A Comparison of Type 2 Diabetes Outcomes Among Persons With and Without Severe Mental Illnesses
Abstract
OBJECTIVE: Type 2 diabetes is an important comorbid medical condition associated with schizophrenia. The objective of this study was to compare glycosylated hemoglobin (HbA1c) levels of patients who had type 2 diabetes and schizophrenia with those of patients who had type 2 diabetes and major mood disorders and those who had type 2 diabetes but who did not have severe mental illness. METHODS: A sample of 300 patients with type 2 diabetes was recruited from community mental health centers in the greater Baltimore region and nearby primary care clinics. Of these, 100 had schizophrenia, 101 had a major mood disorder, and 99 had no identified severe mental illness. HbA1c, the main outcome measure, was compared between the group with schizophrenia and the other two groups. RESULTS: All three groups had HbA1c values above recommended levels. HbA1c levels were significantly lower among patients with schizophrenia than among patients who did not have severe mental illness but were not significantly different from those of patients who had major mood disorders. Patients for whom olanzapine was prescribed had higher HbA1c levels than those for whom other antipsychotic agents were prescribed. CONCLUSIONS: All three groups of patients require improved diabetes treatment to achieve acceptable HbA1c levels. There may be previously unrecognized benefits for diabetes management among persons with severe mental illnesses who are receiving regular mental heath care, but these individuals may also have risk factors that can influence diabetes outcomes and HbA1c levels.
Relative to the general population, persons with schizophrenia have significantly elevated rates of comorbid medical conditions (1,2,3) and a higher mortality rate that is only partially accounted for by increased suicide rates (1,2,4,5,6). Such excess morbidity and mortality are likely to be substantially attributable to modifiable patient behaviors, characteristics of the health care delivery system, and schizophrenia treatments. Side effects of psychiatric medications, high rates of obesity and nicotine use, and neglect of self-care secondary to psychiatric symptoms, such as social withdrawal and psychosis, may adversely affect patients' somatic health status (7,8,9).
An additional cause of poor health outcomes among persons with serious mental illness may be underuse of somatic health care services. Studies in specialized populations of persons with serious mental illness indicate that these individuals' use of medical care may be less than that of comparable individuals who do not have a serious mental illness. Among outpatients with chronic medical conditions at Department of Veterans Affairs (VA) centers, patients with comorbid mental disorders were less likely to receive recommended preventive services than were patients without mental disorders (10). In a study of patients who were hospitalized for an acute myocardial infarction, those with a mental disorder were less likely to receive specialized cardiac procedures than were patients without a mental disorder (11). On the other hand, results of a national study indicate that persons who identified themselves as having a mental disorder were as likely to have a primary care provider as were persons who did not have a mental disorder (12). However, those who had a mental disorder were more likely to report that they had been unable to obtain needed care or that they had to delay care because of cost.
Our study of 200 randomly selected outpatients with severe mental illness showed that general health services are widely used by persons with serious mental illness who are receiving outpatient psychiatric care (13). Dental services were underused, and there was a high rate of perceived barriers to receipt of medical care in this population. Thus existing research does not provide compelling and consistent findings that would explain the elevated mortality rate observed among persons with schizophrenia.
In an attempt to identify factors that might explain poor medical status among persons with schizophrenia, we used a "tracer" condition strategy, focusing on a single medical condition—diabetes—as a prototypical serious, chronic medical problem from which lessons may generalize to other disorders. Type 2 diabetes is a highly prevalent chronic medical condition that affects approximately 4 percent of the U.S. general population (14). Persons with schizophrenia may be at particularly high risk of diabetes (15,16), with an estimated prevalence of 16 to 25 percent (17,18,19).
Persons with schizophrenia have several risk factors and behaviors that are likely to be associated with poor diabetes-related health outcomes. The second-generation antipsychotic medications, especially clozapine and olanzapine (20,21,22), may increase the risk of developing diabetes or worsen its course. Four professional associations, including the American Diabetes Association (ADA), recently published a consensus statement on antipsychotic drugs and obesity and diabetes that acknowledges the linkage of second-generation antipsychotic medications to weight gain, diabetes, and dyslipidemia (23). Other possible causes of poor diabetes outcomes among persons with schizophrenia include poor integration of medical and psychiatric care, sedentary lifestyle, obesity, poor diet, a high prevalence of smoking, cognitive impairment (24,25), psychosocial deficits, and limited family and social supports.
