Clinical Characteristics, Cognitive Functioning, and Criminal Histories of Outpatients With Schizophrenia
Abstract
OBJECTIVE: The authors examined the relationships between clinical characteristics, cognitive functioning, and history of violent behavior and substance use among outpatients with schizophrenia. METHODS: Ninety-six patients with a diagnosis of schizophrenia or schizoaffective disorder completed a clinical and neuropsychiatric battery that included tests of general intelligence, executive and frontal lobe function, visual-motor processing, and motor function. Violent behavior was defined on the basis of arrest records. Self-reported violent behavior and substance use were recorded. The study participants were separated into three groups: history of violent arrest (N=34), nonviolent arrest (N=23), and no arrest (N=39). The three groups were compared for differences in demographic characteristics, clinical symptoms, and scores on neuropsychological tests. RESULTS: Fifty-seven (59 percent) of the 96 participants had a history of arrest. Persons who were arrested for nonviolent crimes had a significantly lower mean±SD number of arrests (3.39±3.7) than those arrested for violent crimes (9.24±8.9). No significant differences in neuropsychological test scores or clinical ratings were found between the three groups. The prevalence of substance use disorders was 65 percent, 57 percent, and 36 percent among patients with a history of violent, nonviolent, and no arrest, respectively. Only 47 percent of participants with a criminal history accurately reported this history, and 11 percent of participants with a history of drug-related arrests acknowledged previous substance use. CONCLUSIONS: Performance on neuropsychiatric tests does not distinguish stable outpatients with schizophrenia who have a history of violent behavior from those who do not have such a history. Two established predictors of violence, a history of arrests and substance abuse, are unreliable when assessed by self-report.
It has become increasingly evident that schizophrenia is associated with an elevated risk of violent behavior (1,2). Efforts to identify factors contributing to this increased risk encompass many domains of psychiatry, including epidemiology (3), brain imaging (4,5), and genetics (6).
Recently our group found that nearly 46 percent of 360 outpatients with psychotic disorders had been arrested (7). The arrest rates of these patients significantly increased at the time of onset of illness, decreased with initiation of treatment, and further decreased with clozapine treatment. Several factors may be responsible for the effect observed with clozapine, including the increased frequency of clinician contact (for biweekly blood draws) and the beneficial effect of clozapine on the cognitive deficits of schizophrenia, including executive function (8,9). In addition, substance abuse has been linked to violent behavior among persons with schizophrenia (2), and clozapine has been reported to decrease substance abuse (10). Clozapine may also decrease impulsivity, which could decrease rates of violence and arrest among persons with schizophrenia. The study reported here was designed to assess the relationship between cognitive deficits and violence among patients with schizophrenia.
Seven studies have examined the relationship between performance on neuropsychological tests and violence among patients with schizophrenia (11,12,13,14,15,16,17). The results of these studies have been inconsistent. Two of the seven studies (11,16) found superior performance on neuropsychological measures among persons with elevated rates of violence. Roy and colleagues (11) found, in a sample of 20 inpatients with chronic schizophrenia, that violent patients (defined on the basis of chart review and ward behavior) outperformed nonviolent patients on several subscales of the Wechsler Adult Intelligence Scale, Revised (WAIS-R): verbal IQ, digit symbol, and block design. A study by Lapierre and colleagues (16) found a correlation between enhanced performance on both the Wisconsin Card Sorting Test (WCST) and a verbal fluency test and lifetime "number of aggressions against another person" among 31 outpatients with schizophrenia. The low-violence group did not achieve significantly better scores than the violent group on any neuropsychological measure in either study.
In contrast, two studies showed that neuropsychological impairment was associated with violence among inpatients with schizophrenia (12,13). Krakowski and colleagues (12) divided a sample of patients into high-violence (N=22), low-violence (N=17), and nonviolent (N=22) groups on the basis of ward behaviors. Patients in the high-violence group were significantly more impaired than those in the nonviolent group, as measured by their scores on the Benton Visual Retention Test and the WAIS-R Performance IQ. An examination of the subtests of the WAIS-R Performance IQ demonstrated significantly lower scores for the high-violence group compared with the nonviolent group on, among others, the digit symbol and block design tests, the same tests on which violent patients received higher scores in the study by Roy and colleagues (11). Adams and associates (13) found that impairment on the Luria-Nebraska Neuropsychological Battery was related to a history of violent arrest but not to inpatient violence in a sample of 37 incarcerated persons with schizophrenia.
