regimen may have been more likely to have adverse events and to be covered by utilization management policies that applied to their medications. For the state comparisons, Spearman rank-order correlation coefficients were used to examine the relationship between the number of prescription drug utilization management features and number of medication access problems and between the number of medication access problems and number of adverse events.
Results
Patient characteristics
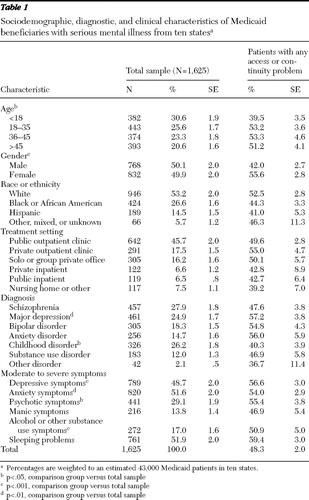
Approximately half the patients were age 35 or under ( Table 1 ). Approximately half were white. Half were female, and nearly half (45.7%) of the patients were treated in public outpatient clinics. The most common diagnoses were schizophrenia (27.9%), childhood disorders (26.2%), and major depression (24.9%).
 |
Medication access problems
Overall, 48.3% of the patients were reported to have experienced at least one medication access problem ( Table 2 ). Rates of access problems varied significantly (p<.001) across the states, with a 37.6% absolute difference between the lowest (New York, 27.1%) and highest (Michigan, 64.7%) rates. A similar pattern of significant differences between states was noted when rates of medication access problems were adjusted for demographic and clinical characteristics of patients (p<.001). The states with the lowest rates of reported medication access problems were New York (27.1%), Texas (31.0%), and California (32.4%), whereas Tennessee (63.3%), Georgia (64.2%), and Michigan (64.7%) had the highest rates. The most common types of medication access problems were as follows: patients were unable to access clinically indicated medication refills or new prescriptions because they were not covered or approved by Medicaid (34.0% of patients overall), clinicians would have preferred to use clinically indicated medications but could not prescribe them because of prescription drug coverage or approval issues or because patients could not make copayments (29.4%), medications were discontinued or temporarily stopped because of prescription drug coverage or administrative or management issues or a problem with patient copayments (25.8%), a medication not clinically preferred was prescribed because another clinically indicated and preferred medication was not covered or approved (25.0%), and problems accessing medications because of copayments were experienced (13.7%).
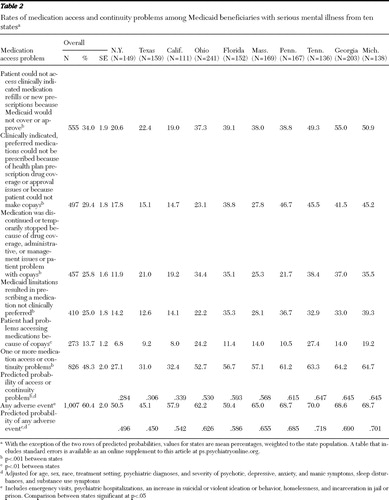 |
Patients in New York, Texas, and California had the lowest rates of problems accessing clinically indicated medication refills or new prescriptions because they were not covered or approved (19.0%–22.4%), whereas patients in Tennessee, Georgia, and Michigan had the highest rates of problems (49.3%–55.0%). Patients in New York, Texas, and California also had the lowest reported rates of problems with accessing medications because of patient copayments (6.8%–9.2%), whereas patients in Ohio and Tennessee had the highest (24.2% and 27.4%, respectively). Patients in New York had the lowest reported rates of discontinuing or temporarily stopping medications because of drug coverage, administrative or management issues, or copayment problems (11.9%), whereas patients in Ohio, Florida, Tennessee, Georgia, and Michigan had significantly higher rates of these problems (34.4%–38.4%).
For 29.4% of patients, the physician listed a specific, clinically indicated medication that he or she would have preferred to use but could not because of health plan prescription drug coverage, approval issues, or issues with patient copayments ( Table 2 ). The medications that most commonly could not be prescribed included second-generation antipsychotics (including clozapine, risperidone, olanzapine, quetiapine, ziprasidone, and aripiprazole; 24.8%±2.6%), sedatives (including eszopiclone, zolpidem tartrate, zaleplon, and ramelteon; 21.5%±3.8%), selective serotonin reuptake inhibitor-type antidepressants (15.3%±2.5%), other types of antidepressants (13.5%±1.8%), and stimulants (6.9%±1.4%). Among the patients currently prescribed sedatives, 59.7%±8.1% had moderate to severe sleep problems, 58.2%±8.1% had moderate to severe anxiety symptoms, and 55.5%±8.2% had moderate to severe depressive symptoms.
