Effects of Divalproex Versus Lithium on Length of Hospital Stay Among Patients With Bipolar Disorder
Abstract
The medical records of all inpatients with bipolar disorder at the Connecticut Mental Health Center in 1997 were examined to compare length of stay for patients who began monotherapy with divalproex (27 treatment starts) and lithium (20 treatment starts). No statistically significant difference was found in length of stay (11.5±6.9 and 10.3±5.2 days for patients on divalproex and lithium, respectively) or other length-of-stay variables. Demographic variables, diagnostic variables, and dosages of neuroleptics and benzodiazepines used adjunctively were similar as well. Dosages and blood levels for divalproex and lithium were consistent with practice guidelines. Prospective randomized studies are needed to compare the cost-effectiveness of divalproex and of lithium in the treatment of bipolar disorder.
An important pharmacological treatment issue for patients with bipolar disorder is whether use of lithium or of divalproex is more cost-effective as the first-line treatment. Length of hospital stay has been reported to be one important parameter for determining the relative cost-effectiveness of treatments for bipolar disorder (1,2). Three studies have reported a shorter length of stay with use of divalproex (1,3,4), although methodological issues in these studies leave the question open. The objective of this retrospective study was to determine whether the reported advantage of using divalproex was reproducible.
Methods
Using an administrative database, we identified all patients who were admitted as inpatients at the Connecticut Mental Health Center in New Haven in 1997 and were diagnosed by the admitting psychiatrist as having bipolar disorder. From this group we selected all patients who began treatment with divalproex or lithium. Five patients were excluded from our analysis because they started two or more mood stabilizers simultaneously. To prevent outliers from biasing the results, we excluded four patients whose length of stay exceeded 31 days; three of these patients were treated with divalproex (hospital stays of 56 days, 68 days, and 88 days, respectively), and one with lithium (59 days).
After these exclusions our sample had 40 patients, six of whom started treatment with one of these drugs on more than one hospital admission: three patients started divalproex treatment twice, and one patient three times, and two patients started lithium treatment twice. Overall, then, our sample included 47 treatment starts—27 with divalproex and 20 with lithium. For three patients a second mood stabilizer was added after the initial start of treatment; one of these patients was taking divalproex, and two were taking lithium.
For each treatment start, length of hospital stay and admission diagnosis were obtained from the database. The following data were collected by chart review for each patient: the patient's weight in the first week of hospitalization; the medication start date; the date on which the mood stabilizer dosage reached the level it would be at discharge; and the dosages of mood stabilizer, along with any antipsychotics (in chlorpromazine equivalents) or benzodiazepines (in lorazepam equivalents) used adjunctively, on day 1, day 5, day 10, and the discharge day. For patients for whom blood levels of mood stabilizer were determined, the blood level measurement taken closest to the discharge day was recorded as well.
For continuous data, equality of variance was determined by the F test, and differences between groups were analyzed with two-tailed t tests for equal or unequal variance, as appropriate. Categorical variables were analyzed with the chi square test.
Results
As Table 1 shows, we did not find any statistically significant differences in length of stay after treatment start or other length-of-stay variables between treatment with lithium and treatment with divalproex for patients with bipolar disorder. The values for demographic and diagnostic variables as well as dosages of neuroleptics and benzodiazepines used adjunctively were also similar for the two treatment groups. The mean±SD dosages and blood levels, as available, for each group are also shown in the table.
The divalproex starting dosages averaged 15.3 mg/kg per day. In six treatment starts, the initial divalproex dosage was 20 mg/kg per day or more (data not shown). For these patients, mean total length of stay and mean length of stay after start of medication were 15.2±10.3 and 12.7±7.5 days, respectively—also very similar to the lithium group.
The type of bipolar episode that led to the patient's admission had no significant effect on relative length of stay. For example, length of stay after start of medication for manic episodes was 10.5±4.9 days for lithium compared with 14.2±7.6 days for divalproex, and 12.2±3.7 versus 11.1± 6.2 days for mixed episodes.
Discussion
Although previous studies have reported shorter hospital stays for patients treated with divalproex, we found no significant difference in any length-of-stay variable between patients treated with divalproex and those treated with lithium. Keck and associates (1) reported a 4.1 day advantage for divalproex, Frye and associates (3) a 7.4 day advantage, and Sajatovic and associates (4) a 2.8 day advantage.
Like previous studies, ours had a number of limitations, including those inherent in naturalistic or retrospective designs. Second, the diagnosis of record was made according to DSM-IV criteria, but no structured interviews were conducted, and therefore some ascertainment errors might have occurred. A third limitation is the possibility of a type II error because of the small sample size—and sample sizes were even smaller when manic, mixed, or depressed bipolar episodes were considered separately.
Our study also incorporated several advantages over previous studies. We identified every monotherapy start in the study interval, and we used several length-of-stay measures; previous studies reported only total length of stay. We minimized any cohort effect by limiting the study interval to one year. In two of the three previous studies that reported the enrollment interval (3,4), the treatment starts occurred over 63 and 24 months. The shorter length of stay in these studies for patients treated with divalproex could have been influenced by secular trends. For example, because divalproex was approved more recently, patients taking the drug may have been admitted later in the study interval, when length of stay was decreasing as a result of cost-containment efforts. Data in the study by Frye and colleagues (3) may have been collected before the managed care era. Our study also was based on a larger number of divalproex starts (N=27) than those of Frye and associates (N=5) and Sajatovic and associates (N=8), although the study by Keck and associates had more (N=42).