Research on diabetes among persons with schizophrenia has focused largely on the incidence and prevalence of diabetes rather than on longer term diabetes outcomes. Our interest in the impact of schizophrenia on diabetes outcomes led us to consider two comparison groups—one group of persons who did not have severe mental illness and another group of individuals with a different severe mental illness, a major mood disorder (either major depression or bipolar disorder), who also were receiving ongoing and intensive mental health care. Although severe mood disorders are associated with deficits that might contribute to poorer (higher) glycosylated hemoglobin (HbA1c) levels, the impairments described for schizophrenia exceed impairments found among persons with major depression or bipolar disorder (26,27). Thus patients with mood disorders provide a reasonable comparison group of persons from the same health care settings as those with schizophrenia, but without the same extent and type of psychiatric morbidity.
Our primary outcome measure was HbA1c, which is a direct biochemical marker of diabetes control, over the preceding two to four months. The ADA recommends an HbA1c value of less than 7 percent among patients with diabetes (28). A 1 percent increase in HbA1c has been associated with a 10 to 20 percent increase in the risk of coronary heart disease and a 10 percent increase in associated mortality (29,30,31). HbA1c levels correlate with advancing age, minority status, obesity, lack of exercise, nonadherence to diabetes medications and diet, use of medications that reduce the production or action of insulin, and stress (32). Clinically significant depression is also associated with poorer glycemic control (33) and increased rates of diabetic complications (34). Because some of the risk factors for elevated HbA1c and poor diabetes management are characteristic of schizophrenia, we hypothesized that persons with schizophrenia would have worse (higher) HbA1c values than the other two groups.
Methods
Study setting and sample
We sought to recruit individuals with diabetes to a sample comprising 100 persons with schizophrenia and two comparison groups of 100 persons who did not have a severe mental illness and 100 who had a major mood disorder. The institutional review boards of the University of Maryland School of Medicine and of each participating facility approved the study. Assessments were conducted between September 1, 1999, and September 30, 2002. Participants met the following inclusion criteria: age of 18 to 65 years, current medical record diagnosis of type 2 diabetes, English speaker, and ability to provide informed consent. In addition, participants with severe mental illnesses had to have a diagnosis of a schizophrenia-spectrum disorder (schizophrenia or schizoaffective disorder) or a major mood disorder (bipolar disorder or major recurrent depression) recorded in their medical chart. Participants who did not have a severe mental illness could not have received treatment for a major psychiatric disorder within the past year, as indicated in their medical record or by their screening interview.
Persons with severe mental illness were recruited from six public and private outpatient mental health clinics in urban and suburban communities across the Baltimore metropolitan area to represent the broad range of individuals receiving schizophrenia treatment. One-quarter of the total sample from each group consisted of veterans recruited from the Baltimore VA Medical Center. To obtain representative groups of patients with the two mental disorders and type 2 diabetes, we attempted to identify every clinic patient who met the study eligibility criteria. Psychiatrists and clinic staff reviewed complete patient rosters to identify participants. Research assistants then received a list of potentially eligible participants. Volunteer recruitment signs were also posted. Research staff approached potential participants. Participants were receiving diabetes care from various primary care providers.
Among patients who had schizophrenia, 22 (18 percent) declined to participate in the study. Ten patients who had a mood disorder (9 percent) also declined to participate. The only difference observed between these two groups was that persons with schizophrenia who declined were significantly older than those who participated (mean±SD age of 62.7±27 compared with 48.1±9.1 years; t=2.50, df=22, p<.05).
Participants who did not have a severe mental illness were recruited from three primary care clinics near the psychiatric clinics and the VA center with a reasonable demographic match to the participants with schizophrenia. We used a frequency matching strategy by selecting a stratum of reference patients who did not have a severe mental illness with matching-factor values equal to our index patients with schizophrenia on age, gender, race, and educational level (35). This goal required a different recruitment protocol from that used in the mental health clinics. On randomly selected recruitment days, all patients with a diagnosis of type 2 diabetes who met inclusion criteria were identified from appointment logs. Primary care providers requested permission from eligible participants to be approached for the study. Research staff verified inclusion criteria for patients who expressed a willingness to participate and provided informed consent. Screening indicated that 19 referred patients were ineligible because of receipt of psychiatric treatment, most commonly antidepressants. Balancing of samples required the selective recruitment of younger individuals. This process was done systematically within the same recruitment protocol described above. Of the patients who did not have a mental illness, 55 (36 percent) refused to participate in the study. No significant differences were found between those who participated and those who declined on race, age, gender, and education.