Of the remaining three studies, none dealt exclusively with schizophrenia. However, in two of the studies a majority of the participants had a diagnosis of schizophrenia. One study compared 23 forensic inpatients, 16 of whom had a diagnosis of schizophrenia, who had committed a violent crime (15). Impairment on several neuropsychological tests—the judgment-of-line orientation test, the symbol digit modalities test, the Stroop Interference Test, and the test of nonverbal intelligence—was correlated with the frequency and severity of violent behavior. In a larger study, Krakowski and colleagues (17) compared 33 inpatients with a history of community violence, as determined by self-report of arrest for violent crime and chart review for such arrests, with 69 inpatients who denied arrests for violent crimes. Of the 102 patients included in the study, 72 percent had a diagnosis of schizophrenia or schizoaffective disorder. A history of community violence was significantly related to impairment on some WCST subtests as well as impairment in the finger-tapping test and the Perdue pegboard test (both left handed).
It is apparent that discrepancies exist between previous studies with regard to the neuropsychological characteristics that may be associated with violence among patients with schizophrenia. Some of the inconsistency in findings may reflect methodologic limitations. Only two of the studies that were specific to schizophrenia (12,17) included more than 50 participants, and in all but one study (16) the participants either were inpatients or were incarcerated at the time of the neuropsychological testing. The validity of the methods used to ascertain violence is also problematic.
The goal of the study reported here was to assess whether performance on a battery of neuropsychological tests would differentiate patients who had a documented history of violence in a larger sample of stable outpatients with schizophrenia. We hypothesized that patients who had a history of violent arrest would perform poorly on tests of frontal lobe function. We also expected that patients who had a history of arrest for violent behavior would be more likely to have a comorbid substance use disorder, given that data from the Epidemiologic Catchment Area study found that 21 percent of persons with substance use disorders and 30 percent with both schizophrenia and substance abuse reported violent behavior in the previous year (2).
Methods
Study participants
The study participants were outpatients at an urban community outpatient mental health clinic. Approximately 550 psychotic patients are followed through this clinic, which has a catchment area with a population of 160,000. The clinic population consists largely of patients with low socioeconomic status and chronic mental illnesses.
The study was approved by the local institutional review board. Written informed consent was obtained from all participants. Study participants were referred to the research study by their primary treater in the clinic. No attempts were made to control the selection of participants referred by the clinician. The clinicians were aware only that the research study involved clinical and neuropsychological ratings. These referrals resulted in 112 participants with DSM-IV diagnoses of schizophrenia (N=110) or schizoaffective disorder, depressed type (N=2) who participated in the study and underwent a battery of clinical and neuropsychological measures. Diagnosis was established with use of the Structured Clinical Interview for DSM-IV (SCID) by psychiatrists who were trained in the use of this instrument.
Clinical symptoms were assessed with the Positive and Negative Symptom Scale (PANSS) and the Scale for the Assessment of Negative Symptoms (SANS). Self-reports of criminal records and substance abuse were collected by using the Violence and Suicide Assessment Scale (18). Criminal background checks were used to identify a history of violent behavior. Participants' names, dates of birth, and Social Security numbers were submitted to the Commonwealth of Massachusetts Criminal History Systems Board in compliance with the regulations pertaining to research. These regulations allow for criminal background checks to be performed in the Commonwealth of Massachusetts without consent, provided that the research is conducted for valid educational, scientific, or other public purposes. The researchers are held to a strict standard for maintaining subject anonymity; in accordance with this standard, only one investigator (WGF) had access to the criminal background data.
The neuropsychological battery included the WAIS-IIIR, the Stroop Interference Test, the WCST, the Trails A and B, the semantic verbal fluency test, and the finger-tapping test. This battery allowed for the testing of various domains, including general intelligence (WAIS-IIIR), executive and frontal lobe function (Stroop test, WCST, and Trails B), temporoparietal function (semantic verbal fluency), visual and motor processing (Trails A and B), and motor function (finger-tapping test).
