Trends in the Use of Antidepressants in a National Sample of Commercially Insured Pediatric Patients, 1998 to 2002
Abstract
OBJECTIVE: The aim of this study was to estimate the prevalence of use of prescription antidepressants among children and adolescents by using nationwide data and to examine trends in use from 1998 to 2002. METHODS: Ambulatory prescription claims data for a nationwide random sample of more than 1.9 million life-years of commercially insured children (aged 18 years or younger) for the years 1998, 1999, 2000, 2001, and 2002 were examined retrospectively. The main outcome measure was trend in prevalence of antidepressant use. RESULTS: The overall prevalence of antidepressant use among children increased from 160 per 10,000 (1.6 percent) in 1998 to 240 per 10,000 (2.4 percent) in 2002, for an adjusted annual increase of 9.2 percent. The growth in the overall prevalence of antidepressant use was greater among girls (a 68 percent increase) than boys (a 34 percent increase). In 2002, antidepressant use was highest among girls aged 15 to 18 years, at 640 per 10,000 (6.4 percent). The trend of increasing overall use of antidepressants among children and adolescents appears to have been driven primarily by greater use of selective serotonin reuptake inhibitors. CONCLUSIONS: The growth in the prevalence of use of antidepressant medications among youths appears to be continuing, and the rate of increase between 1998 and 2002 is similar to the rate of increase seen in the period of the second-generation antidepressants (late 1980s to mid-1990s).
Estimates of the prevalence of depression range from 2.5 percent among children to 8.3 percent among adolescents (1). The onset of depression appears to be occurring earlier in life (2). Early-onset depression, if left untreated, can persist, recur, and continue into adulthood and may lead to more severe depression (3). Depression among children and adolescents is associated with an increased risk of suicidal behavior and suicide (3).
A limited number of antidepressant medications have been approved by the U.S. Food and Drug Administration (FDA) for treatment of pediatric obsessive-compulsive disorder (4). Before 2003, no antidepressant had FDA approval for the treatment of depression in the pediatric age group (5). Although psychotherapy is considered the first-line therapy for mild to moderate depressive disorders in this patient population (6,7), off-label use of antidepressants for depression appears to be widespread (8,9) and gaining in acceptance. Studies have reported three- to tenfold increases in the prevalence of antidepressant use among children and adolescents from 1987 to 1996 (10,11).
The use of antidepressants to treat depression among pediatric patients remains controversial (12). Off-label drug use in this age group has been described as "an experiment with every use (13)." Published studies of antidepressant treatment of depression among youths have shown these agents to be only modestly effective; many treated patients continue to experience symptoms (14,15,16). Tricyclic antidepressants have not been found to be effective for treating depression among prepubescent children (17) and have been implicated in pediatric sudden death (18). Recently, the FDA, the United Kingdom's Medicines and Healthcare Products Regulatory Authority, and Health Canada recommended that paroxetine, a selective serotonin reuptake inhibitor (SSRI), not be used among children and adolescents, because its efficacy has not been established for depression among youths and its use is associated with increased risk of suicidal thinking and suicide attempts (19). This issue is currently the subject of great debate. The evidence of increased risk of suicide with use of the other SSRIs in pediatric populations currently is being reviewed.
Recent data on trends in the ambulatory use of antidepressants overall and by class in nationwide samples of young patients are limited. Given this fact along with the dearth of data on the safety and efficacy of antidepressants for the pediatric age group and the fact that approximately 65 percent of all children were commercially insured beneficiaries in 2000 (20), a contemporary description of the patterns of use of antidepressants in this population is warranted. The objectives of the population-based, retrospective study reported here were to estimate the prevalence of use of the different classes of prescription antidepressant medications among commercially insured children and adolescents in ambulatory care by using national pharmacy claims data to examine trends in antidepressant use from 1998 to 2002.
Methods
Participants
Data were extracted from five one-year databases (1998 to 2002) constructed for research purposes by Express Scripts, Inc. (ESI). The databases comprised ambulatory administrative pharmacy claims and eligibility information (including age and gender) of random samples of 3 million commercially insured beneficiaries whose pharmacy benefit was administered by ESI in each year the databases were constructed. The health plan sponsors for these beneficiaries included private- and public-sector employer groups, managed care organizations, third-party administrators, and unions.