For 26.7%±1.7% of patients, the physicians reported initiating prescription drug exceptions and appeals processes, whereas for 20.3%±1.7% of patients, the physicians reported changing or discontinuing medications rather than pursuing prescription drug exceptions and appeals processes. The mean number of medication access problems was 1.3±.06 per patient, with patients prescribed 2.1±.05 medications. Patients who were female or age 18 or older were more likely to have had medication access problems ( Table 1 ). Although patients with major depressive disorder, more severe depressive symptoms, or sleep problems were more likely to have medication access problems, rates of access problems were high across all diagnostic groups.
Adverse events
All five medication access problems studied were strongly associated with increased odds of reported adverse events ( Table 3 ). Adjusting for patient case mix, we found that patients with a reported medication access or continuity problem had 3.6 times greater likelihood of a reported significant adverse event (p<.001), including an emergency visit, psychiatric hospitalization, increase in suicidal or violent ideation or behavior, homelessness, or incarceration in prison or detention in jail. Overall, 72.2% of patients with medication access problems were reported to have experienced an adverse event, compared with 49.4% for patients with no reported access problems. Adjusting for patient case mix, we also found that patients with problems accessing medications because of copayments had 7.8 times greater odds of experiencing an adverse event (p<.001) and that patients who discontinued or temporarily stopped their medications as a result of prescription drug coverage or management issues had 4.4 times greater odds of experiencing an adverse event (p<.001).
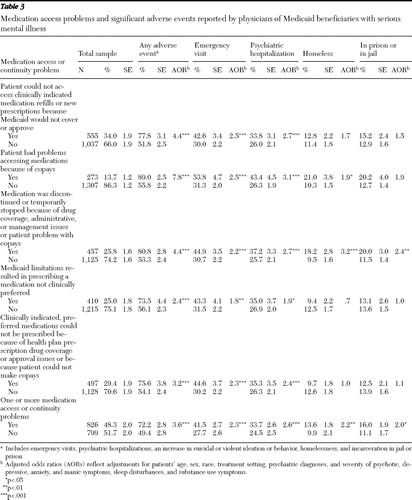 |
With patient case mix adjusted, all five of the access problems studied were found to be associated with increased odds of emergency visits and psychiatric hospitalizations. Patients who were reported to have discontinued or temporarily stopped taking their medications because of prescription drug coverage, utilization management, or copayment issues also had 3.2 times greater odds of being homeless (p<.001). Patients reported to have discontinued or temporarily stopped their medications had more than twice the odds of being incarcerated in prison or detained in jail (p<.01).
Prescription drug policies and access and adverse events
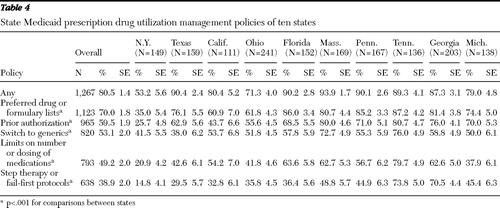
Use of preferred drug or formulary lists was the most commonly reported prescription drug utilization management feature (70.0%; state range 35.0%–87.2%), followed by prior authorization (59.5%; state range 25.7%–80.7%), requirements to switch to generics (53.1%; state range 38.0%–76.0%), limits on the number or dosing of medications (49.2%; state range 20.9%–79.7%), and use of step therapy or fail-first protocols (38.9%; state range 14.8%–73.8%) ( Table 4 ). Overall, patients in New York, Texas, and California generally had significantly lower rates of having these management features apply to their medications compared with patients residing in the other states.
 |
With adjustments for patient case mix, all five prescription drug utilization management features studied were highly associated with significantly increased adjusted odds of medication access problems (p<.001) ( Table 5 ). Overall, among patients reported to have a utilization management policy apply to their prescription drugs, 56.7% had a medication access or continuity problem; among patients without prescription drug utilization management, 13.6% had a medication access problem. Prior authorization was associated with 7.8 times higher adjusted odds of experiencing a medication access problem (p<.001). Use of preferred drug or formulary lists was associated with 5.4 times higher adjusted odds (p<.001). Step therapy and fail-first protocols were associated with 4.7 times greater odds of a medication access or continuity problem (p<.001). Adjusting for patient case mix and all the utilization management features studied, we found that patients required to have prior authorization had 4.4 times greater odds (AOR=4.4, CI=4.4–2.7) of a reported medication access problem, whereas patients with step therapy had 1.6 times greater odds (AOR=1.6, CI=1.1–2.4).