Assessments
After providing written informed consent, each participant met in person with research staff for a 2.5-hour assessment. Research assistants met weekly throughout the study to ensure standardization of the interview procedures.
HbA1c was the primary outcome measure. Normal HbA1c values range from 4.6 percent to 6 percent. HbA1c was measured by using the Bayer DCA 2000+.
The Summary of Diabetes Self-Care Activities was used to measure the patient's self-reported frequency of completion of prescribed diabetes self-care activities, including diet, exercise, glucose testing, and use of medications in the past week (36).
The Diabetes Knowledge Test was used to assess knowledge about diabetes. The test is appropriate for adults with type 1 or type 2 diabetes. The general test subscale is calculated as the percentage of correct answers out of 14 items (37). Diabetes education, an element of the ADA assessment of quality of diabetes care (38), was also measured. Study participants provided self-reports of receipt of any form of diabetes education—written material, informal information, counseling, or formal classes—during the previous six months. Diabetes services were assessed by participants' self-report of diabetes outpatient visits, inpatient hospitalizations, and emergency department visits over the preceding six months.
Body mass index (BMI) was calculated on the basis of the patient's weight and height at the time of visit. Smoking status and number of cigarettes smoked per day were based on self-report. Alcohol use was based on self-report of frequency of alcohol use in the previous six months. Blood pressure and hypertension was measured by using a portable, digital, self-inflating blood pressure cuff. We classified individuals who had a systolic blood pressure reading greater than or equal to 130 or a diastolic reading greater than or equal to 80 as having current elevated blood pressure (28) and classified those who had current elevated blood pressure or who self-reported a diagnosis of hypertension as having current hypertension.
Use of mental health services was based on self-reported use of services—outpatient visits, day program participation, and inpatient hospitalizations—provided by a psychiatrist or a mental health treatment team over the preceding six months.
Information about psychiatric medications was obtained from the psychiatric record for patients who had a mental illness at the time of interview.
Psychiatric symptoms were measured with the Colorado Symptom Index (CSI), which asks respondents the frequency with which they experience psychiatric symptoms (39). The anxiety-depression subscale was used.
Statistical analysis
We first compared patients who had schizophrenia with the other two groups on demographic characteristics and various diabetes-related and other health-related characteristics and treatments. Because the primary aim of this study was to evaluate whether the patients with schizophrenia differed in HbA1c values from those who did not have a mental illness and those who had a mood disorder, we did not compare patients who did not have a mental illness with those who had a mood disorder.
We initially compared the HbA1c values of patients who had schizophrenia with those of patients who did not have a mental illness and those who had a mood disorder separately by using unadjusted t tests; p values of less than .025, using an a priori alpha level of .05 and a standard Bonferroni correction, were considered statistically significant. We then used multiple linear regression models (analysis of covariance type) to compare HbA1c values of the patients who had schizophrenia with those of the patients in the other two groups while controlling for group differences on potentially confounding factors—for example, demographic characteristics. The schizophrenia group served as the reference category. The models included covariates that we thought were most likely to be related to both mental illness and HbA1c levels on the basis of previous research. Age, gender, race, education, duration of diabetes, BMI, smoking status, current hypertension, diabetes knowledge test score, receipt of diabetes education in the previous six months, number of outpatient visits for diabetes in the previous six months, adherence to diet and exercise regimens, prescription of hypoglycemic medication, and depressive symptoms were included. This full multivariate model was designated as model A. The subset of the study sample for whom hypoglycemic medications were prescribed was included in a separate multivariate model adjusted for adherence to hypoglycemic medications (model B).
To assess the association between selected antipsychotic medications and HbA1c levels, we conducted two analogous multivariate analyses of HbA1c levels that included only patients for whom antipsychotic medications were prescribed. Given the small sample of patients for whom clozapine was prescribed (nine patients) and clozapine's link to disordered glucose regulation, we excluded these nine patients from the analyses. Given that olanzapine was prescribed, by far, to the largest number of patients (63 patients), and given its association with weight gain and metabolic disturbances, we compared patients for whom olanzapine was prescribed with patients for whom antipsychotic medications other than olanzapine were prescribed. For all analyses, we report model-based adjusted mean HbA1c values (least-square means) for each group.
Results
The final study sample consisted of 100 persons with schizophrenia, 101 persons with a major mood disorder, and 99 persons who did not have a mental illness. The patients with schizophrenia and those without a mental illness did not differ on gender, race, or education, although the patients with schizophrenia were somewhat younger than those who did not have a mental illness (mean age difference of 4.7 years) (Table 1).