ESI is the third largest pharmacy benefit manager in the United States. ESI administers pharmacy benefits to more than 50 million persons in approximately 1,300 commercially insured health plans—that is, not Medicare or Medicaid—representing all 50 states and the District of Columbia. Approximately 7 million of those for whom ESI administers pharmacy benefits are beneficiaries aged 18 years or younger. In 2000 there were approximately 80 million persons aged 19 years or younger in the United States (21), and approximately 65 percent of them were commercially insured (20).
Beneficiaries aged 18 years or younger who were continuously eligible for pharmacy benefits during the year the database was constructed were included in the analysis, for a final sample of more than 1.9 million person-years. Age categories were preschool (birth to five years), elementary school (six to ten years), middle school (11 to 14 years), and high school (15 to 18 years). The research was performed under the principles outlined in the Declaration of Helsinki and recently approved regulations of the Health Insurance Portability and Accountability Act (HIPAA) pertaining to the use of personal health information.
Procedures and measures
Claims for antidepressants were identified on the basis of Generic Product Identifier (GPI) codes starting with 58 (22). The GPI categorizes medications by a hierarchical therapeutic classification scheme. An "antidepressant user" was defined as a pediatric beneficiary with one or more prescription claims for an antidepressant during a calendar year. Prevalence was estimated for each antidepressant class for each year of the study and was stratified by age and gender (race information was unavailable in the claims data). Antidepressant classes included SSRIs (citalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline), tricyclics (amitriptyline, amoxapine, clomipramine, desipramine, doxepin, imipramine hydrochloride, imipramine pamoate, nortriptyline, protriptyline, and trimipramine), modified cyclics (nefazodone and trazodone), tetracyclics (mirtazapine), and miscellaneous antidepressants (maprotiline, bupropion, and venlafaxine). In light of the FDA's recent warning on the use of paroxetine among pediatric patients (19), annual prevalence estimates for paroxetine were also examined. Prevalence estimates for monoamine oxidase inhibitors are not reported because of the limited number of users.
An antidepressant class prevalence time trend analysis was performed with use of basic log-rate regression modeling (23). Differences in the number of person-years between the cohorts were accounted for by an offset term in the model. The offset is a constant term that does not require an estimation of any unknown parameters, thus providing a weighting for each value of the dependent variable by including the logarithm of person-years as an additive effect in the model. An interaction term between gender and age was included in the model, because the relationship between gender and antidepressant use has been reported to depend on patient age (8,10). Given the large sample studied, statistical analyses were not performed on the individual year-to-year changes, because small differences would have been significant.
Results
The overall sample of commercially insured children and adolescents increased from approximately 370,000 in 1998 to more than 380,000 in 2002, as can be seen from Table 1. Over the study period, the proportion of girls increased, but the mean age of children in the sample remained relatively constant in both the overall sample and the subsample of antidepressant users. This resulted in a sample of child and adolescent antidepressant users in 2002 that was older and had a slightly higher proportion of girls relative to the overall sample.
From 1998 to 2002, the overall prevalence of antidepressant use increased by 49 percent, as can be seen from Table 2. Log-rate modeling indicated that the overall prevalence of antidepressant use increased significantly (p<.001) at an adjusted rate of 9.2 percent per year over the study period (model goodness of fit p=.180 with a deviance of 37.9, df=31). The largest year-to-year proportional increase in overall prevalence occurred between 2001 and 2002 (16 percent).
The prevalence of antidepressant use increased in all strata of age and gender in 2002 compared with 1998. However, prevalence increased more markedly among girls (68 percent) than among boys (34 percent). The prevalence was higher for boys at younger ages, but girls had a higher prevalence by the time they reached high school age. When 2003 was compared with 1998, girls and boys aged 15 to 18 years showed the largest absolute changes in prevalence (2.6 and 1.2 percentage point increases, respectively). However, the youngest age group (aged zero to five years) showed the largest proportional increase in prevalence within the respective gender categories (100 and 62 percent for girls and boys, respectively).
The overall prevalence of use for all antidepressant classes increased during the study period except in the case of tricyclics (a decrease of 29 percent), as can be seen from Table 3. In all five years of the study, SSRIs were the most commonly prescribed antidepressants, and tetracyclics were the least prescribed. Although tetracyclics (mirtazapine) had the lowest prevalence of use of all antidepressant classes, this class had the largest proportional increase in prevalence over the study period—400 percent overall. The largest absolute increase in overall prevalence over the five years was for SSRIs (.7 percentage points). The prevalence of use among girls increased by 97 percent for SSRIs and 114 percent for miscellaneous antidepressants—including the serotonin and norepinephrine reuptake inhibitor venlafaxine—over the study period. The prevalence of use of the SSRI paroxetine increased by 113 percent and 91 percent among girls and boys, respectively, from 1998 to 2002.