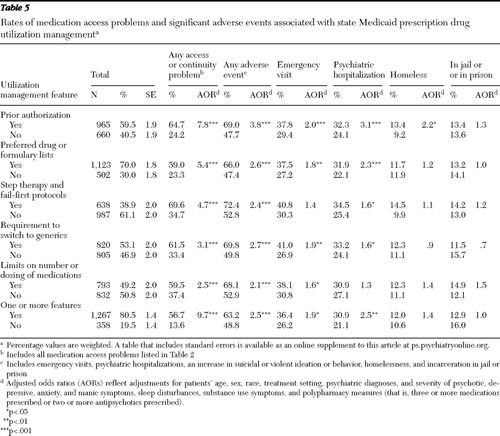 |
With adjustment for patient case mix, all the prescription drug utilization management policies studied were associated with increased odds of adverse events. Rates of adverse events ranged from 66.0% to 72.4% among patients with prescription drug utilization management and from 47.4% to 52.9% among patients without ( Table 5 ). Four of the five prescription drug utilization management features studied were associated with increased emergency visits and psychiatric hospitalizations. Patients with prior authorization had 2.2 times greater likelihood of being reported homeless (p<.05). Adjusting for patient case mix and all the utilization management features studied, we found that patients subject to prior authorization had 3.8 times the odds (AOR=2.3, CI=1.5–3.6) of experiencing a significant adverse event, and those with requirements to switch to generics had 2.7 times greater odds (AOR=1.7, CI=1.1–2.4).
Patients in states with more reported medication access problems had significantly higher rates of adverse events (p<.001). With adjustment for case mix, patients in the seven states with the highest rates of medication access problems had 2.3 times greater odds of experiencing an adverse event (AOR=2.3, CI=1.6–3.5) than patients in the three states with the lowest rates. Spearman rank-order correlations indicated that the number of prescription drug management features was correlated with the number of access problems in states (r=.70, p=.025), which was correlated with the number of adverse events observed in the states (r=.79, p=.006).
Discussion
Strengths and limitations
This study provided clinically detailed data on the experiences of a large, ten-state sample of psychiatric patients receiving Medicaid benefits. The primary limitation is this study's exclusive reliance on physician-reported, cross-sectional, observational data, which have potential response and recall biases. However, it is important to note that for many of the primary medication access problems of interest (such as clinicians' inability to prescribe a clinically indicated and preferred medication because of drug coverage or management issues), physicians would likely be the best source for this type of information. The physicians were compensated to help increase the response rates; however, physicians whose patients were experiencing medication access problems may have been more likely to respond to our survey or to deviate from the systematic patient sampling protocol.
The physicians may have lacked accurate information on their states' utilization management methods and policies or misattributed medication access problems to these policies, which are complex and can vary across and within medication classes. Because respondents were asked about plan features in general (that is, not specific to particular medication types or classes), the responses are best interpreted as general perceptions by clinicians of policies affecting their patients. Clinicians may be more inclined to report prescription drug policies when they encounter them or a medication access problem.
Although some data on state Medicaid prescription drug management policies are publicly available, the sources of information we identified were limited and not readily comparable across the states or specific to psychopharmacologic medications ( 5 , 15 , 16 ). State prescription drug policies also vary between managed and fee-for-service plans within states; this variance was not assessed in this study. Pharmacies may also vary in implementation of prescription drug copayment or other policies. With a particular policy, such as prior authorization, utilization management practices may vary widely in application among patients. The ability to capture these complexities through provider or key informant reports and other sources is limited given the complexity of these arrangements and variations in policy implementation.