The study participants were tested over a two-year period (April 1999 to April 2001). Interrater reliability on all clinical scales was established and was tested quarterly over the course of data collection. A correlation coefficient greater than .8 for total PANSS and SANS scores was maintained throughout the study.
Statistical analysis
The study participants were separated into three groups on the basis of the arrest data: history of violent arrest, history of nonviolent arrest, and no history of arrest. Violent arrests, defined according to the Department of Justice Uniform Crime Reporting Categories (19), are for murder, forcible rape, robbery, and aggravated assault. Participants were categorized as violent if they had a history of arrest for at least one violent crime. If several charges occurred on the same date, the most serious charge was selected. In addition, if a drug-related charge occurred, it was coded separately (within the nonviolent category).
The three groups were compared for differences in demographic characteristics, clinical symptoms, and scores on the neuropsychological tests. Chi square tests were used to compare categorical variables, and analysis of variance (ANOVA) was used for continuous measures. For the clinical and neuropsychological variables, the alpha level was set at .003, incorporating a Bonferroni correction for multiple comparisons (.05 divided by 18). Self-reports of arrests and substance abuse were compared with the criminal background data to determine the percentage of patients who accurately reported criminal histories and substance abuse.
Results
We received arrest data for all 112 study participants from the Criminal Histories Systems Board. Of this sample, 96 individuals completed all clinical and neuropsychological testing. Fifty-seven (59 percent) of the 96 participants had at least one previous arrest, for a total of 392 arrests for all participants. Twenty-three participants (24 percent) had arrests for nonviolent crimes only, and 34 (35 percent) had arrests for violent crimes. There was a significant difference in the mean±SD number of arrests between the nonviolent-arrest group (3.39±3.7) and the violent-arrest group (9.24±8.9) (t=3.5, df=48, p=.001).
For the entire group of 96, the mean age was 44±9.4 years and the mean age at onset of illness was 24±7.4 years. The sample was 26 percent female (25 patients), 25 percent African American (24 patients), and 72 percent Caucasian (69 patients). The mean PANSS total score was 62.1±14, and the mean SANS total score was 42.3±14.9. The demographic data for each group are summarized in Table 1. No differences in demographic variables were found between the three groups except for a lower percentage of women in the two arrest groups (χ2=7.8, df=2, p=.02).
No differences in neuropsychological test scores or clinical ratings were found between the three groups. The mean scores are shown in Table 2 (20,21,22). A subsequent analysis (data not shown), dividing the study participants into two groups—participants with arrests (N=57) and those without (N=39)—also did not find any differences on these variables.
Of the 57 participants who had been arrested, 19 (33 percent) had a history of a drug- or alcohol-related arrest. Seventeen of the 19 participants denied a lifetime history of even recreational use of substances. These 19 individuals accounted for 219 (56 percent) of the 392 total arrests (violent and nonviolent) in the study. Table 3 lists the frequency of self-report of arrest as well as substance-related arrests and substance use disorders in the three groups. A total of 49 participants (51 percent) had a history of at least one substance use disorder (substance abuse or dependence) established by SCID interview and chart review; there were no significant differences between the violent and nonviolent groups. The prevalence of a substance use disorder was 65 percent among persons with a history of violent crime, 57 percent among those with a history of nonviolent arrest, and 36 percent among those without a history of arrest; the differences between groups were not significant.
Analysis of the accuracy of self-report of criminal history indicated that 27 (47 percent) of the 57 study participants who had a history of an arrest reported the arrest to the examiners; the other 30 (53 percent) denied that they had been arrested. Thirty-two (82 percent) of the 39 participants who had no history of arrest correctly reported this; the other seven (18 percent) reported a history of arrest despite the fact that there was no record of their arrest in Massachusetts.
