Augmenting Evidence-Based Care With a Texting Mobile Interventionist: A Pilot Randomized Controlled Trial
Abstract
Objective:
This study aimed to evaluate the feasibility and clinical utility of training intensive psychiatric community care team members to serve as “mobile interventionists” who engage patients in recovery-oriented texting exchanges.
Methods:
A 3-month pilot randomized controlled trial was conducted to compare the mobile interventionist approach as an add-on to assertive community treatment (ACT) versus ACT alone. Participants were 49 individuals with serious mental illness (62% with schizophrenia/schizoaffective disorder, 24% with bipolar disorder, and 14% with depression). Clinical outcomes were evaluated at baseline, posttreatment, and 6-month follow-up, and satisfaction was evaluated posttreatment.
Results:
The intervention appeared feasible (95% of participants assigned to the mobile interventionist arm initiated the intervention, texting on 69% of possible days and averaging four messages per day), acceptable (91% reported satisfaction), and safe (no adverse events reported). Exploratory posttreatment clinical effect estimations suggested greater reductions in the severity of paranoid thoughts (Cohen’s d=–0.61) and depression (d=–0.59) and improved illness management (d=0.31) and recovery (d=0.23) in the mobile interventionist group.
Conclusions:
Augmentation of care with a texting mobile interventionist proved to be feasible, acceptable, safe, and clinically promising. The findings are encouraging given the relative ease of training practitioners to serve as mobile interventionists, the low burden placed on patients and practitioners, and the simplicity of the technology. The technical resources are widely accessible to patients and practitioners, boding well for potential intervention scalability. When pandemics such as COVID-19 block the possibility of in-person patient-provider contact, evidence-based texting interventions can serve a crucial role in supporting continuity of care.
HIGHLIGHTS
This is the first pilot randomized controlled trial examining the feasibility of augmenting assertive community treatment (ACT) with a texting mobile interventionist.
The texting intervention proved to be feasible, acceptable, and safe.
Preliminary findings suggest better symptom management and recovery outcomes for individuals randomly assigned to receive texting-augmented ACT than for those receiving ACT alone.
When in-person patient-provider contact is difficult or impossible (e.g., distance from a clinic, social distancing restrictions imposed as a result of the COVID-19 pandemic), evidence-based texting interventions may serve a crucial role in supporting continuity of mental health care.
Technology is helping to reshape mental health services. Wearable sensors, natural language processing, machine learning, augmented reality, and other innovations may usher in an era when patient monitoring, illness detection, and interventions are deployed with reduced need for time-sensitive involvement of human providers (1). If such approaches prove to be feasible and clinically useful, these cutting-edge technologies would provide opportunities for dramatic innovation of mental health services. But more pressingly and pragmatically, innovative use of existent and widely accessible technologies may already enable us to expand the reach and potency of evidence-based services (2, 3).
Clinic-based services models often fail to reach those in need of care (4). When individuals with serious mental illness experience symptom exacerbations, they may find going to a treatment center to be challenging. Those who seek care must contend with the challenges that are intrinsic to clinic-based services, including distance from their residence, limited hours of operation, stigma associated with going to a mental health center, apprehension about interacting with others in group settings, gaps between meetings, and varying quality of services (5–7). The recent COVID-19 global pandemic has made starkly clear that in-person services may be abruptly discontinued. Mobile technologies may help overcome these barriers by providing opportunities to deliver mental health services to patients in their communities (8).
Approximately seven billion mobile phones are in use worldwide (9). Access to mobile phones is one of the few areas where the resource gap between people with serious mental illness and the general population is almost nonexistent; community-based studies consistently find that most people with severe psychopathology have mobile phones and are open to using their devices as recovery support tools (10–12). Text messaging is a simple, feasible, and technically robust digital communication modality that has emerged as one of the most widely used health technology resources on the planet (13). People with serious mental illness use short message service text messages at rates similar to those of the general population (14, 15). A recent meta-analysis suggested that use of a range of texting interventions is feasible among people with serious mental illness (16). A majority of these approaches involved automated or semiautomated messaging rather than targeted personalized interventions.