Discussion
Our results suggest a continuing growth in the use of antidepressants among patients aged 18 years or younger. The adjusted trend in the prevalence of use of antidepressants among children and adolescents increased at an annual rate of 9.2 percent. The proportional increase in overall prevalence of use that we observed during the study period (about 50 percent over five years) was less than the three- to tenfold (10,11) increases over ten years that were reported in studies performed in earlier periods in a variety of study populations. However, the rates of increase observed in these other studies (10,11) reflect increases from lower base prevalences. These findings suggest that rates of growth, in terms of absolute prevalence increases observed from the late 1980s to mid-1990s, are continuing.
The prevalence of overall use of antidepressants in our pediatric population (160 per 10,000 in 1998) was similar to rates reported for 1996 by Olfson and associates (11) (100 per 10,000) in a national survey and by Zito and associates (10) (160 per 10,000) in a health maintenance organization. The 1999 prevalence of use of SSRIs (110 per 10,000) and paroxetine (30 per 10,000) in our pediatric population was similar to that estimated by Shatin and Drinkard (24) for the same year (130 per 10,000 and 38 per 10,000, respectively) in a nationwide sample of employer-insured patients less than 20 years of age.
A number of factors acting together or independently may have led to the escalated use of antidepressants among children and adolescents, including increasing rates of depression in successive age cohorts (2), a growing awareness of and screening for depression by pediatricians (25), emergent extrapolation of the safety and efficacy of antidepressant pharmacotherapy seen by clinicians among adults to the needs of pediatric patients (11), and greater reliance on pharmacotherapy by general practitioners as coverage for mental health services, such as psychotherapy, becomes more limited (26). The increasing trend we observed in overall antidepressant use appears to have been driven primarily by greater use of SSRIs.
As in other studies, we found that the use of antidepressants was higher among younger male and older female pediatric patients (8,24). We also found substantial proportional increases in the prevalence of overall antidepressant use among female and male preschoolers (10), increasing prevalence with each successive age group (27), and a marked increase in the prevalence of SSRI use (9,24) and decrease in tricyclic use (24). The prevalence of paroxetine use in our sample increased by approximately 100 percent from 1998 to 2002, a finding that is similar to that reported by Shatin and Drinkard (24) (a 102 percent increase from 1995 to 1999), which suggests a continued growth in use of paroxetine through the late 1990s and into the 21st century. This finding is of note given FDA's recent recommendation that paroxetine not be prescribed for depression among pediatric patients (19).
Several factors should be considered in interpreting our findings. Our data did not enable us to document whether youths who received antidepressants had a diagnosis of depression. However, available data suggest that antidepressants are prescribed for children and adolescents for depression more than for anxiety disorders (28), and prescription claims have been shown to be a reliable and valid source of data for documenting treatment for depression among adults (29). As with any analysis involving the use of administrative claims data, a claim does not indicate whether the patient actually took the medication. In addition, although this study did exclude publicly insured pediatric patients, the generalizability of the results is likely to be robust given that previous investigations have documented similar if not higher rates of growth in antidepressant use among Medicaid beneficiaries than among commercially insured pediatric beneficiaries (10,30).
Although we were unable to assess the community prevalence of depression in our sample, the proportions of youths we observed receiving any antidepressants (.2 percent to 1.6 percent of children and 2.4 percent to 6.4 percent of adolescents in 2002) are substantially lower than the estimated U.S. national prevalence of depression in these age groups (2.5 percent of children and 8.3 percent of adolescents) (1). Even if all the children and adolescents with an antidepressant claim in our study were being treated for depression, these findings suggest that the use of prescription antidepressants among pediatric patients will continue to grow as more children and adolescents with depression have their illness diagnosed and treated. This potential growth highlights two differing but not mutually exclusive beliefs: first, the concern that antidepressants are being prescribed for children and adolescents in the face of insufficient information about their safety and efficacy in this population (31) and, second, the inference that advocacy work to identify and treat depression among children and adolescents has begun to pay off (32). We were unable to assess the merit of these two views in our study.