Table 1 shows the differences between the schizophrenia group and one or both of the comparison groups on mean age at diabetes diagnosis, diabetes knowledge, diabetes education, BMI, smoking status, hypertension, and depression. The patients with schizophrenia had a younger age of diagnosis of diabetes, lower scores on the diabetes knowledge test, lower BMIs, greater rates of smoking, and lower rates of self-reported hypertension than the two other groups. The patients with schizophrenia were less likely than those who did not have a mental illness to receive diabetes education. In addition, the depression scores of the patients with schizophrenia were lower than those of the patients with mood disorders but higher than those of the patients who did not have a mental illness.
The patients with schizophrenia had significantly lower HbA1c values than those who did not have a mental illness, but their HbA1c values did not differ from those of patients with mood disorders. The differences in HbA1c values persisted in both model A (the full model) and model B (Table 2). The overall F values were significant in all models. The unadjusted mean±SE HbA1c values were 7.83± .23 for the schizophrenia group, 7.87±.23 for the mood disorder group, and 8.64±.23 for the group without a mental illness. The adjusted values are shown in Table 2.
In models A and B, Caucasians and those with a shorter duration of diabetes had lower HbA1c values than their respective comparison categories. In model A, patients for whom hypoglycemic medications were prescribed had higher HbA1c values. In model B, patients who had fewer outpatient visits for diabetes and better adherence to hypoglycemic medications had lower HbA1c values. Because use of diabetes-related services could have been influenced by HbA1c levels—our dependent variable—we conducted analyses that assessed use of services separately from other factors. Our results generally did not change.
In the analyses of patients who were receiving antipsychotic medications, both the unadjusted model and model A showed a trend that linked the prescription of olanzapine with elevated HbA1c values. This difference was statistically significant in model B. In both adjusted models, Caucasian patients had lower HbA1c values than their non-Caucasian counterparts. In model A, patients for whom a hypoglycemic medication was prescribed had higher HbA1c values than those for whom these agents were not prescribed.
Discussion and conclusions
All three patient groups we studied had mean HbA1c values that exceeded recommended levels and that would be associated with at least a 10 to 20 percent increase in the risk of coronary heart disease and a 10 percent increase in associated mortality (29,30,31). Long-term, intensified glycemic control can markedly reduce the development of diabetes complications (40,41). Multifaceted interventions are needed that combine patient education, case management, and system-level efforts to better integrate and coordinate diabetes-related services and other medical or psychiatric care for the populations we studied.
Although all three groups of patients had HbA1c values that exceeded recommended levels, the patients with schizophrenia had clinically significantly lower HbA1c values than a frequency-matched comparison group of patients who did not have a severe mental illness. Several possible explanations for this finding have implications for diabetes care. Although the patients with schizophrenia were frequency-matched on demographic characteristics with the group of patients who did not have a mental illness, they were recruited from mental heath centers and received mental health treatments, whereas those who did not have a mental illness were recruited from primary care settings, which suggests two distinct possibilities.
In the two groups of patients with mental illness, mental health services could have contributed to stability and adherence to diabetes treatment, even though the use of diabetes-related services was comparable in all three groups. The finding that HbA1c values did not differ significantly between patients with schizophrenia and patients with mood disorders supports the possible role of mental health services in lower HbA1c values. Another possibility is that the different sampling methods led to the selection of non-mentally ill patients who had more poorly controlled diabetes than the patients in the two other groups. The fact that patients with mental illnesses were recruited from sites where they were seeking mental health treatment and patients without a mental illness were recruited from primary care clinics where they were seeking medical care may have produced a comparison group that was apparently more severely ill.
Although it is difficult to make direct comparisons, a review of published studies of community-based and clinic-based samples of persons with type 2 diabetes suggests that the non-mentally ill sample in this study resembled comparable clinic samples of patients seeking medical treatment for diabetes in HbA1c values and weight. For example, outpatients with type 2 diabetes in a large, urban, public hospital had mean HbA1c values of 9 percent in a clinic staffed by general medicine residents, 8.2 percent in a clinic staffed by general medicine faculty, and 8.8 percent in a specialty diabetes clinic (42). A study of patients who made a first visit to a diabetes clinic in a large, urban public hospital showed a mean HbA1c value of 9.1 percent (43). This finding is consistent with the notion that our sample of patients without a mental illness had higher HbA1c values than those with a mental illness, in part because the former group was recruited from settings where they were seeking diabetes care. However, it is important to emphasize that the comparability of the HbA1c values in our sample of patients who did not have a severe mental illness with published values for other clinic samples suggests that this sample is not necessarily more ill than patients found in other urban primary care settings but, rather, reasonably represents the clinical status of typical patients with diabetes seeking care in the primary care sector.