Our group developed a technology-assisted illness management strategy that is delivered by a texting “mobile interventionist.” In this model, a trained community-based mental health worker engages people with serious mental illness in daily recovery-oriented texting exchanges tailored to their individual needs. Unlike digital health apps or therapeutic websites that can be accessed only on smartphones with computational capacities, practically all mobile phones in use today can technically support this type of texting intervention. Furthermore, the model incorporates the expertise of a clinical workforce that can be trained to deliver targeted patient-centered mobile health (mHealth). Repurposing rather than replacing existing clinical personnel may be more appealing to agencies that are looking to modernize their models of care and may provide a more seamless transition to remote care. A previous proof-of-concept study found the mobile interventionist approach was engaging to people with serious mental illness and that it facilitated a strong patient-provider therapeutic alliance that was rated as superior to in-person care (17). We report on the next phase in this program of research: a pilot randomized controlled trial designed to evaluate real-world feasibility and acceptability and to estimate clinical effects of the mobile interventionist approach when added to an existing practice.
Methods
We conducted an assessor-blind, two-arm, randomized controlled trial between December 2017 and October 2019 in partnership with assertive community treatment (ACT) teams providing services to individuals with serious mental illness in the Midwest and Pacific Northwest regions of the United States. The study was approved by the institutional review boards of the University of Washington and Dartmouth College. The research was monitored by an independent safety monitoring board at the University of Washington’s School of Medicine. Participants provided informed consent. All participants were receiving ACT services and were randomly assigned to one of two treatment arms: mobile interventionist (ACT augmented with mobile phone texting) or treatment as usual (standard ACT). Interventions were deployed for 3 months. We conducted assessments at baseline (0 months), posttrial (3 months), and follow-up (6 months). Participants were compensated for completing assessments ($30 per assessment, plus reimbursement for travel). The study is registered at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT03062267).
Participants
Participants were identified by ACT teams at the study site and screened by research staff. Inclusion criteria were chart diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, or major depressive disorder; 18 years or older; currently receiving ACT services; a rating of 3 or higher on one of three items from the Recovery Assessment Scale indicating need for additional support (“I don’t have the skills to stop my symptoms from getting worse,” “I want to make changes need to help my recovery,” and “I am not able to handle my symptoms on my own most of the time”); and ownership of a mobile phone with data plan. Exclusion criteria were hearing, vision, or motor impairment affecting operation of a mobile device, determined by using the candidate’s device for screening; and less than fourth grade English reading ability, determined by using the reading section of the Wide Range Achievement Test, fourth edition (18).
Random Assignment and Blinding
The study statistician created a computer-generated random assignment list organized in blocks of four participants (3:1 ratio of mobile interventionist versus treatment as usual, respectively). Study assessors were blinded to group assignment.
Intervention Description
All participants received ACT services, which have been described extensively (19, 20). ACT is an intensive team-based treatment model designed to provide comprehensive psychiatric, rehabilitation, and support services to people with serious mental illness residing in the community (21–23). In the experimental arm, the mobile intervention was tested as an add-on to ACT. Practitioners embedded in ACT teams were trained to serve as mobile interventionists in a half-day training seminar. They participated in weekly supervision calls with a clinical psychologist trained in the intervention model. Interventionists were given guidance on how to introduce the intervention to clients, establish rapport via text, and optimize engagement over time. Interventionists were encouraged to infuse their approach with their own “personal touch” so that the texts did not seem bland or robotic. They were instructed to provide daily texting support over 12 weeks to clients assigned to them. The study involved seven ACT teams. Each team had one mobile interventionist. Study participants allocated to the experimental arm received texting services from their assigned mobile interventionist but did not receive any other services from this individual for the duration of the study. Participants received services from only one ACT team and were not exposed to other team interventionists.
At a baseline visit, mobile interventionists met with each participant to build rapport, review the treatment model, identify target areas, and complete a training session regarding basic phone functions (e.g., charging the battery, making calls) and texting. After this visit, mobile interventionists provided daily support via text messages during the ACT team’s hours of operation, typically Monday–Friday between 9 a.m. and 5 p.m. (after-hours ACT crisis management resources continued to be available). During this period, mobile interventionists continued as members of the ACT team, regularly consulted with other team members on client issues, and provided weekly reports in person or via secure e-mail. If clients indicated a crisis via text message (despite being directed that the intervention was not meant as a time-sensitive tool to communicate urgent matters), the mobile interventionists would relay the information to their ACT team colleagues, who would engage in standard crisis management protocols.
The maximum intervention dose was three “exchanges,” wherein an exchange was defined as a cluster of thematically connected back-and-forth messages between mobile interventionist and participant. Texting strategies included reminders (e.g., appointments, prescription refills), information provision (e.g., psychoeducation, links to regional events and resources), cognitive challenges (e.g., restructuring dysfunctional beliefs about voices, questioning the validity of self-sabotaging automatic beliefs), self-monitoring and self-reflection (e.g., guidance on self-evaluation of affect, journaling of symptomatic experiences), relaxation techniques (e.g., diaphragmatic breathing, guided imagery), social skills training (e.g., initiating conversations, maintaining eye contact), supportive messages (e.g., affirmations, inspirational quotes), and in vivo instruction (e.g., prescheduled real-time support as the patient attempted a new activity).