Discussion
Violent behavior is difficult to predict. Neuropsychological impairment, specifically in executive function, has been associated with violent behavior in nonpsychiatric populations (23). This study found no significant differences in mean scores on a battery of neuropsychological tests between patients with schizophrenia who had a history of violent arrest, those who had a history of nonviolent arrest, and those who had no history of arrest. Our results differ from those of studies that found differences between violent-arrest and nonviolent-arrest groups on some measures of cognition. The participants in our study demonstrated overall impaired performance in neuropsychological function that is characteristic of patients with schizophrenia (24), but the severity of impairment was not greater among those with a history of violent arrest. It is possible that neuropsychological deficits play less of a role in violent behavior among patients with schizophrenia than they do in nonpsychiatric populations. The acuity of psychiatric symptoms or active substance abuse may make more of a contribution to the likelihood of arrest than do cognitive deficits.
Interestingly, in our study, the results of the WCST were in the predicted direction: patients in the violent-arrest group performed more poorly than did those without a history of violent arrest. We highlight this finding because a similar result on the WCST was found in another study (17). In the study reported here the effect size for the comparison between the three groups was within range of .2, a relatively low effect size. Our study was not powered to detect a difference at this level. However, a larger study may find this effect to be statistically significant.
One drawback of our study was that we were unable to control for use of specific antipsychotic medications, given the large number of different medications used in the clinical treatment of this population. From our previous research we know that medication status can affect arrest rates. This potential effect, combined with evidence that antipsychotic medications differ in their effect on neurocognitive performance (25), may have limited our ability to detect differences between the groups, because the arrests may have occurred during episodes of psychotic exacerbation or substance intoxication when patients were not taking medications.
This study had several other limitations. First, arrests result from multiple factors and may not be a sensitive measure of total violent or criminal behavior. The police officer on the street makes the decision to either arrest the person or bring the person to a hospital and, if the person is arrested, decides what that individual will be charged with. This decision is influenced by a complex interaction between the symptoms of mental illness overtly demonstrated by the individual and the severity of the crime (26). This process may have an impact on the number of study participants that we recognized as violent, because the nature of the charges may or may not have adequately reflected the individual's behavior at the time. In addition, behaviors occurring among family members or acquaintances may go unseen by the police and therefore were not included in this study.
Another limitation was the lack of access to arrest data from outside the Commonwealth of Massachusetts. Some of the 23 percent of the study participants for whom there was no record of an arrest in Massachusetts but who reported a history of arrest may have been arrested in another state. The use of aliases or erroneous self-reporting may also have contributed to this finding.
Another factor that may contribute to discrepancies in the results of studies in this area is the use of self-report data to determine history of violence and substance abuse. The accuracy of self-report not only is important for researchers but, because self-reported data are frequently the only available data in the clinical setting, affects a clinician's ability to assess risk. We hypothesized that the accuracy of self-reporting of both arrest history and substance use would be poor, because patients would be likely to perceive high risk and little benefit in providing these details. In addition, among patients who have schizophrenia, inaccurate self-report of nonstigmatizing details, such as age (27), can occur, which suggests that cognitive deficits may also play a role in the discrepancies in study results.
The overall accuracy of self-reported criminal history among outpatients with schizophrenia in our sample (61 percent) was similar to the rate of 66 percent described in the only other published study of psychiatric patients (28) and was lower than accuracy rates described in populations without mental illness (75 to 91 percent) (29). Although there are limitations inherent in using arrest data as the sole criterion for violence history, the results of our study suggest that problems can arise, depending on the accuracy of self-report of criminal histories in this population.
Substance abuse is an important factor contributing to violent behavior (2). Among patients with both schizophrenia and a history of violent arrest, 65 percent met criteria for a comorbid substance use disorder and 38 percent had a history of arrest for a substance-related crime; however, only 18 percent of them reported active substance use or a history of substance abuse at the time of assessment. Interestingly, we also found that only 11 percent of patients who had been arrested for a drug-related crime admitted to a history of substance use. This finding suggests very low accuracy of self-report for one of the few consistently valid predictors of violence.
Conclusions
This study did not establish a relationship between performance on a neuropsychological battery and history of violent arrest. It did demonstrate that self-report of arrest and substance use tends to be quite inaccurate among patients with schizophrenia. Further studies would ideally include both inpatient and outpatient populations, and neuropsychological and clinical assessments would occur around the time of violent behaviors, in order to evaluate the contribution of disinhibition, psychotic symptoms, and substance use to the violent behavior and the circumstances leading up to those behaviors. Identifying episodes of violent behavior through a variety of sources, including self-report, forensic records, and interviews with family members and treaters, would provide a more complete description of history of violence than arrest records or self-report alone.
