Outcomes of Obese, Clozapine-Treated Inpatients With Schizophrenia Placed on a Six-Month Diet and Physical Activity Program
An enhanced appetite with a consequent gain in weight is common among patients with schizophrenia who are being treated with antipsychotic drugs. Other obesity-related conditions among these patients are diabetes mellitus, hypertension, and cardiovascular disease, all of which may be seriously detrimental to good health ( 1 ).
Clozapine is a dibenzodiazepine derivative and a second-generation antipsychotic drug for treatment of patients with schizophrenia ( 2 ). Among the second-generation antipsychotics, clozapine appears to have the greatest potential to induce weight gain. For example, Allison and colleagues ( 3 ) found that clozapine and olanzapine led to a mean weight gain of 4.45 kg and 4.15 kg, respectively, after ten weeks. Lamberti and coauthors ( 4 ) reported a mean weight gain of 7.7 kg for a group of 36 patients receiving a mean dosage of 380 mg of clozapine a day for six months. A retrospective study of 82 patients who received 500 to 600 mg of clozapine a day for up to 90 months found that about 50% of them became substantially overweight ( 5 ). Weight gain induced by antipsychotics is a common cause of noncompliance, resulting in discontinuation of antipsychotic treatment and return of psychotic symptoms ( 6 ).
However, weight gain and metabolic changes, such as higher levels of glucose, insulin, triglyceride, and cholesterol and reduced levels of insulin-like growth factor 1 (IGF-1), have been reported to be more significant among patients on long-term clozapine therapy than among those being treated with other first- and second-generation antipsychotics ( 7 , 8 , 9 ). Therefore, it is important to manage weight gain among these patients, especially for those on long-term treatment with clozapine without alternative medication.
Growth hormone and IGF-1 are both powerful regulatory agents for maintaining the effective functioning of the cardiovascular system ( 10 , 11 ). IGF-1 appears to improve cardiac function because low levels of this growth factor are associated with an increased risk of heart disease ( 11 , 12 ). The insulin-like growth factor-binding protein-3 (IGFBP-3) is the most abundant carrying protein and carries most of the circulating IGFs (90% in adult serum) ( 13 , 14 ). In circulation only a minor fraction of IGF-1 occurs in the free, unbound form. The free form of IGF-1 has greater physiological and clinical relevance than total IGF-1, and it has anabolic, endocrine, and autocrine actions ( 13 , 14 ). IGFBP-3 is a key carrying protein for regulating the distribution and bioavailability of IGF-1 to target tissues ( 14 ). The molar ratio of IGF-1 and IGFBP-3 (IGF-1/IGFBP-3) was considered to be a marker of free IGF-1 ( 14 ). However, the role of growth hormone, IGF-1, and IGFBP-3 among patients with schizophrenia receiving long-term clozapine therapy has not yet been investigated.
Weight control has been reported to be useful for reducing health risks among overweight or obese patients with schizophrenia ( 15 , 16 ). In one study all patients taking second-generation antipsychotics except those taking clozapine were able to lose weight ( 17 ). In a randomized study, patients with schizophrenia were first referred to a wellness clinic, where a rigorous evaluation of exercise and dietary habits was conducted, and they were then placed on a weight management program, which led to weight reduction among outpatients taking olanzapine ( 18 ). It is known that weight management by way of caloric restriction and regular physical activity can prevent morbidity among obese individuals. However, results of studies of weight management programs for clozapine-treated patients with schizophrenia appear to contradict each other. Wirshing and colleagues ( 17 ) reported unsuccessful weight loss in a six-year study of 20 male outpatients with schizophrenia taking clozapine. The therapeutic strategies used in that study were habit evolution, education, exercise classes, and group support. In contrast, Heimberg and associates ( 19 ) reported positive results using dietary restriction (1,400 to 2,500 kcal a day) with ten inpatients for six months, although no information was provided about metabolic and hormonal changes.
The randomized, controlled study reported here was undertaken to investigate the effect of six months of continuous dietary control and regular physical activity on obese patients with schizophrenia who were taking clozapine. The study assessed anthropometric and biochemical parameters (serum glucose, triglyceride, cholesterol, insulin, cortisol, prolactin, growth hormone, IGF-1, and IGFBP- 3) at three and six months.