By contrast, a recent study that used 1999-2000 data from a population-based sample of persons with diabetes in the National Health and Nutrition Examination Survey (NHANES) showed a mean HbA1c value of 7.8 and a mean BMI of 32 (44). HbA1c values and BMIs were similar between the mentally ill patients in our study and this population-based sample. However, the patients in the non-mentally ill group exhibited higher HbA1c values and were more overweight. It might be useful to consider our samples of patients with mental illness as being more similar to a community sample with respect to their diabetes care.
Additional considerations in interpreting these findings are differences in diabetes- and health-related characteristics between the groups. Patients with schizophrenia were less likely to be obese than those without a mental illness, which may have contributed to their better diabetes control (32). On the other hand, the patients with schizophrenia were more likely to smoke and had higher levels of depressive symptoms, and almost all had antipsychotic medications prescribed, which would have impaired their glucose control. Patients with schizophrenia also had less diabetes education and less diabetes knowledge than those without a mental illness. Further research must assess the consistency and significance of these diverse influences on HbA1c levels in these populations. Although space precludes full discussion of these differences here, our study is the first to describe the clinical characteristics and service use of a large cohort of persons with mental illness who have a long-standing diagnosis of diabetes.
Nevertheless, it is important to note that we found no evidence that patients with schizophrenia had worse diabetes outcomes than those without a severe mental illness. These findings underscore the fact that the patients with schizophrenia were not specifically disadvantaged in their glucose control and that these patients had more strengths in medical self-care than has previously been noted. Persons with schizophrenia are frequently regarded as incapable of participating in their medical care, and this assumption was not supported by our data. Patients with schizophrenia who receive regular mental health care may have skills in managing diabetes that are related to their experience in managing their psychiatric disorder.
Direct comparison of patients with schizophrenia and those with mood disorders did not yield evidence of differences in HbA1c values, although the patients with mood disorders were slightly older, more likely to be obese, and more likely to be depressed. Comorbid depression has been associated with poorer glycemic control among persons with diabetes (33). On the other hand, the patients with mood disorders in our study also had certain characteristics that would be expected to be associated with reduced HbA1c values. Fewer of the patients with mood disorders were receiving antipsychotic medications, they were more likely to be Caucasian, and they had more diabetes knowledge. It is possible that these conflicting influences balanced each other out in comparisons of the two groups of patients with mental illness that were sampled from the same clinics. Here the comparison is not subject to the potential sampling bias involved in comparing the schizophrenia and non-mentally ill groups.
Patients for whom olanzapine was prescribed had higher HbA1c values than those for whom other antipsychotic medications were prescribed. This finding is consistent with other reports of glucose dysregulation associated with olanzapine (20,21,22,23) as well as the Food and Drug Administration's recent mandate that product labeling for all second-generation antipsychotics contain warnings about hyperglycemia and diabetes. However, ours is the first study to examine the influence of antipsychotic medications on diabetes outcomes among persons with a diagnosis of type 2 diabetes. Our study sample has an average duration of diabetes of 8.6±7.9 years. Although preliminary, these results have significant implications for prescribing decisions about antipsychotic medications and underscore the need for clinicians to conduct careful risk-benefit analyses when making medication choices. This finding must also be considered in light of the fact that the study was cross-sectional, that we do not know the length of time over which antipsychotic medications were prescribed, and that we do not know the overall level of adherence. The effect of antipsychotic medications on other short-term diabetes outcomes—for example, lipid abnormalities—as well as the long-term complications of diabetes warrants further attention.
The limitations of this study should be taken into consideration. Selection bias could account for some of the differences we observed. We took measures to prevent this bias by sampling from community settings in which the groups of patients with schizophrenia or a major mood disorder would likely be followed had they not developed a mental disorder. Another potential limitation is surveillance bias due to the known risk of glucose dysregulation associated with certain psychiatric medications. We do not believe that this possible bias explains our findings, because there is consistent evidence that persons with mental illness do not receive adequate monitoring for medical conditions (45,10).