Conclusions
The growth in the prevalence of use of antidepressant medications among children and adolescents appears to be continuing and is similar to that reported for the late 1980s to mid-1990s. However, given that children and adolescents represent one of the most vulnerable patient populations, further research is needed to examine the appropriateness of antidepressant prescribing to an ambulatory pediatric population.
Acknowledgment
This study was funded by Express Scripts, Inc.
Dr. Delate, Ms. Simmons, and Dr. Motheral are affiliated with the office of evidence-based pharmacy benefit design at Express Scripts, Inc., 13900 Riverport Drive, STL12N, Maryland Heights, Missouri 63043 (e-mail, [email protected]). Dr. Gelenberg is head of the department of psychiatry of the University of Arizona in Tuscon.
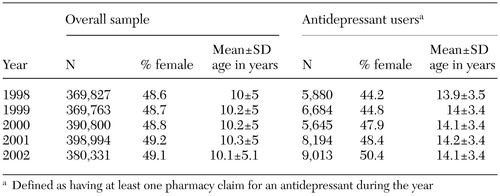 |
Table 1. Characteristics of a national sample of commercially insured children and adolescents and a subsample of those using antidepressants
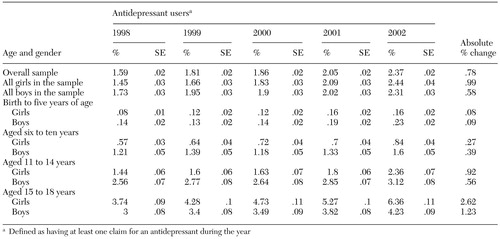 |
Table 2. Prevalence of use of antidepressants in a national random sample of commercially insured children and adolescents stratified by age and gender
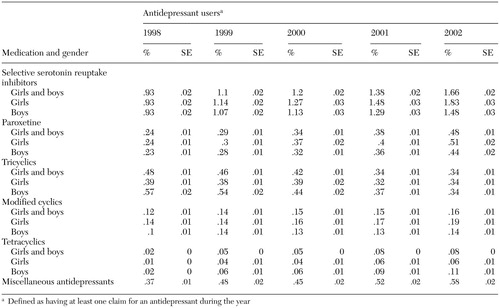 |
Table 3. Use of subclasses of antidepressants and of paroxetine in a national random sample of commercially insured children and adolescents stratified by gender
1. Birmaher B, Ryan ND, Williamson DE, et al: Childhood and adolescent depression: I. a review of the last 10 years. Journal of the American Academy of Child and Adolescent Psychiatry 35:1427–1439, 1996Crossref, Medline, Google Scholar
2. Klerman GL, Weissman MM: Increasing rates of depression. JAMA 261:2229–2235, 1989Crossref, Medline, Google Scholar
3. Weissman MM, Wolk S, Goldstein RB, et al: Depressed adolescents grown up. JAMA 281:1701–1713, 1999Crossref, Medline, Google Scholar
4. National Institute of Mental Health: Medications. Available at www.nimh.nih.gov/publicat/medicate.cfm#ptdep16. Accessed Dec 10, 2003Google Scholar
5. US Food and Drug Administration: FDA approves Prozac for pediatric use to treat depression and OCD. Available at www.fda.gov/bbs/topics/answers/2003/ans01187.html. Accessed Dec 10, 2003Google Scholar
6. Brent DA, Holder D, Kolko D, et al: A clinical psychotherapy trial for adolescent depression, comparing cognitive, family, and supportive therapy. Archives of General Psychiatry 54:877–885, 1997Crossref, Medline, Google Scholar
7. Birmaher B, Brent DA, Benson RS: Summary of the practice parameters for the assessment and treatment of children and adolescents with depressive disorders. Journal of the American Academy of Child and Adolescent Psychiatry 37:1234–1238, 1998Crossref, Medline, Google Scholar
8. Zito JM, Safer DJ, Riddle MA, et al: Prevalence variations in psychotropic treatment of children. Journal of Child and Adolescent Psychopharmacology 8:99–105, 1998Crossref, Medline, Google Scholar
9. Pincus HA, Tanielian TL, Marcus SC, et al: Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA 279:526–531, 1998Crossref, Medline, Google Scholar
10. Zito JM, Safer DJ, dosReis S, et al: Psychotropic practice patterns for youth: a 10-year perspective. Archives of Pediatric and Adolescent Medicine 157:17–25, 2003Crossref, Medline, Google Scholar
11. Olfson M, Marcus SC, Weissman MM, et al: National trends in the use of psychotropic medications by children. Journal of the American Academy of Child and Adolescent Psychiatry 41:514–521, 2002Crossref, Medline, Google Scholar
12. Riddle MA, Labellarte MJ, Walkup JT: Pediatric psychopharmacology: problems and prospects. Journal of Child and Adolescent Psychopharmacology 8:87–97, 1998Crossref, Medline, Google Scholar