Methods
Participants
The sample consisted of participants selected from 753 hospitalized patients from September 2003 to February 2004. All patients in the sample had a DSM-IV diagnosis of schizophrenia and were 18 to 65 years old. Inclusion criteria consisted of taking at least 300 mg of clozapine orally per day for at least a year and having a body mass index (BMI) greater than 27 kg/m 2 . Asian individuals with a BMI greater than 27 kg/m 2 are considered to be obese ( 20 , 21 ). Patients were excluded if they were taking any antipsychotic (not clozapine) or lipid-lowering medications, had any abnormal ambulatory functions or organ failure, had severe mental illness or mental retardation, were in an acute phase of mental illness, were pregnant or lactating, had a disability that prevented walking, or were not interested in the program. The 56 patients selected by using the inclusion and exclusion criteria were randomly divided between the intervention (study) group and the control group. The study was performed in accordance with the Declaration of Helsinki and was approved by the Yu-Li Veterans Hospital's Ethics Review Committee. All patients were completely informed about the study and provided written consent before participating.
Intervention
Dietary control was implemented by a registered dietitian, who ensured that caloric intake was restricted to 1,300 to 1,500 kcal per day for women and to 1,600 to 1,800 kcal per day for men. The minimum respective dietary requirements were 1,200 kcal per day for women and 1,500 kcal per day for men ( 22 ). We measured caloric intake and assessed the types of foods that the patients ate, including fruit and vegetables (up to 7.5 servings per day), sugar-free versions of foods and drinks, and artificial sweeteners. This intake of macronutrients ensured that participants were compliant with the expected changes of 20%, 25%, and 55% in energy from protein, fat, and carbohydrate, respectively ( 22 ). The current average macronutrient composition of the Taiwanese diet is 10% to 14% of calories from protein, 20% to 30% from fat, and 58% to 68% from carbohydrate.
The 1996 U.S. Surgeon General's Report ( 23 ) recommended that persons of all ages obtain "a minimum of 30 minutes of physical activity of moderate intensity (e.g., brisk walking) on most, if not all, days of the week." The physical activities in our study were to be sustained for six months and performed three days per week. The program, designed to fit the hospital environment in which the patients would be exercising, consisted of level walking for 1.62 km for about 40 minutes and walking up 231 stairs (14 cm per stair) and down 330 stairs (13.5 cm per stair) for about 20 minutes under supervision. The walking speed and distances were kept constant during the six-month intervention, except for warm-up during the first week, and we encouraged participants to complete it in about 60 minutes but not to force themselves. The patients consequently expended energy at an approximate rate of 600 to 750 kcal per week, which was estimated by using the formula in the guidelines of the American College of Sports Medicine ( 24 ). To motivate participants, different rewards were offered, such as toilet paper, soap, and sugar-free drinks.
Anthropometric measurements
Anthropometric and body parameters were assessed after participants had fasted overnight. Body weight and body fat percentage were measured with a body composition analyzer by using bioelectrical impedance analysis ( 25 ). Height was measured using a calibrated stadiometer. Waist and hip circumference were measured in centimeters in a standing position after gentle expiration by using calibrated plastic tapes midway between the lowest rib and the iliac crest and at the greater trochanters, respectively. BMI was calculated as weight divided by height squared (kg/m 2 ). The waist-to-hip ratio was calculated as waist circumference divided by hip circumference.
Blood sampling
Overnight fasting blood samples were drawn from patients between 7 and 9 a.m. by a trained phlebotomist who used a venipuncture of an antecubital vein. Part of each blood sample was immediately used for the metabolic analysis and enzyme assay, and the other part of the sample was centrifuged at 3,000 rpm for 15 minutes at 4° C within one hour of drawing and subsequently frozen at -80° C until an enzyme-linked immunosorbent assay (ELISA) analysis could be performed.
Metabolic analysis and enzyme immunoassay
The freshly drawn blood was immediately used to measure serum glucose, triglyceride, cholesterol, insulin, prolactin, and cortisol. Serum glucose, triglyceride, and cholesterol were assessed by using an autoanalyzer that measures glucose oxidase, triglyceride enzyme, and cholesterol oxidase, respectively ( 26 ). Serum cortisol, prolactin, and insulin were measured with an enzyme immunoassay system ( 27 ).
ELISA measurements of growth hormone, IGF-1, and IGFBP-3 levels were determined by using commercially available ELISA kits ( 28 ) with a VersaMax Tunable Microplate Reader ( 29 ). These assays were noncompetitive and involved the horseradish peroxidase-labeled detection antibody as previously described ( 30 ).