The intervention continued for 12 weeks for all participants. To avoid abrupt discontinuation, mobile interventionists led a tapered termination during the last 2 weeks of this period. At the end of treatment, the mobile interventionist and participant met to review gains in their personal goals, summarize the helpful tools acquired, and discuss how the participant could use skills independently.
Measures
Feasibility.
Feasibility was assessed on the basis of the number of participants in the experimental arm who initiated texting, the number of days in which texting occurred, the average number of texts sent and received per day on days in which texting occurred, and the number of text messages sent and received per participant.
Acceptability.
Treatment acceptability was assessed with a five-item measure rated on a 7-point scale adapted from previous research examining mHealth for people with serious mental illness (17, 24). All items appear in Figure 1.
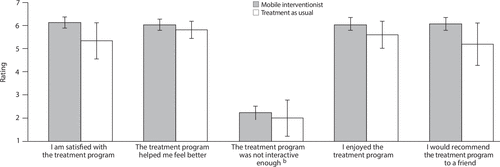
FIGURE 1. Ratings of treatment acceptability measures among patients receiving mobile interventionist treatment (N=37) and treatment as usual (N=12)a
aTreatment acceptability was assessed with a five-item measure rated on a 7-point scale, with higher scores indicating greater acceptability, except where noted. Vertical lines indicate standard error.
bLower scores on this item indicate greater acceptability.
Clinical effects.
Depression and anxiety were assessed with the Beck Depression Inventory–II (BDI-II) (25) and Beck Anxiety Inventory (BAI) (26), both of which include 21 items rated on a 4-point scale, summed for total severity scores. Psychosis was assessed with the 17-item Psychotic Symptom Rating Scales (PSYRATS) (27). The PSYRATS includes dimensions of auditory hallucinations and delusions that are rated on a 5-point scale. Persecutory ideation was assessed with the Green et al. Paranoid Thought Scale (GPTS)–persecution subscale (28), a 16-item questionnaire rated on a 5-point scale that is summed to generate a total score. Illness management was measured with the Illness Management Recovery Scale (IMRS) (29), a 15-item self-report measure that captures different aspects of illness management, including coping with symptoms, medication use, utilization of social and familial support, and relapse prevention planning. IMRS items are scored on a 5-point scale and summed to represent a composite score. Recovery was assessed by using the Recovery Assessment Scale (RAS) (30, 31), a 24-item, 5-point Likert scale assessing self-rated recovery.
Adverse events were monitored by site clinical staff who interacted with participants regularly and had access to agencywide reports on the electronic health record. Participant issues were discussed during weekly calls with the study clinical supervisor and reported to the study team during project management calls. Negative events (e.g., marked deterioration in physical health, hospitalization, death) were evaluated by the investigative team and the study’s safety monitoring board to determine whether they might be associated with participation in the study.
Because this study focused on piloting the intervention and estimating preliminary effect sizes for a larger-scale trial, rather than robustly testing effectiveness, our sample size was designed to examine real-world feasibility. We had 0.80 power to detect only very large clinical effect sizes of d≥0.94.
Analytic Approach
Feasibility was modeled descriptively on the basis of frequency of texting. We used an intent-to-treat approach in preliminary outcomes analyses. Measures were analyzed by using linear regression, with treatment condition and baseline values included as covariates. Participants with missing follow-up measures were included by using multiple imputation, which reduces bias in effect estimates (32). Attrition analyses were used to evaluate rates and predictors of missingness.
Results
The study enrolled 51 individuals, but 49 were included in analyses (see study CONSORT diagram in the online supplement). Table 1 presents the demographic and clinical characteristics of participants. The average age of participants was 44.8 years, 55% (N=27) were male, 52% white, 26% Black/African American, 17% multiracial, and 5% (N=2) other racial groups. Thirty-nine percent of the sample were patients with schizophrenia, 22% with schizoaffective disorder, 24% with bipolar disorder, and 14% with major depressive disorder. Participants did not differ on demographic variables between groups. Of the 49 patients, 43 (88%) provided 3-month follow-up data, and 42 (86%) provided 6-month follow-up data. Follow-up rates did not differ between the treatment conditions and were not significantly associated with baseline demographic or clinical measures.
