A Case Series of Nicotine Nasal Spray in the Treatment of Tobacco Dependence Among Patients With Schizophrenia
Abstract
A retrospective case series of 12 smokers with schizophrenia or schizoaffective disorder who had not successfully quit smoking with previous treatments for tobacco dependence were treated with nicotine nasal spray. All but one patient (92 percent) tolerated the nasal spray well, and nine (75 percent) used it at maximal doses for prolonged periods. After treatment five patients (42 percent) were abstinent from smoking for more than 90 days, and four patients (33 percent) substantially reduced the amount that they smoked. Ten patients (83 percent) used the spray in combination with other medications, and all received psychosocial support. Nicotine nasal spray was found to be well tolerated.
Individuals with schizophrenia smoke at alarmingly high rates, are less likely to try to quit smoking, and are only half as successful at quitting as other smokers (1). Previous trials of nicotine replacement for smokers with schizophrenia have used only the nicotine patch, even though the unique neurobiological abnormalities among individuals with schizophrenia suggest that treatments that provide rapid, pulsatile delivery of nicotine, which closely mimics the effects of smoking, may be more effective.
Abnormal electrophysiological (P50) measures among patients with schizophrenia, which are normalized with doses of nicotine, are linked to the alpha-7 nicotinic cholinergic receptor (nAChR). Constant nicotine dosing may result in the desensitization of nicotinic receptors. The alpha-7 nAChR, which is among the most rapidly desensitizing nicotinic receptor, requires high and intermittent doses of nicotine to be most effectively activated (2,3). Strategies of intermittent nicotine delivery remain unstudied among patients with schizophrenia, and the obvious hazards of tobacco smoke point to a need for a safer nicotine delivery device. This research supports an intermittent dose administration strategy, such as can be delivered by nicotine nasal spay, as possibly being more effective for modification of cigarette craving among patients with schizophrenia.
Nicotine nasal spray has unique features, such as a rapid onset of action, intermittent dosing, and more immediate relief from cravings. In clinical trials nasal spray has been found to be particularly effective among heavily dependent smokers (4), most likely because of its rapid nicotine delivery. Smokers with schizophrenia may be more willing to use nicotine nasal spray because of this property.
Case series
We report on a retrospective case series of 12 smokers with schizophrenia or schizoaffective disorder who were treated from July 2001 to October 2003 with nicotine nasal spray in our clinical practice at the tobacco dependence program at the University of Medicine and Dentistry of New Jersey. The institutional review board at the University of Medicine and Dentistry of New Jersey approved the study. As shown in Table 1, the group consisted of six men and six women and had a mean±SD age of 45±7.5 years. The group had smoked for a mean of 25.9±11.1 years. The mean Fagerstrom score for nicotine dependence was 7.8±1.25, which is indicative of moderate to severe dependence. The smokers smoked a mean of 26.7±10.1 cigarettes per day and had a mean expired level of carbon monoxide (CO) of 22.3±8 parts per million when they began treatment with the nasal spray.
All 12 patients had attempted to quit during the past year by using methods that included medications and psychosocial treatment; all had trials of high-dose nicotine replacement, either with combination treatment or a 42 mg patch. Before beginning treatment with the nasal spray, patients were instructed to fill the nasal spray prescription so that they could receive their first dose during an individual counseling appointment. Patients were encouraged to set a quit date and use the spray at least once an hour, up to 40 doses daily (one dose equals one spray per nostril). Patients were given reassurance and education about the product's side effects and were told that they would likely remit with continued use.
All 12 patients received monthly individual psychosocial treatment for tobacco dependence, and eight (67 percent) attended group treatment for smokers with mental illness, although attendance was highly variable. Combination pharmacotherapy was common in this group. The nasal spray was viewed as the primary form of nicotine replacement.
Five patients (42 percent) were abstinent for more than 90 days (mean of 168±118 days). Self-report of abstinence was verified by measuring expired CO levels at every monthly visit. Four of the seven patients who did not quit (57 percent) had substantial reductions in the number of cigarettes that they smoked and in expired CO levels (mean CO level of 21±2.6 parts per million before beginning treatment with nicotine spray and mean CO level of 3.5±1.9 parts per million at the last visit). Three patients (25 percent) continued at the same rate of smoking.
Nine patients (75 percent) used at least 30 doses of nicotine nasal spray per day; three of the five patients who were continuously abstinent (60 percent) still used it at 40 sprays per day, with one 10 mL bottle consumed every three days. Increased use of the spray seemed to be correlated with better outcomes. Three patients (25 percent) preferred to use more nasal spray and reported spraying two to three sprays per nostril, especially in times of stress, without any adverse effect. Spray usage was restricted by prescribing four to five 10 mL bottles every two weeks. Smith and colleagues (5) employed a regimen in a laboratory setting that used multiple sprays per nostril for smokers with schizophrenia to mimic the effects of cigarettes; no adverse reactions were found in that study.
The mean length of time that all 12 patients were treated with nasal spray was 257±245.2 days (range, 14 to 811 days), and three of the five who were abstinent (60 percent) used the spray for months before achieving abstinence. Nine (75 percent) asked to use the spray for long periods, even when they continued to smoke, although all but one had intermittent periods of abstinence. Three (25 percent) were sensitive to brief discontinuations of spray and quickly relapsed back to smoking when the spray was not available. Brief substitution of another form of nicotine medication was not helpful in these cases. It was only when treatment with the spray was reinitiated that these patients were able to return to abstinence.