It is somewhat surprising that our study did not demonstrate a shorter length of stay for patients treated with divalproex, given this agent's shorter half-life and consequently the shorter time required to reach steady-state levels. It does not appear that divalproex dosing was inadequate. The dosage of 15.3 mg/kg per day is similar to one study's recommended initial dosage of 15 mg/kg per day (5), but lower than another's recommendation of 20 mg/kg per day (6). None of the previous studies reported the divalproex dosages that were actually achieved in practice, so we cannot compare ours with others (1,3,4).
The divalproex dosages prescribed for the patients in our study did produce therapeutic blood levels; because these levels were slightly higher than the levels reported in previous studies (3,4), the difference in length of stay between our study and the previous studies cannot be attributed to drug levels.
The differences between previous findings and ours do not appear to be the result of lithium dosing, use of adjunctive medication, or demographic or clinical characteristics. The initial lithium dosages in our study were somewhat higher than the traditional starting dosage of 600 to 900 mg per day. Our facility does not use rapid loading for lithium. The dosages may have been higher as a result of physicians' prescribing an optimal dose at the outset, as the maintenance dose was known from previous treatment experience (5).
Like the divalproex blood levels, the lithium levels on average were in the lower part of the recommended range. Patients' clinical characteristics were similar for divalproex and lithium starts, as were dosages of adjunctive medications at all four measurement points.
One possible reason for our finding no difference in length of stay between lithium and divalproex is adverse selection for divalproex. Because it is a relatively new mood stabilizer, divalproex could have been tried with more severely ill or treatment-resistant patients, even though the lifetime number of hospitalizations and the Global Assessment of Functioning scores over the past year were similar in the two groups.
Recently Goldberg and coworkers (7) reported comparable time to remission for patients with bipolar disorder treated with rapid titration of divalproex, lithium, or carbamazepine. To the extent that time to remission correlates with length of stay, these data are consistent with ours.
Conclusions
In a cost-effectiveness comparison of divalproex and lithium, shorter length of stay has been proposed as an important balancing factor for divalproex's higher acquisition cost (1). At our hospital the length of stay of patients with bipolar disorder treated with divalproex was similar to that of patients treated with lithium. Prospective randomized studies are needed to explore more definitively the cost-effectiveness of prescribing divalproex or lithium in the treatment of patients with bipolar disorder.
Acknowledgments
This research was supported by U.S. Public Health Service grant MH-54446. The authors thank Roy Money and Michael Levine at the Connecticut Mental Health Center and Charles Hoadley, Joan Forrester, and Cheryl Croll at the Connecticut Department of Mental Health and Addiction Services for assistance with the database queries.
The authors are affiliated with the treatment research program at the Connecticut Mental Health Center and the division of services research and treatment outcomes in the department of psychiatry of the Yale University School of Medicine in New Haven, Connecticut. Send correspondence to Dr. Woods, Department of Psychiatry, Yale University School of Medicine, 34 Park Street, New Haven, Connecticut 06508 (e-mail, [email protected]).
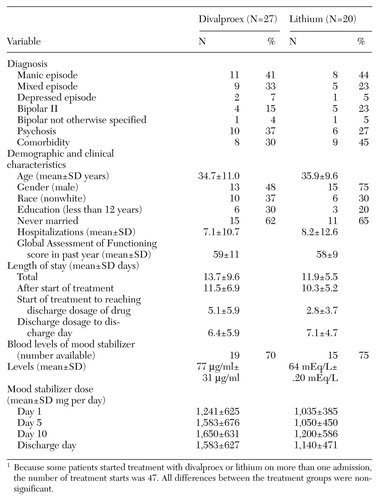 |
Table 1. Comparison of length of stay and other variables for inpatients with bipolar disorder starting treatment with divalproex and lithium1
1 Because some patients started treatment with divalproex or lithium on more than one admission, the number of treatment starts was 47All differences between the treament groups were non-significant.
1. Keck PE, Nabulsi AA, Taylor LJ, et al: A pharmacoeconomic model of divalproex vs lithium in the acute and prophylactic treatment of bipolar I disorder. Journal of Clinical Psychiatry 57:213-222, 1996Medline, Google Scholar
2. Baker CB, Woods SW, Sernyak MJ: Cost-effectiveness of divalproex versus lithium (ltr). Journal of Clinical Psychiatry 58:363-364,1997Crossref, Medline, Google Scholar
3. Frye MA, Altshuler LL, Szuba MP, et al: The relationship between antimanic agent for treatment of classic or dysphoric mania and length of hospital stay. Journal of Clinical Psychiatry 57:17-21, 1996Medline, Google Scholar
4. Sajatovic M, Gerhart C, Semple W: Association between mood-stabilizing medication and mental health resource use in the management of acute mania. Psychiatric Services 48:1037-1041, 1997Link, Google Scholar
5. Bowden CL: Dosing strategies and time course of response to antimanic drugs. Journal of Clinical Psychiatry 57(suppl 13):4-9, 1996Medline, Google Scholar
6. Keck PE, McElroy SL, Tugrul KC, et al: Valproate oral loading in the treatment of acute mania. Journal of Clinical Psychiatry 54:305-308, 1993Medline, Google Scholar
7. Goldberg JF, Garno JL, Leon AC, et al: Rapid titration of mood stabilizers predicts remission from mixed or pure mania in bipolar patients. Journal of Clinical Psychiatry 59:151-158, 1998Crossref, Medline, Google Scholar