This observational study did not capture data on the timing of medication access problems and adverse events and allowed examination of only the associations between Medicaid policies, medication access problems, and adverse events (as reported by the physicians), thus limiting the ability to make causal inferences. Patients with more severe illness, who may require more complex medication regimens, may be more likely to experience medication access problems, to be subject to prescription drug utilization management policies, and to experience adverse events. The logistic regression analyses did, however, adjust for available patient-related covariates. In addition, the logistic regression analyses of the association between adverse events and utilization management features adjusted for two polypharmacy measures because patients with a polypharmacy regimen may be more likely to face adverse events and to have utilization management apply to their medications. Finally, although we presented p values as large as p<.05 to convey general patterns of associations, if a Bonferroni correction was used to adjust for the multiple tests (66 in total) in Tables 3 and 5 , which provide results from the primary study analyses, only those findings with a p value <.00078 would be considered statistically significant.
Key findings and policy implications
Approximately half the Medicaid patients were reported by their physician to have experienced at least one medication access or continuity problem, with one-quarter discontinuing or temporarily stopping their medications because of drug coverage, prescription drug utilization management, or copayment issues. Reported rates of medication access problems varied substantially across states, ranging from 27.1% to 64.7% of sampled patients. Clinician-reported adverse events, which were strongly associated with medication access problems, also varied significantly across states, from 45.1% to 70.0%.
States with the highest rates of prescription drug utilization management had significantly higher medication access problems. With adjustment for patient case mix, Medicaid patients in states with the highest rates of medication access problems had 2.3 times greater likelihood of experiencing adverse events compared with patients in states with the lowest rates of access problems. Overall, the prescription drug policies in New York, Texas, and California warrant careful consideration, because they appeared to have a favorable impact on medication access and continuity and on adverse events. Patients in these states generally had significantly lower rates of having the prescription drug utilization management features we studied (including step therapy and fail-first protocols, limits on the number or dosing of medications, generic requirements, prior authorization, and preferred drug or formulary lists) apply to their care, compared with patients in the other states. Other state policies and factors may also be important. For example, better communication through interagency state information systems, more effective policies for waiving copayments, and prompter responses to prior authorization or appeals and exemption requests may also play a role in the lower observed rates of medication access problems and adverse events in these states.
Our study showed a strong, consistent pattern in which all the clinician-reported prescription drug utilization management policies and all the medication access problems studied were highly associated with significant adverse events. Patients reported to have a medication access problem were 3.6 times more likely than patients without access problems to experience an adverse event. Although rates of access problems in this study were generally lower than in our previous study of psychiatric patients who were dually eligible for Medicaid and Medicare Part D ( 11 ), the patterns of associations were highly similar. The transition of patients with dual eligibility to Medicare Part D caused significant problems for states, which may have contributed to access problems observed in this study.
Although prior research has identified significant opportunities for management strategies to improve quality and continuity of psychopharmacologic treatment ( 16 , 17 , 18 , 19 ), this study indicated that current Medicaid prescription drug management features as reported by physicians were not associated with enhanced continuity of medication. Patients reported to be subject to prescription drug utilization management policies had 9.7 times greater odds of having a medication access problem. Medicaid prescription drug utilization management features—such as prior authorization, preferred drug lists, step therapy, and limits on the number and dosing of medications—raise concerns about their effect on quality and continuity of care, given their strong associations with medication access problems, discontinuations, and adverse events. Our data are consistent with prior research indicating that prescription drug utilization management strategies may have significant cost implications; medication access problems have been associated with greater health care services utilization and costs ( 12 , 13 , 20 ), as well as costs to the social services sector (for example, unemployment and workers' disability and compensation associated with impaired functional status) and criminal justice sector. Given significant research highlighting the challenges of medication adherence in this seriously ill and vulnerable population ( 21 , 22 , 23 ) and the deleterious clinical and other consequences of discontinuing or switching psychopharmacologic medications ( 22 , 24 , 25 , 26 , 27 ), these findings raise important concerns, particularly given the clinical challenges of restabilizing patients who relapse.
The high rates at which patients were reported not to be able to access clinically indicated, preferred medications because of drug coverage or management issues (affecting 29.4% of patients) or to get medication refills or new prescriptions because they were not covered or approved (34.0%) are of particular concern. It is noteworthy that for 25% of patients, clinicians reported prescribing a medication not clinically preferred because drug coverage or management issues prevented them from doing so and that 20.3% of physicians reported changing or discontinuing medications rather than pursuing exceptions or appeals, possibly because of administrative burdens ( 28 ).