Statistical analysis
The effectiveness of the treatment was assessed by using variance and covariance analysis (ANCOVA) with SPSS statistical software (version 10.0) and was based on a general linear model. The anthropometric, metabolic, and hormonal data obtained for the study group were compared with those obtained for the control group at the beginning of the study and again after three and six months. The data collected at the beginning of the assessment period (baseline) were used as the covariate. The comparison was done by using a repeated-measures ANCOVA adjusted for all baseline values. A two-way mixed-design ANCOVA was used to correct for potentially confounding variables and to test for correlation between variables. In all cases, a p value of .05 was considered to be statistically significant.
Results
Fifty-three patients completed the study; three withdrew from the control group because they were discharged from the hospital in the second month of the study. The 53 inpatients, all of whom were clozapine-treated obese patients with schizophrenia, were randomly assigned to one of the two groups. Twenty-five were assigned to the control group, which included 11 men (44%) and 14 women (56%) with a mean±SD age of 39.0±6.7 years. Twenty-eight were assigned to the study group, which included 11 men (39%) and 17 women (61%) with a mean age of 42.2±7.5 years. No significant differences were found between the study group and the control group in gender distribution or mean age.
Anthropometric measures
BMI, body weight, waist and hip circumference, waist-to-hip ratio, and fat percentage of body weight did not differ significantly between the study group and the control group at baseline ( Table 1 ). In the control and study groups at baseline, the mean body fat percentages among men (30.9%±4.8% in the study group and 30.0%±5.1% in the control group) and among women (43.6%±10.7% and 41.4%±5.7%, respectively) were similar. At baseline in both the study and the control groups the body fat percentage of men was significantly lower (p<.001) than that of the women but free fat mass among men was significantly higher (p<.001).
 |
As shown in Table 1 , body fat percentages at three and six months were not significantly lower within the groups nor was there a difference between the control and study groups ( Table 1 ). When data for men and women were analyzed separately, we did not observe significant changes in fat percentage or in free fat mass after three months or even after six months. However, BMI, body weight, and waist and hip circumference measures in the study group decreased significantly ( Table 1 ). BMI, body weight, and waist circumference decreased significantly (p<.05) after both three months and six months for the study group compared with the control group, whereas hip circumference decreased only after six months of the intervention. We observed a significant decrease from baseline values (p<.05) in BMI, body weight, and hip circumference in the study group even after three months, whereas waist circumference was significantly reduced only after six months ( Table 1 ). Furthermore, BMI, body weight, and waist-to-hip ratio values were significantly reduced (p<.05) from the third to the sixth month of the intervention.
Metabolic analysis and enzyme immunoassay
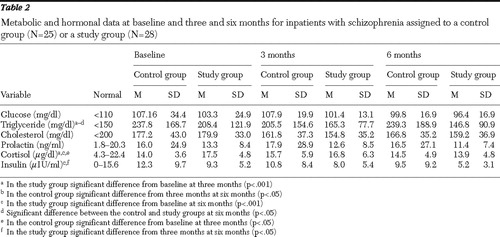
As Table 2 shows, no significant difference was found at baseline between the study and control groups in serum glucose, triglyceride, cholesterol, prolactin, cortisol, and insulin levels. During the intervention, serum glucose, cholesterol, prolactin, cortisol, and insulin did not significantly differ between the control and study groups ( Table 2 ). At six months the triglyceride level of the study group was significantly lower than that of the control group (p<.05). No significant differences between the control and study groups were found in glucose, cholesterol, prolactin, cortisol, and insulin levels after three months and six months of the intervention ( Table 2 ). In the study group we observed a significant decrease (p<.05) from baseline levels for triglyceride and cortisol concentration at three and six months, whereas the insulin level was significantly lower (p<.05) only after six months (compared with the three-month level). At the same time, the triglyceride level of the control group significantly increased (p<.05) from the third to the sixth months.