There are two tensions inherent in this study. The first is the tension between the surprising results (the superior outcome of the schizophrenia group) and the limitations of the sampling. Is this finding merely a result of sampling, of "comparing apples and oranges"? There is no certainty here, and it is necessary to conduct more research. Such research will be a challenge. In our study, we would have preferred to draw our samples of patients with schizophrenia and those without a mental illness from the same primary care clinics, but this was not feasible given the typical segmentation of the mental health and primary care systems.
The second tension is between the temptation to focus only on the first tension—the surprising results of the comparison among the patient groups—and the temptation to ignore the other important findings, the validity of which is less threatened by the sampling strategy. Examples of important findings that could be overlooked as a result of focusing on the first tension include the overall poor outcomes of all groups; the racial differences in HbA1c; the potential linkage of olanzapine with higher HbA1c levels, even among patients who have had diabetes for an average of nine years; and the importance of adherence to diabetes medication for HbA1c.
This study raises many issues for future research. Strategies to enhance the glycemic control of persons with diabetes are necessary for those with schizophrenia as well as for those who do not have a severe mental illness but who may have similar socioeconomic characteristics. The relationship between diabetes and schizophrenia demands more extensive investigation. Future studies should consider following cohorts of patients over time to capture differences in health outcomes longitudinally. Clinicians should be aware of the diabetes risk among persons with severe mental illness, should consider the impact of different medications in treating these patients, and should monitor glucose levels.
Dr. Dixon, Dr. Kreyenbuhl, Dr. Goldberg, Ms. Wolheiter, Ms. Postrado, Ms. Fang, and Dr. Marano are affiliated with the department of psychiatry at the University of Maryland School of Medicine, 685 West Baltimore Street, MSTF/300, Baltimore, Maryland 21201 (e-mail, [email protected]). Dr. Dickerson is with the Sheppard Pratt Health System in Baltimore. Dr. Donner is with the department of medicine and Dr. Brown with the department of epidemiology and preventive medicine at the University of Maryland School of Medicin. Dr. Messias is with the department of psychiatry at Johns Hopkins School of Medicine in Baltimore. This paper was presented in part at the Academy Health Annual Research Meeting held June 23 to 25, 2002, in Washington, D.C.
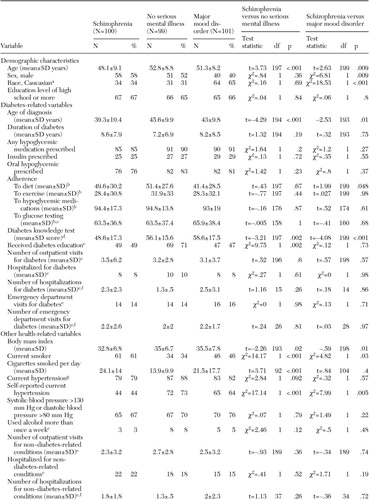 |
Table 1. Characteristics of three samples in a study of outcomes among patients with type 2 diabetes
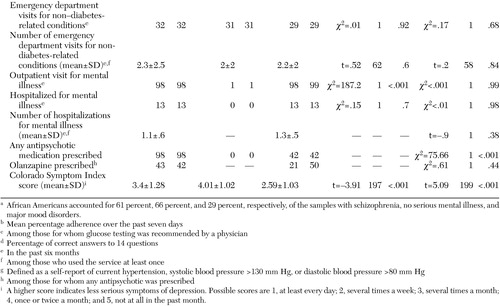 |
Table 1b.