Acknowledgment
This study was supported in part by a Young Investigator Award provided by the American Psychiatric Institute for Research and Education and Janssen Pharmaceuticals to Dr. Lafayette.
Dr. Lafayette, Ms. Dyer, and Dr. Goff are affiliated with the schizophrenia research program of Massachusetts General Hospital and Harvard Medical School, Warren 605, 33 Fruit Street, Boston, Massachusetts 02114 (e-mail, [email protected]). Dr. Frankle is with the New York State Psychiatric Institute and with the department of psychiatry of Columbia University in New York City. Ms. Pollock is with the department of internal medicine of Massachusetts General Hospital in Boston.
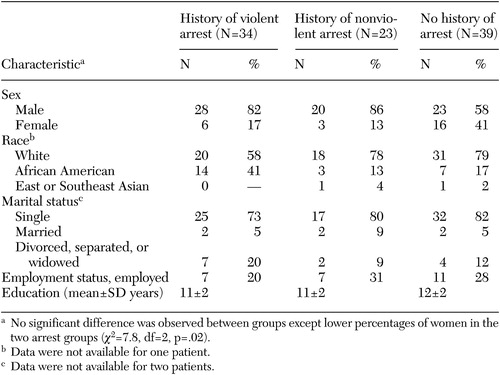 |
Table 1. Demographic characteristics of three groups of patients with schizophrenia
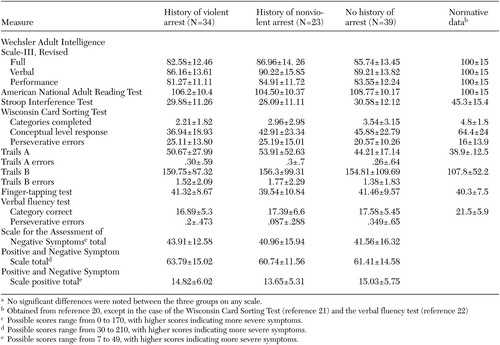 |
Table 2. Mean±SD scores on neuropsychological and clinical scales in three groups of patients with schizophreniaa
a No significant differences were noted between the three groups on any scale.
 |
Table 3. Accuracy of self-report of history of arrest and substance use in three groups of patients with schizophrenia
1. Link BG, Stueve A, Phelan J: Psychotic symptoms and violent behaviors: probing the components of "threat/control-override" symptoms. Social Psychiatry and Psychiatric Epidemiology 33(suppl 1):S55–60, 1998Google Scholar
2. Swanson JW, Holzer CE, Ganju VK, et al: Violence and psychiatric disorder in the community: evidence from the epidemiologic catchment area surveys. Hospital and Community Psychiatry 41:761–770, 1990Abstract, Google Scholar
3. Eronen M, Angermeyer MC, Schulze B: The psychiatric epidemiology of violent behaviour. Social Psychiatry and Psychiatric Epidemiology 33(suppl 1):S13–23, 1998Google Scholar
4. Raine A, Stoddard J, Bihrle S, et al: Prefrontal glucose deficits in murderers lacking psychosocial deprivation. Neuropsychiatry, Neuropsychology, and Behavioral Neurology 11:1–7, 1998Medline, Google Scholar
5. Raine A, Buchsbaum M, LaCasse L: Brain abnormalities in murderers indicated by positron emission tomography. Biological Psychiatry 42:495–508, 1997Crossref, Medline, Google Scholar
6. Kotler M, Barak P, Cohen H, et al: Homicidal behavior in schizophrenia associated with a genetic polymorphism determining low catechol O-methyltransferase (COMT) activity. American Journal of Medical Genetics 88:628–633, 1999Crossref, Medline, Google Scholar
7. Frankle W, Shera D, Berger-Hershkowitz H, et al: Clozapine-associated reduction in arrest rates of psychotic patients with criminal histories. American Journal of Psychiatry 158:270–274, 2001Link, Google Scholar
8. Cuesta M, Peralta V, Zarzuela A: Effects of olanzapine and other antipsychotics on cognitive function in chronic schizophrenia: a longitudinal study. Schizophrenia Research 48:17–28, 2001Crossref, Medline, Google Scholar
9. Meltzer HY, McGurk SR: The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophrenia Bulletin 25:233–255, 1999Crossref, Medline, Google Scholar
10. Buckley P, Thompson P, Way L, et al: Substance abuse and clozapine treatment. Journal of Clinical Psychiatry 55(9 suppl B):114–116, 1994Google Scholar
11. Roy S, Herrera J, Parent M, et al: Violent and nonviolent schizophrenic patients: clinical and developmental characteristics. Psychological Reports 61:855–861, 1987Crossref, Medline, Google Scholar