 |
Intervention and growth hormone-IGF-1 axis
At baseline there were no significant differences between the control and the study groups in growth hormone, IGF-1, IGFBP-3, and the molar ratio of IGF-1 and IGFBP-3. As Table 3 shows, no significant changes were observed for either group in the levels of growth hormone and IGF-1 after three months or even after six months. Similarly, no significant changes in IGFBP-3 level after three months were observed, although the IGFBP-3 level of the study group was significantly lower (p<.05) than that of the control group after six months ( Table 3 ). IGFBP-3 concentration in the study group was significantly lower (p<.05) than the baseline level at three months. The control group, on the other hand, had a significantly higher level (p<.05) of this growth factor at six months compared with baseline. After six months, the molar ratio of IGF-1 to IGFBP-3 of the study group was not only significantly higher than that of the control group ( Table 3 ), but it had also increased significantly (p<.05) at three months compared with baseline.
 |
Discussion
This study demonstrates the benefits of a six-month intervention consisting of integrated dietary control and regular physical activity for obese patients with schizophrenia being treated with clozapine. Our intervention resulted in significant decreases in BMI, body fat percentage, and waist and hip circumference. In addition, participating patients showed improved metabolic profiles of triglyceride, insulin, IGFBP-3 levels, and the IGF-1 to IGFBP-3 molar ratio. In contrast, the control group showed no improvement in anthropometric measurements and no amelioration in triglyceride and insulin levels and had a lower molar ratio of IGF-1 to IGFBP-3.
Dietary guidelines suggest that weight loss at the rate of .5–1.0 kg per week (diet reduction of 500–1,000 kilocalories per day) occurs safely for up to six months. Our participants' diets contained approximately 200 to 300 fewer kilocalories per day than they normally consumed, and they were expected to expend approximately 600 to 750 kcal per week more energy by increasing their physical activity. These levels were chosen in an effort to minimize any possible adverse effects from diet alone, which we observed in the form of mental and emotional instability among inpatients who consumed much fewer calories. The particular physical activities we selected were suitable for obese patients with schizophrenia on clozapine treatment, because they are mild and uncomplicated and hold no danger for these patients. Walking does not require expensive equipment or a designated athletic facility, and it can be done alone or with another person with minimal or no instruction. Patients with severe mental illness are more likely to walk as their sole form of physical activity ( 31 ).
All 28 patients in the study group completed the six-month diet control intervention and at least 90% of the physical activity program. The level of compliance and consequently our success rate may have been lower if participants had been outpatients rather than inpatients. However, the high success rate made it easier to interpret our results at the end of the program. We are, therefore, able to assure patients who rigorously follow the program that they can reap many health benefits. Also, within the hospital context, key health care professionals were on hand to ensure proper implementation of our weight-management intervention.
The prevention and treatment of weight gain in psychiatric patients is difficult but not impossible. Even a modest 5% to 10% reduction in body weight has significant health benefits. Our results show that for obese patients with schizophrenia who are taking clozapine, the intervention resulted in a significant reduction in BMI (5.4%), body weight (5.4%), waist circumference (3.3 cm), and hip circumference (3.3 cm), suggesting that the program had successfully reduced BMI and improved various measures by the end of the six-month intervention period. Some of these parameters were reduced after three months but others only after six months of intervention. All participants' anthropometry (including body weight, BMI, and waist and hip circumference), physical activity, and dietary behavior were monitored regularly throughout the study by investigators and ward staff. The psychiatric condition of none of the participants in the control group appeared to have worsened.
In this and previous studies, clozapine-treated patients with schizophrenia have been reported to gain weight and to have increased BMI and fat deposits ( 4 , 5 , 32 ). These patients often exhibit a marked increase in central adiposity ( 32 , 33 ), although the waist-to-hip ratio is a better predictor of cardiovascular disease and death than the actual amount of adiposity ( 20 , 34 ). In our current study, the average waist-to-hip ratio of clozapine-treated patients with schizophrenia was .88 for women and .97 for men. Japanese criteria for central obesity are .8 for women and .9 for men. Therefore, the women and the men in our study could be said to have central obesity. However, on the basis of World Health Organization criteria of .85 for women and 1.0 for men, only our female patients would have been classified as exhibiting central obesity ( 20 ). An abrupt increase in the prevalence of coronary artery disease was found among Indian patients who had a fat percentage of more than 25 ( 35 ). Among our study participants, the average body fat percentage was as high as 37.4%. Consequently, obese patients with schizophrenia being treated with clozapine would seem to have a higher risk of developing cardiovascular diseases, especially women. Of course, additional studies are required to address possible cardiovascular abnormalities among these patients.