13. Holdsworth MT: Pediatric drug research: the road less traveled. Annals of Pharmacotherapy 37:586–591, 2003Crossref, Medline, Google Scholar
14. Emslie GJ, Rush AJ, Weinberg WA, et al: A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Archives of General Psychiatry 54:1031–1037, 1997Crossref, Medline, Google Scholar
15. Mohr WK: Updating what we know about depression in adolescents. Journal of Psychosocial Nursing and Mental Health Services 36:12–19, 1998Medline, Google Scholar
16. Wagner KD, Ambrosini PJ: Childhood depression: pharmacological therapy/treatment (pharmacology of childhood depression). Journal of Clinical Child Psychology 30:88–97, 2001Crossref, Medline, Google Scholar
17. Hazell P, O'Connell D, Heathcote D, et al: Tricyclic drugs for depression in children and adolescents. Cochrane Database of Systematic Reviews 2:CD002317, 2002Google Scholar
18. Varley CK: Sudden death related to select tricyclic antidepressants in children: epidemiology, mechanisms, and clinical implications. Pediatric Drugs 3:613–627, 2001Crossref, Medline, Google Scholar
19. Elliot VS: FDA warning on one drug spurs concern over others. American Medical News, Sept 8, 2003. Available at www.ama-assn.org/amednews/2003/09/08/hlsc0908.htm. Accessed Dec 10, 2003Google Scholar
20. Elixhauser A, Machlin SR, Zodet MW, et al: Health care for children and youth in the United States:2001 annual report on access, utilization, quality, and expenditures. Ambulatory Pediatrics 2:419–437, 2002Google Scholar
21. US Census Bureau: Profile General Demographic Characteristics:2000. Available at http://factfinder.census.gov/servlet/qttable? ds_name=dec_2000_sf1_u&geo_id=01000us&qr_name=dec_2000_sf1_u_dp1. Accessed Dec 10, 2003Google Scholar
22. Generic Product Identifier Number. Indianapolis, First DataBank (Medi-Span), 2001Google Scholar
23. Agresti A: Categorical Data Analysis. New York, Wiley, 1990Google Scholar
24. Shatin D, Drinkard CR: Ambulatory use of psychotropics by employer-insured children and adolescents in a national managed care organization. Ambulatory Pediatrics 2:111–119, 2002Crossref, Medline, Google Scholar
25. Halpern-Felscher BL, Ozer EM, Millstein SG, et al: Preventive services in a health maintenance organization: how well do pediatricians screen and educate adolescent patients? Archives of Pediatric and Adolescent Medicine 154:173–179, 2000Google Scholar
26. Sturm R, Wells K: Health insurance may be improving—but not for individuals with mental illness. Health Services Research 35:253–262, 2000Medline, Google Scholar
27. Kelleher KJ, Hohmann AA, Larson DB: Prescription of psychotropics to children in office-based practice. American Journal of Diseases of Children 143:855–859, 1989Medline, Google Scholar
28. Shireman TI, Olson BM, Dewan NA: Patterns of antidepressant use among children and adolescents. Psychiatric Services 53:1444–1450, 2002Link, Google Scholar
29. Kwon A, Bungay KM, Pei Y, et al: Antidepressant use: concordance between self-report and claims records. Medical Care 41:368–374, 2003Medline, Google Scholar
30. Rushton JL, Whitmire JT: Pediatric stimulant and selective serotonin reuptake inhibitor prescription trends:1992 to 1998. Archives of Pediatric and Adolescent Medicine 155:560–565, 2001Google Scholar
31. Moldenhauer Z, Melnyk BM: Use of antidepressants in the treatment of child and adolescent depression: are they effective? Pediatric Nursing 25:643–647, 1999Google Scholar
32. Walkup JT: Increasing use of psychotropic medications in children and adolescents: what does it mean? Journal of Child and Adolescent Psychopharmacology 13:1–3, 2003Google Scholar