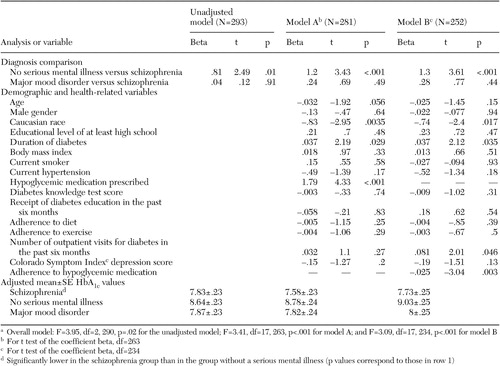 |
Table 2. Glycosylated hemoglobin (HbA1c) levels among patients with schizophrenia, patients with major mood disorders, and patients without a serious mental illnessa
a Overall model: F=3.95, df=2, 290, p=.02 for the unadjusted model; F=3.41, df=17, 263, p<.001 for model A; and F=3.09, df=17, 234, p<.001 for model B
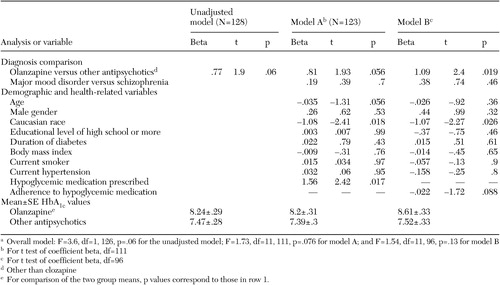 |
Table 3. Glycosylated hemoglobin (HbA1c) levels among patients for whom olanzapine was prescribed and patients for whom other antipsychotics were prescribeda
a Overall model: F=3.6, df=1, 126, p=.06 for the unadjusted model; F=1.73, df=11, 111, p=.076 for model A; and F=1.54, df=11, 96, p=.13 for model B
1. Jeste DV, Gladsjo JA, Lindamer LA, et al: Medical comorbidity in schizophrenia. Schizophrenia Bulletin 22:413–430, 1996Crossref, Medline, Google Scholar
2. Felker B, Yazel JJ, Short D: Mortality and medical comorbidity among psychiatric patients: a review. Psychiatric Services 47:1356–1363, 1996Link, Google Scholar
3. Muck-Jorgensen P, Mors O, Mortensen PB, et al: The schizophrenic patient in the somatic hospital. Acta Psychiatrica Scandinavica Suppl 102:96–99, 2000Crossref, Google Scholar
4. Baxter DN: The mortality experience of individuals on the Salford psychiatric case register: I. all-cause mortality. British Journal of Psychiatry 168:772–779, 1996Crossref, Medline, Google Scholar
5. Simpson JC, Tsuang MT: Mortality among patients with schizophrenia. Schizophrenia Bulletin 22:485–499, 1996Crossref, Medline, Google Scholar
6. Harris EC, Barraclough B: Excess mortality of mental disorder. British Journal of Psychiatry 173:11–53, 1998Crossref, Medline, Google Scholar
7. Allison DB, Fontaine KR, Mentore JL, et al: The distribution of body mass index among individuals with and without schizophrenia. Journal of Clinical Psychiatry 60:215–220, 1999Crossref, Medline, Google Scholar
8. Dalack GW, Healy DJ, Meador-Woodruff JH: Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. American Journal of Psychiatry 155:1490–1501, 1998Link, Google Scholar
9. Goldman LS: Medical illness in patients with schizophrenia. Journal of Clinical Psychiatry 60(suppl 21):10–15, 1999Medline, Google Scholar
10. Druss BG, Rosenheck RA, Desai MM, et al: Quality of preventive medical care for patients with mental disorders. Medical Care 40:129–136, 2002Crossref, Medline, Google Scholar
11. Druss BG, Bradford DW, Rosenheck RA, et al: Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA 283:506–511, 2000Crossref, Medline, Google Scholar
12. Druss BG, Rosenheck RA: Mental disorders and access to medical care in the United States. American Journal of Psychiatry 155:1775–1777, 1998Link, Google Scholar
13. Dickerson FB, McNary SW, Brown CH, et al:Somatic healthcare utilization among adults with serious mental illness who are receiving community psychiatric services. Medical Care 4:560–570, 2003Google Scholar
14. CDC Diabetes Surveillance System. Available at www.cdc.gov/diabetes/statistics/prev/national/fig2.htmGoogle Scholar
15. Dynes JB: Diabetes in schizophrenia and diabetes in nonpsychotic medical patients. Diseases of the Nervous System 30:341–344, 1969Medline, Google Scholar
16. Wilkinson DG: Psychiatric aspects of diabetes mellitus. British Journal of Psychiatry 138:1–9, 1981Crossref, Medline, Google Scholar
17. Mukherjee S, Decina P, Bocola V, et al: Diabetes mellitus in schizophrenic patients. Comprehensive Psychiatry 37:68–73, 1996Crossref, Medline, Google Scholar
18. Mukherjee S: High prevalence of type II diabetes in schizophrenic patients. Schizophrenia Research 15:195, 1995Google Scholar
19. Dixon L, Weiden P, Delahanty J, et al: Prevalence and correlates of diabetes in national schizophrenia samples. Schizophrenia Bulletin 26:903–912, 2000Crossref, Medline, Google Scholar
20. Koro CE, Fedder DO, L'Italien GJ, et al: Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. British Medical Journal 325:243–224, 2002Crossref, Medline, Google Scholar
21. Sernyak MJ, Leslie DL, Alarcon RD, et al: Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. American Journal of Psychiatry 159:561–566, 2002Link, Google Scholar