Prescription drug utilization management strategies that are based primarily on cost ( 29 ) rather than on clinical considerations, such as patients' symptomatology, comorbidities, and prior treatment history and response, as well as the therapeutic risks and benefits of different medications, can result in suboptimal care and pose serious risks to patients ( 9 , 10 , 30 ). Psychopharmacologic medications within a class (such as antidepressants, antipsychotic medications, anticonvulsants, or antianxiety medications) are not directly interchangeable. Psychiatric patients generally have differential responses and tolerance or side effect reactions to different medications within a class. Patients with psychiatric illnesses often do not respond to their initial medication but do respond to subsequent trials with a different medication within the same or different class ( 30 ). Although the impact of prescription drug utilization management varies depending on the drug and available alternatives, access to a full range of medications facilitates clinical management for this population. Especially worrisome are policies that could lead to medication discontinuations or treatment gaps among stabilized patients, which affected one-quarter of the patents in this study. Fail-first policies requiring documentation within current or past claims databases of failing to improve with a preferred medication can also have major unintended consequences. Such policies may require repeating a medication trial in which a patient previously did poorly or switching a clinically stable patient's medication, which may result in patient relapse or other adverse consequences.
Treatment protocols for evidence-based prescription drug utilization management, such as the Texas Medication Algorithm Project ( 31 ), offer potential to improve outcomes of care. Prescription drug utilization management strategies should be based on evidence-based, patient-centered, clinically appropriate care management strategies and rendered in "real time" (without delay) to minimize disruptions in continuity of medication. Continued investments in comparative clinical effectiveness studies and publicly available databases of results of clinical trials are needed to inform evidence-based treatment protocols ( 32 , 33 , 34 , 35 ). More effective information systems are also needed to better utilize existing Medicaid administrative data to systematically evaluate medication patterns and outcomes and correlate findings with prescription drug utilization management policies ( 36 ).
The association between requirements to switch to generics and increased adverse events raises concerns. This study did not, however, distinguish between requirements to switch to the same versus different generic compounds. If generic medications are true bioequivalents to branded products, one would not expect differential treatment responses. In a seriously ill, cognitively impaired population, switching to generics may create confusion among patients ( 9 ), which may be associated with dosing or administration changes and result in medication discontinuations. Further investigation is needed to understand this dynamic.
Our findings that psychiatric patients with Medicaid coverage that places limits on the number or dosing of medications had higher rates of adverse events are consistent with prior research ( 11 , 12 , 13 , 14 , 20 ). Although dosing limits may provide some clinical safety protections for patients, there may be clinical risks to psychiatric patients, particularly for those prescribed second-generation antipsychotics, given that the upper dosing limits have not been well established ( 30 ).
The finding that one in seven patients was reported to have problems accessing medications because of copayments is troubling, particularly because this consumer issue frequently does not come to clinicians' attention ( 37 ). States should strengthen policies and pharmacy practices to waive copayments for Medicaid patients for whom copayments provide a financial barrier to clinically needed medications, particularly for beneficiaries with severe mental illness who are at risk of decompensation and adverse events.
States should consider other best practices to improve the management and continuity of prescription drugs for this population. The use of medication support and adherence strategies shown to be effective in assertive community treatment models ( 23 , 38 , 39 ) should be explored. This includes ordering and delivering medications to patients, providing education about medications, and monitoring medication compliance and side effects. Disease management strategies ( 40 ) and more effective use of technology, such as pill boxes with paging systems, have also been suggested to enhance medication continuity ( 41 ).
Conclusions
Medications are among the first-line, evidence-based treatments for most mental illnesses ( 30 ). Although prescription drugs are an increasingly costly component of state Medicaid budgets, current state prescription drug utilization management strategies are associated with significant adverse clinical consequences for this population. Medication disruptions or switches that are not clinically indicated have been shown in this and other studies to be associated with significant adverse effects for psychiatric patients. It is therefore of concern that reported rates of these problems varied widely across the states we studied, even after we adjusted for patient case mix. These patterns of associations suggest that state prescription drug policies may have a major impact on outcomes for beneficiaries with mental illness and highlight the need for more effective prescription drug management strategies and policies to promote medication continuity and more cost-effective treatment. Clinical and fiscal accountability and transparency are critical in pharmacy benefit management, especially with the limited evidence base for current utilization management strategies. Further data development and sharing are vital in establishing an evidence base to inform these formulary management approaches. Medicaid prescription drug utilization management policies based primarily on cost rather than on clinical considerations may ultimately result in significant human, economic, and social costs.