Only one patient (1 percent) reported discontinuing the nasal spray because of side effects (facial rash), and no patients developed nicotine toxicity, even when the spray was used while smoking or with another nicotine replacement. Although nearly all patients experienced some initial irritating side effects, such as sneezing, cough, and rhinnorhea, none discontinued the spray for these reasons. These patients developed tolerance to these side effects in the first week with repeated use.
All patients were covered by Medicare or Medicaid, which covers nicotine nasal spray in New Jersey. One patient had his nasal spray treatment terminated by a managed Medicaid plan, rather than by clinical need, and two other patients experienced lapses in treatment because of inadequate pharmacy supply, which was related to their high use of the product.
Discussion
It is our clinical experience that smokers with schizophrenia responded well to nicotine nasal spray, with good tolerability, few side effects, and high satisfaction. Patients immediately liked the product and wanted to continue using it, with one remarking that it "felt good in his brain." Many expressed a preference for the spray, having already tried other nicotine medications. The low discontinuation rates that we observed are in contrast to those in the general population (6), and our patients commonly used the spray at the maximum dose and for extended periods. Although it appears that dependence on the nasal spray may develop among smokers with schizophrenia, ongoing use of the spray seems preferable to continued smoking. Nicotine nasal spray has a tendency for longer use and more potential for dependence than other forms of nicotine replacement (7). Increased use of the spray—that is, more doses per day—seemed to be correlated with better outcomes (8). However, the nasal spray was not effective for everyone in this case series: two patients used it for extended periods without achieving clinical gains.
Conclusions
Although all patients were receiving adjunctive psychosocial treatment and nearly all patients were receiving additional pharmacotherapy for tobacco, we observed significant clinical gains when nasal spray was added to the regimen. The role of bupropion SR or clozapine could not be determined in our study, although other studies have found a positive effect (9,10). Adjunctive nicotine replacement usually was with an inhaler and was added when the nasal spray was being used at the maximum dose, which suggests that the total dose of nicotine replacement may be a key factor in the success of these patients. Combination pharmacotherapy that involves the nicotine nasal spray and psychosocial support may be a promising treatment for tobacco dependence among persons with schizophrenia.
Acknowledgments
This work supported in part by grant K-DA-14009-01 from the National Institute on Drug Abuse and by the New Jersey Department of Health and Senior Services through the Comprehensive Tobacco Control Program.
The authors are affiliated with the department of psychiatry at the University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, 671 Hoes Lane, D339, Piscataway, New Jersey 08855 (e-mail, [email protected]). They are also with the tobacco dependence program at the University of Medicine and Dentistry of New Jersey-School of Public Health in New Brunswick.
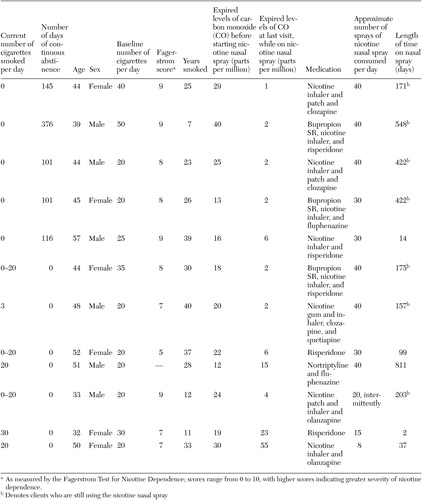 |
Table 1. Characteristics of 12 patients with schizophrenia in a case series of nicotine nasal spray in the treatment of tobacco dependence
1. Ziedonis DM, George TP: Schizophrenia and nicotine use: report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophrenia Bulletin 23:247–254, 1997Crossref, Medline, Google Scholar
2. Griffith JM, O'Neill JE, Petty F, et al: Nicotinic receptor desensitization and sensory gating deficits in schizophrenia. Biological Psychiatry 44:98–106, 1998Crossref, Medline, Google Scholar
3. Freedman R, Adams CE, Leonard S: The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. Journal of Chemical Neuroanatomy 20:299–306, 2000Crossref, Medline, Google Scholar
4. Sutherland G, Stapleton JA, Russell MAH, et al: Randomized controlled trial of nicotine nasal spray in smoking cessation. Lancet 340:324–329, 1992Crossref, Medline, Google Scholar
5. Smith RC, Singh A, Infante M, et al: Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology 27:479–497, 2002Crossref, Medline, Google Scholar
6. Hajek P, West R, Foulds J, et al: Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Archives of Internal Medicine 159:2033–2038, 1999Crossref, Medline, Google Scholar
7. West R, Hajek P, Foulds J, et al: A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray, and inhaler. Psychopharmacology 149:198–202, 2000Crossref, Medline, Google Scholar
8. Schneider MP, van Melle G, Uldry C, et al: Electronic monitoring of long-term use of the nicotine nasal spray and predictors of success in a smoking cessation program. Nicotine and Tobacco Research 5:719–727, 2003Crossref, Medline, Google Scholar
9. Evins AE, Mays VK, Rigotti NA, et al: A pilot trial of bupropion added to cognitive behavioral therapy for smoking cessation in schizophrenia. Nicotine and Tobacco Research 3:397–403, 2001Crossref, Medline, Google Scholar
10. George TP, Sernyak MJ, Ziedonis DM, et al: Effects of clozapine on smoking in chronic schizophrenic outpatients. Journal of Clinical Psychiatry 56:344–346, 1995Medline, Google Scholar