12. Krakowski MI, Convit A, Jaeger J, et al: Neurological impairment in violent schizophrenic inpatients. American Journal of Psychiatry 146:849–853, 1989Link, Google Scholar
13. Adams JJ, Meloy JR, Moritz MS: Neuropsychological deficits and violent behavior in incarcerated schizophrenics. Journal of Nervous and Mental Disease 178:253–256, 1990Crossref, Medline, Google Scholar
14. Nestor PG: Neuropsychological and clinical correlates of murder and other forms of extreme violence in a forensic psychiatric population. Journal of Nervous and Mental Disease 180:418–423, 1992Crossref, Medline, Google Scholar
15. Foster HG, Hillbrand M, Silverstein M: Neuropsychological deficit and aggressive behavior: a prospective study. Progress in Neuropsychopharmacology and Biological Psychiatry 17:939–946, 1993Crossref, Medline, Google Scholar
16. Lapierre D, Braun CM, Hodgins S, et al: Neuropsychological correlates of violence in schizophrenia. Schizophrenia Bulletin 21:253–262, 1995Crossref, Medline, Google Scholar
17. Krakowski M, Czobor P, Carpenter MD, et al: Community violence and inpatient assaults: neurobiological deficits. Journal of Neuropsychiatry and Clinical Neuroscience 9:549–555, 1997Crossref, Medline, Google Scholar
18. Feinstein R, Plutchik R: Violence and suicide risk assessment in the psychiatric emergency room. Comprehensive Psychiatry 31:337–343, 1990Crossref, Medline, Google Scholar
19. National Incident-Based Reporting System. Clarksburg, WV, Federal Bureau of Investigation, Criminal Justice Information Services Division, 2000, pp 1–128Google Scholar
20. Mitrushina M, Boone K, D'Elia L: Handbook of Normative Data for Neuropsychological Assessment. New York, Oxford University Press, 1999Google Scholar
21. Heaton R: A Manual for the Wisconsin Card Sorting Test. Odessa, Fla, Psychological Assessment Resources, 1981Google Scholar
22. Harrison JE, Buxton P, Husain M, et al: Short test of semantic and phonological fluency: normal performance, validity, and test-retest reliability. British Journal of Clinical Psychology 39(pt 2):181–191, 2000Medline, Google Scholar
23. Morgan AB, Lilienfeld SO: A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review 20:113–136, 2000Crossref, Medline, Google Scholar
24. Andreasen NC, Paradiso S, O'Leary DS: "Cognitive dysmetria" as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin 24:203–218, 1998Google Scholar
25. Bilder RM, Goldman RS, Volavka J, et al: Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. American Journal of Psychiatry 159:1018–1028, 2002Link, Google Scholar
26. Lamb HR, Grant RW: The mentally ill in an urban county jail. Archives of General Psychiatry 39:17–22, 1982Crossref, Medline, Google Scholar
27. Manschreck TC, Maher BA, Winzig L, et al: Age disorientation in schizophrenia: an indicator of progressive and severe psychopathology, not institutional isolation. Journal of Neuropsychiatry and Clinical Neuroscience 12:350–358, 2000Crossref, Medline, Google Scholar
28. Convit A, O'Donnell J, Volavka J: Validity of self-reports of criminal activity in psychiatric inpatients. Journal of Nervous and Mental Disease 178:48–51, 1990Crossref, Medline, Google Scholar
29. Blackmore J: The relationship between self-reported delinquency and official convictions amongst adolescent boys. British Journal of Criminology 14:172–176, 1974Crossref, Google Scholar