Acknowledgments and disclosures
This study was funded by grants from the American Psychiatric Foundation (APF), the National Institute of Mental Health (NIMH), and the Agency for Healthcare Research and Quality under subcontract U18HS016097 with Rutgers University's Center for Education and Research on Mental Health Therapeutics. Although a consortium of industry supporters, including AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, Forest, Janssen, Pfizer, and Wyeth, provided financial support to APF for this research, APIRE had complete discretion and control over the design and conduct of this study and the analyses of the resulting database. Input or comments on the manuscript were neither solicited nor obtained from benefactors before the manuscript was submitted for consideration.
The authors report no competing interests.
1. Mark TL, Coffey RM, McKusick DR, et al: National Estimates of Expenditures for Mental Health Services and Substance Abuse Treatment, 1991–2001. Pub no SMA 05-3999. Rockville, Md, Substance Abuse and Mental Health Services Administration, 2005Google Scholar
2. Mark TL, Levit K, Buck J, et al: Mental health treatment expenditure trends, 1986–2003. Psychiatric Services 58:1041–1048, 2007Google Scholar
3. Kaiser Commission on Medicaid and the Uninsured: Medicaid: a Primer. Washington, DC, Kaiser Family Foundation, March 2007. Available at www.kff.org/Medicaid Google Scholar
4. Catlin A, Cowan C, Heffler S, et al: National health spending in 2005: the slowdown continues. Health Affairs 26:142–153, 2007Google Scholar
5. Crowley JS, Ashner D, Elam L: State Medicaid Outpatient Prescription Drug Policies: Findings From a National Survey, 2005 Update. Washington, DC, Kaiser Family Foundation, Kaiser Commission on Medicaid and the Uninsured, Oct 2005. Available at www.kff.org/medicaid/7381.cfm Google Scholar
6. Fischer MA, Schneeweiss S, Avorn J, et al: Medicaid prior-authorization programs and the use of cyclooxygenase-2 inhibitors. New England Journal of Medicine 351:2187–2194, 2004Google Scholar
7. Smalley WE, Griffin MR, Fought RL, et al: Effect of a prior-authorization requirement on the use of nonsteroidal antiinflammatory drugs by Medicaid patients. New England Journal of Medicine 332:1612–1617, 1995Google Scholar
8. Motheral BR, Henderson R, Cox ER: Plan-sponsor savings and member experience with point-of-service prescription step therapy. American Journal of Managed Care 10(7 pt 1):457–464, 2004Google Scholar
9. Soumerai SB: Benefits and risks of increasing restrictions on access to costly drugs in Medicaid. Health Affairs 23:135–146, 2004Google Scholar
10. Soumerai SB, Zhang F, Ross-Degan D, et al: Use of atypical antipsychotic drugs for schizophrenia in Maine Medicaid following a policy change. Health Affairs 27:w185–w195, 2008Google Scholar
11. West JC, Wilk JE, Muszynski IL, et al: Medication access and continuity: the experiences of dual-eligible psychiatric patients during the first 4 months of the Medicare prescription drug benefit. American Journal of Psychiatry 164:789–796, 2007Google Scholar
12. Goldman DP, Joyce GF, Zheng Y: Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA 298:61–69, 2007Google Scholar
13. Soumerai SB, McLaughlin TJ, Ross-Degnan D, et al: Effects of a limit on Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. New England Journal of Medicine 331:650–655, 1994Google Scholar
14. Tamblyn R, Laprise R, Hanley JA, et al: Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA 285:421–429, 2001Google Scholar
15. Koyanagi C, Forquer S, Alfano E: Medicaid policies to contain psychiatric drug costs. Health Affairs 24:536–544, 2005Google Scholar
16. Polinski JM, Wang PS, Fischer MA: Medicaid's prior authorization program and access to atypical antipsychotic medications. Health Affairs 26:750–760, 2007Google Scholar
17. West JC, Wilk J, Olfson M, et al: Patterns and quality of treatment for patients with schizophrenia in routine psychiatric practice. Psychiatric Services 56:283–291, 2005Google Scholar