22. Newcomer JW, Haupt DW, Fucetola R, et al: Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Archives of General Psychiatry 59:337–345, 2002Crossref, Medline, Google Scholar
23. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity: Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care 27:596–601, 2004Crossref, Medline, Google Scholar
24. Seidman LJ, Cassens GP, Kremen WS, et al: Neuropsychology in schizophrenia, in Clinical Syndromes in Adult Neuropsychology: The Practitioner's Handbook. Edited by White RF. New York, Elsevier, 1992Google Scholar
25. Braff DL, Heaton R, Kuck J, et al: The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Archives of General Psychiatry 48:891–898, 1991Crossref, Medline, Google Scholar
26. Goldberg TE, Gold JM, Greenberg R, et al: Contrasts between patients with affective disorders and patients with schizophrenia on a neuropsychological test battery. American Journal of Psychiatry 150:1355–1362, 1993Link, Google Scholar
27. Marneros A, Deister A, Rohde A: Psychopathological and social status of patients with affective, schizophrenic, and schizoaffective disorders after long-term course. Acta Psychiatrica Scandinavica 82:352–358, 1990Crossref, Medline, Google Scholar
28. American Diabetes Association: Clinical Practice Recommendations 2003. Diabetes Care 26:S80-S82, 2003Google Scholar
29. Klein R: Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care 18:258–268, 1995Crossref, Medline, Google Scholar
30. Haffner SJ, Cassells H: Hyperglycemia as a cardiovascular risk factor. American Journal of Medicine 115(8A):6S-11S, 2003Google Scholar
31. Turner RC, Millns H, Neil HAW, et al: Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom prospective diabetes study (UKPDS:23). British Medical Journal 316:823–828, 1998Google Scholar
32. Beck-Nielson H, Hother-Nielson O: Obesity in type 2 diabetes mellitus, in Diabetes Mellitus: A Fundamental and Clinical Text. Edited by LeRoith D, Taylor SI, Olefsky JM. Philadelphia, Lippincott Williams & Wilkins, 2000Google Scholar
33. Lustman PJ, Anderson RJ, Freedland KE, et al: Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 23:434–442, 2000Crossref, Medline, Google Scholar
34. De Groot M, Anderson RJ, Freedland KE, et al: Association of depression and diabetes complications: a meta-analysis. Psychosomatic Medicine 63:619–630, 2001Crossref, Medline, Google Scholar
35. Rothman KJ, Greenland S: Modern Epidemiology, 2nd ed. New York, Lippincott Williams & Wilkins, 1998Google Scholar
36. Toobert DJ, Glasgow RE: Assessing diabetes self-management: the summary of diabetes self-care activities questionnaire, in Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Edited by Bradley C. Amsterdam, Harwood, 1994Google Scholar
37. Fitzgerald JT, Funnell M, Hess GE, et al: The reliability and validity of the Brief Diabetes Knowledge Test. Diabetes Care 21:706–710, 1998Crossref, Medline, Google Scholar
38. Provider Recognition Program. Alexandria, Va, American Diabetes Association, 1997. Available at www.diabetes.orgGoogle Scholar
39. Shern DL, Wilson NC, Coen AS, et al: Client outcomes: II. longitudinal client data from the Colorado Treatment Outcome Study. Milbank Quarterly 72:123–148, 1994Crossref, Medline, Google Scholar
40. Diabetes Control and Complications Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependent diabetes mellitus. New England Journal of Medicine 329:977–986, 1993Crossref, Medline, Google Scholar
41. UK Prospective Diabetes Study (UKPDS) group: Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–852, 1998Crossref, Medline, Google Scholar
42. Suwattee P, Lynch JC, Pendegrass ML: Quality of care for diabetic patients in a large urban public hospital. Diabetes Care 26:563–568, 2003Crossref, Medline, Google Scholar
43. Rhee M, Cook C, Dunbar V, et al: Limited health care access impairs glycemic control in urban blacks with type 2 diabetes. Diabetes 52:A269, 2003Google Scholar
44. Saydah SH, Fradkin J, Cowie CC: Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 291:335–342, 2004Crossref, Medline, Google Scholar
45. Phelan M, Strardins L, Morrison S: Physical health of people with severe mental illness. British Medical Journal 322:443–444, 2001Crossref, Medline, Google Scholar