Our weight management program consisting of dietary control (a reduction of 200 to 300 kcal a day) and physical activity (an expenditure of 600 to 750 kcal a week) was successful in reducing clozapine-related weight gain among patients with schizophrenia. Only two studies involving weight management programs for clozapine-treated patients with schizophrenia have been published ( 17 , 19 ). Heimberg and colleagues ( 19 ) observed that of ten inpatients taking clozapine who were prescribed a diet of 1,400 to 2,500 kcal a day, the men lost an average of 7.1 kg, but the women gained less than .5 kg after six months. In contrast, Wirshing and associates ( 17 ) observed that 20 outpatients taking clozapine gained weight even after therapeutic strategies were used that included feedback about their weight, a more rigorous diet and exercise evaluation, education, and an exercise class with group support for six years.
The benefits of reduced levels of insulin were found in the study group. Among healthy obese persons who do not have schizophrenia or glucose intolerance, blood glucose concentrations do not usually decrease after exercise. In addition, it has been found that an improvement in glucose or insulin concentration achieved as a consequence of weight loss may gradually be negated when weight is regained ( 36 ). Our results show that participants in the study group had significantly lower concentrations of insulin as a consequence of our intervention, a result similar to those of previous studies of persons with no psychiatric illness ( 37 , 38 , 39 ). Keeping in mind that obese patients with elevated insulin and triglyceride levels face a considerably enhanced risk of cardiovascular morbidity and mortality ( 40 ), it is clear that our program of dietary control and regular physical activity lowered insulin levels and may help to lower cardiovascular risk among obese patients with schizophrenia who are taking clozapine.
IGFBPs are synthesized by the kidney and may modulate the local autocrine or paracrine actions of IGF-1 ( 14 ). IGFBP-3 is the most abundant IGF-binding protein in human serum and is now believed to be a critical element in numerous cellular processes and a key factor in several disease states via IGF-dependent or IGF-independent mechanisms ( 41 ). In our study, we detected lower levels of IGFBP-3 and consequently a higher IGF-1 to IGFBP-3 molar ratio after six months of the intervention. These results are in agreement with those of previous studies in which exercise was also found to reduce IGFBP-3 levels ( 42 , 43 ). These findings imply that "active IGF-1" appears to be enhanced by a long-term (longer than six months) intervention involving dietary control and physical activity. The effect of reduced IGFBP-3 levels on the availability of IGF-1 is still unclear and further studies are needed. In our study, growth hormone and IGF-1 levels were not found to be altered by the intervention. However, growth hormone and IGF-1 levels have been reported to be elevated by vigorous exercise among persons with no mental illness ( 44 ), and it may be that the exercise intensity (fast walking) we used in our study was insufficient to induce changes in growth hormone and IGF-1 concentrations.
There are some difficulties and weaknesses in the study to address. The motivation for weight reduction (dietary restriction and physical activity) is very low for psychiatric patients if these patients are not under institutional supervision. In addition, suppression of appetite for long-term continuance among psychiatric patients was difficult, so the dietary reduction was controlled to only 200–300 kcal per day in the study. Therefore, manpower and work-site support such as dietitians and physical therapists were very important for long-term monitoring of risk factors and for continuously encouraging the patient to continue with weight reduction.
Conclusions
We found that an intervention involving dietary control and physical activity for six months significantly decreased the body weight, BMI, waist and hip circumference, triglyceride, insulin, and IGFBP-3 levels of obese inpatients with schizophrenia who were being treated with clozapine. In addition, we observed a significantly increased IGF-1 to IGFBP-3 molar ratio among these patients. Dietary control and physical activity seemed to normalize some metabolic abnormalities, minimize hormonal changes, and attenuate some neuroleptic-related side effects, such as sedation and reduced daily activity.
Clozapine appears to present great risk for weight gain. However, previous reports have shown that nearly 50% of patients with schizophrenia have comorbid medical conditions, and many of these illnesses are misdiagnosed or undiagnosed ( 45 ). We propose that it is crucially important to monitor the health of obese patients with schizophrenia who are being treated with clozapine. Because some other metabolic benefits of the diet and the exercise program were not realized until six months of intervention, long-term adherence to such a program is necessary. We further propose that lifestyle modification (continuous dietary control and routine physical activity) be prescribed for these patients so that they can avoid obesity-related abnormalities and enjoy long-term benefits.