18. West JC, Duffy FF, Wilk J, et al: Patterns and quality of treatment for patients with major depressive disorder in routine psychiatric practice. Focus 3:43–50, 2005Google Scholar
19. Wang PS, Simon GE, Avorn J, et al: Telephone screening, outreach, and care management for depressed workers and impact on clinical and work productivity outcomes: a randomized controlled trial. JAMA 298: 1401–1411, 2007Google Scholar
20. Cunningham PJ: Medicaid cost containment and access to prescription drugs. Health Affairs 24:780–789, 2005Google Scholar
21. Lieberman JA, Stroup TS, McEvoy JP, et al: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 353:1209–1223, 2005Google Scholar
22. Svarstad BL, Shireman TI, Sweeney JK: Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatric Services 52: 805–811, 2001Google Scholar
23. Zygmunt A, Olfson M, Boyer CA, et al: Interventions to improve medication adherence in schizophrenia. American Journal of Psychiatry 159:1653–1664, 2002Google Scholar
24. Robinson D, Woerner MG, Alvir JM, et al: Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Archives of General Psychiatry 56:241–247, 1999Google Scholar
25. Valenstein M, Copeland LA, Blow FC, et al: Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Medical Care 40:630–639, 2002Google Scholar
26. Gilmer TP, Dolder CR, Lacro JP, et al: Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. American Journal of Psychiatry 161:692–699, 2004Google Scholar
27. Essock SM, Covell NH, Davis SM, et al: Effectiveness of switching antipsychotic medications. American Journal of Psychiatry 163:2090–2095, 2006Google Scholar
28. Wilk JE, West JC, Rae DS, et al: Medicare Part D prescription drug benefits and administrative burden in care of dually eligible psychiatric patients. Psychiatric Services 59:34–39, 2008Google Scholar
29. Gencarelli DM: Medicaid prescription drug coverage: state efforts to control costs. National Health Policy Forum Issue Brief 790:1–17, 2003Google Scholar
30. American Psychiatric Association Practice Guidelines for the Treatment of Psychiatric Disorders Compendium. Arlington, Va, American Psychiatric Association, 2006Google Scholar
31. Kashner TM, Rush AJ, Crismon ML, et al: An empirical analysis of cost outcomes of the Texas Medication Algorithm Project. Psychiatric Services 57:648–659, 2006Google Scholar
32. Sachs GS, Nierenberg AA, Calabrese JR, et al: Effectiveness of adjunctive antidepressant treatment for bipolar depression. New England Journal of Medicine 356:1711–1722, 2007Google Scholar
33. Rush AJ, Trivedi MH, Wisniewski SR, et al: Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal of Psychiatry 163:1905–1917, 2006Google Scholar
34. March JS, Silva SG, Compton S, et al: The case for practical clinical trials in psychiatry. American Journal of Psychiatry 162:836–846, 2005Google Scholar
35. Swartz MS, Perkins DO, Stroup TS, et al: Assessing clinical and functional outcomes in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial. Schizophrenia Bulletin 29:33–43, 2003Google Scholar
36. Crystal S, Akincigil A, Bilder S, et al: Studying prescription drug use and outcomes with Medicaid claims data: strengths, limitations, and strategies. Medical Care 45:S58–S65, 2007Google Scholar
37. Piette, JD, Heisler M: Problems due to medication costs among VA and non-VA patients with chronic illnesses. American Journal of Managed Care 10:861–868, 2004Google Scholar
38. Phillips SD, Burns BJ, Edgar ER, et al: Moving assertive community treatment into standard practice. Psychiatric Services 52:771–779, 2001Google Scholar
39. Coldwell CM, Bender WS: The effectiveness of assertive community treatment for homeless populations with severe mental illness: a meta-analysis. American Journal of Psychiatry 164:393–399, 2007Google Scholar
40. Foote SM: Population-based disease management under fee-for-service Medicare. Health Affairs Web Exclusives W3:342–356, July 30, 2003Google Scholar
41. Osterberg L, Blaschke T: Adherence to medication. New England Journal of Medicine 353:487–497, 2005Google Scholar