Acknowledgments and disclosures
The study was supported by grant VHYL-92-10 from Yu Li Veterans Hospital and grant CMU95-262 from China Medical University, Taiwan.
The authors report no competing interests.
1. Aronne LJ: Epidemiology, morbidity, and treatment of overweight and obesity. Journal of Clinical Psychiatry 62(suppl 23):13–22, 2001Google Scholar
2. Gaszner P, Makkos Z: Clozapine maintenance therapy in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry 28:465–469, 2004Google Scholar
3. Allison DB, Mentore JL, Heo M, et al: Antipsychotic-induced weight gain: a comprehensive research synthesis. American Journal of Psychiatry 156:1686–1696, 1999Google Scholar
4. Lamberti JS, Bellnier T, Schwarzkopf SB: Weight gain among schizophrenic patients treated with clozapine. American Journal of Psychiatry 149:689–690, 1992Google Scholar
5. Umbricht DS, Pollack S, Kane JM: Clozapine and weight gain. Journal of Clinical Psychiatry 55(suppl B):157–160, 1994Google Scholar
6. Silverstone T, Smith G, Goodall E: Prevalence of obesity in patients receiving depot antipsychotics. British Journal of Psychiatry 153:214–217, 1988Google Scholar
7. Kelly DL, Kreyenbuhl J, Love RC, et al: Six-month review of weight and metabolic parameters in patients receiving clozapine, risperidone, olanzapine, or quetiapine. Journal of Clinical Psychiatry 64:1133–1134, 2003Google Scholar
8. Baymiller SP, Ball P, McMahon RP, et al: Serum glucose and lipid changes during the course of clozapine treatment: the effect of concurrent beta-adrenergic antagonist treatment. Schizophrenia Research 59:49–57, 2002Google Scholar
9. Melkersson KI, Hulting AL, Brismar KE: Different influences of classical antipsychotics and clozapine on glucose-insulin homeostasis in patients with schizophrenia or related psychoses. Journal of Clinical Psychiatry 60:783–791, 1999Google Scholar
10. Opgaard OS, Wang PH: IGF-I is a matter of heart. Growth Hormone and IGF Research 15:89–94, 2005Google Scholar
11. Ren J, Samson WK, Sowers JR: Insulin-like growth factor I as a cardiac hormone: physiological and pathophysiological implications in heart disease. Journal of Molecular and Cellular Cardiology 31:2049–2061, 1999Google Scholar
12. Bleumink GS, Rietveld I, Janssen JA, et al: Insulin-like growth factor-I gene polymorphism and risk of heart failure (the Rotterdam Study). American Journal of Cardiology 94:384–386, 2004Google Scholar
13. Binoux M: The IGF system in metabolism regulation. Diabetes and Metabolism 21:330–337, 1995Google Scholar
14. Kelly KM, Oh Y, Gargosky SE, et al: Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. International Journal of Biochemistry and Cell Biology 28:619–637, 1996Google Scholar
15. Menza M, Vreeland B, Minsky S, et al: Managing atypical antipsychotic-associated weight gain: 12-month data on a multimodal weight control program. Journal of Clinical Psychiatry 65:471–477, 2004Google Scholar
16. Vreeland B, Minsky S, Menza M, et al: A program for managing weight gain associated with atypical antipsychotics. Psychiatric Services 54:1155–1157, 2003Google Scholar
17. Wirshing DA, Wirshing WC, Kysar L, et al: Novel antipsychotics: comparison of weight gain liabilities. Journal of Clinical Psychiatry 60:358–363, 1999Google Scholar
18. Soo Kwon J, Choi JS, Bahk WM, et al: Weight management program for treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: a 12-week randomized controlled clinical trial. Journal of Clinical Psychiatry 67:547–553, 2006Google Scholar
19. Heimberg C, Gallacher F, Gur RC, et al: Diet and gender moderate clozapine-related weight gain. Human Psychopharmacology: Clinical and Experimental 10:367–371, 1995Google Scholar
20. Obesity: Preventing and Managing the Global Epidemic. WHO technical report series no 894. Geneva, World Health Organization. 2000Google Scholar
21. WHO Expert Consultation: Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363:157–163, 2004Google Scholar
22. St Jeor ST, Howard BV, Prewitt TE, et al: Dietary protein and weight reduction: a statement for healthcare professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation 104:1869–1874, 2001Google Scholar
23. Physical Activity and Health: A Report of the Surgeon General. Washington, DC, US Department of Health and Human Services, US Public Health Service, 1996Google Scholar
24. American College of Sports Medicine Guidelines for Exercise Testing and Prescription, 7th ed. Philadelphia, Lippincott Williams & Wilkins, 2006Google Scholar
25. TBF-410 body composition analyzer. Tokyo, Tanita Corp, 2001Google Scholar
26. Dimension RxL autoanalyzer. Newark, NJ, DADE Behring, 2001Google Scholar
27. ACCESS system. Minneapolis, Minn, Beckman, 2000Google Scholar
28. ELISA kit. Webster, Tex, Diagnostic Systems Laboratories, 2004Google Scholar
29. VersaMax Tunable Microplate Reader. Sunnyvale, Calif, Molecular Devices, 1998Google Scholar
30. Khosravi MJ, Papanastasiou-Diamandi A, Mistry J: An ultrasensitive immunoassay for prostate-specific antigen based on conventional colorimetric detection. Clinical Biochemistry 28:407–414, 1995Google Scholar
31. Daumit GL, Goldberg RW, Anthony C, et al: Physical activity patterns in adults with severe mental illness. Journal of Nervous and Mental Disease 193:641–646, 2005Google Scholar
32. Frankenburg FR, Zanarini MC, Kando J, et al: Clozapine and body mass change. Biological Psychiatry 43:520–524, 1998Google Scholar
33. Sharpe JK, Hills AP: Anthropometry and adiposity in a group of people with chronic mental illness. Australian and New Zealand Journal of Psychiatry 32:77–81, 1998Google Scholar
34. Ito H, Nakasuga K, Ohshima A, et al: Detection of cardiovascular risk factors by indices of obesity obtained from anthropometry and dual-energy X-ray absorptiometry in Japanese individuals. International Journal of Obesity and Related Metabolic Disorders 27:232–237, 2003Google Scholar
35. Singh RB, Niaz MA, Beegom R, et al: Body fat percent by bioelectrical impedance analysis and risk of coronary artery disease among urban men with low rates of obesity: the Indian paradox. Journal of the American College of Nutrition 18:268–273, 1999Google Scholar
36. Fogelholm M, Kukkonen-Harjula K, Nenonen A, et al: Effects of walking training on weight maintenance after a very-low-energy diet in premenopausal obese women: a randomized controlled trial. Archives of Internal Medicine 160:2177–2184, 2000Google Scholar
37. Ditschuneit HH, Flechtner-Mors M, Johnson TD, et al: Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. American Journal of Clinical Nutrition 69:198–204, 1999Google Scholar
38. Chadan SG, Dill RP, Vanderhoek K, et al: Influence of physical activity on plasma insulin-like growth factor-1 and insulin-like growth factor binding proteins in healthy older women. Mechanisms of Ageing and Development 109:21–34, 1999Google Scholar
39. Nicklas BJ, Dennis KE, Berman DM, et al: Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African-American and Caucasian women. Journal of Gerontology, Series A 58:181–189, 2003Google Scholar
40. Hecker KD, Kris-Etherton PM, Zhao G, et al: Impact of body weight and weight loss on cardiovascular risk factors. Current Atherosclerosis Reports 1:236–242, 1999Google Scholar
41. Ali O, Cohen P, Lee KW: Epidemiology and biology of insulin-like growth factor binding protein-3 (IGFBP-3) as an anti-cancer molecule. Hormone and Metabolic Research 35:726–733, 2003Google Scholar
42. Filaire E, Jouanel P, Colombier M, et al: Effects of 16 weeks of training prior to a major competition on hormonal and biochemical parameters in young elite gymnasts. Journal of Pediatric Endocrinology and Metabolism 16:741–750, 2003Google Scholar
43. Eliakim A, Scheett TP, Newcomb R, et al: Fitness, training, and the growth hormone-insulin-like growth factor I axis in prepubertal girls. Journal of Clinical Endocrinology and Metabolism 86:2797–2802, 2001Google Scholar
44. Kraemer RR, Durand RJ, Acevedo EO, et al: Rigorous running increases growth hormone and insulin-like growth factor-I without altering ghrelin. Experimental Biology and Medicine 229:240–246, 2004Google Scholar
45. Goldman LS: Medical illness in patients with schizophrenia. Journal of Clinical Psychiatry 60(suppl 21):10–15, 1999Google Scholar