| Total (N=49) | Mobile interventionist (N=37) | Treatment as usual (N=12) | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % |
| Female | 22 | 45 | 15 | 41 | 7 | 58 |
| White | 24 | 52 | 17 | 49 | 7 | 64 |
| Black/African American | 12 | 26 | 10 | 29 | 2 | 18 |
| Multiracial | 8 | 17 | 6 | 17 | 2 | 18 |
| Hispanic/Latinx | 6 | 13 | 3 | 8 | 3 | 25 |
| Schizophrenia | 19 | 39 | 17 | 46 | 2 | 17 |
| Schizoaffective disorder | 11 | 22 | 9 | 24 | 2 | 17 |
| Major depressive disorder | 7 | 14 | 4 | 11 | 3 | 25 |
| Bipolar disorder | 12 | 24 | 7 | 19 | 5 | 42 |
| M | SD | M | SD | M | SD | |
| Age | 44.8 | 11.2 | 45.4 | 11.1 | 43.3 | 12.0 |
| Education (years) | 12.9 | 3.0 | 12.8 | 2.4 | 13.1 | 4.5 |
| Lifetime hospitalizations | 2.8 | 3.0 | 2.8 | 3.4 | 2.7 | 1.7 |
| Past-year hospitalizations | .4 | .6 | .4 | .6 | .3 | .5 |
TABLE 1. Baseline demographic and clinical characteristics of individuals with serious mental illnessa
Patients randomly assigned to the mobile interventionist arm had significantly higher PSYRATS scores (difference=14.4, t=2.32, df=47, p=0.03) and GPTS scores (difference=12.7, t=2.05, df=47, p=0.047) (Table 2). To control for this difference, baseline PSYRATS scores were included as covariates in analyses, which obviated the need to control for baseline GPTS scores due to the strong correlation between these measures (r=0.60, df=47, p<0.001).
| Mobile interventionist | Treatment as usual | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | M | SD | M | SD | t | df | p | Cohen's d | 95% CI |
| PSYRATSb | |||||||||
| Baseline | 19.6 | 20.4 | 5.2 | 11.2 | 2.32 | 47 | .03 | ||
| 3 month | 15.1 | 16.7 | 6.2 | 14.4 | .18 | 34 | .86 | .06 | −.61, .73 |
| 6 month | 17.9 | 19.1 | .6 | 1.9 | 1.63 | 39 | .11 | .62 | −.15, 1.40 |
| GPTS–persecutory ideation subscalec | |||||||||
| Baseline | 38.4 | 20.6 | 25.7 | 10.6 | 2.05 | 47 | .047 | ||
| 3 month | 36.6 | 19.6 | 41.5 | 20.7 | –1.80 | 30 | .08 | −.61 | –1.31, .08 |
| 6 month | 36.9 | 20.5 | 22.5 | 14.2 | 1.04 | 36 | .30 | .40 | −.38, 1.17 |
| BDI-IId | |||||||||
| Baseline | 18.5 | 13.7 | 18.5 | 11.4 | .00 | 47 | 1.00 | ||
| 3 month | 17.2 | 11.3 | 23.4 | 12.4 | –2.05 | 30 | .049 | −.63 | –1.25, −.002 |
| 6 month | 15.5 | 11.2 | 14.8 | 10.3 | .22 | 33 | .83 | .07 | −.57, .71 |
| BAIe | |||||||||
| Baseline | 21.9 | 14.0 | 18.9 | 13.3 | .66 | 47 | .52 | ||
| 3 month | 19.7 | 12.9 | 22.0 | 15.5 | −.49 | 37 | .63 | −.17 | −.87, .53 |
| 6 month | 20.7 | 12.5 | 18.3 | 9.9 | .09 | 36 | .93 | .03 | −.63, .69 |
| IMRSf | |||||||||
| Baseline | 48.8 | 9.0 | 48.8 | 7.5 | .03 | 47 | .98 | ||
| 3 month | 47.8 | 9.7 | 43.3 | 6.5 | .58 | 31 | .57 | .23 | −.59, 1.05 |
| 6 month | 51.6 | 9.6 | 51.6 | 9.8 | .11 | 32 | .92 | .04 | −.68, .76 |
| RASg | |||||||||
| Baseline | 89.8 | 17.3 | 91.8 | 14.6 | −.35 | 47 | .73 | ||
| 3 month | 92.9 | 15.5 | 90.0 | 13.8 | .85 | 34 | .40 | .24 | −.33, .80 |
| 6 month | 94.4 | 14.9 | 93.8 | 13.1 | .43 | 32 | .67 | .15 | −.55, .85 |
TABLE 2. Clinical outcomes of individuals with serious mental illness (N=49) at 3 and 6 months, by treatment groupa
Feasibility
No adverse events were reported in either intervention arm. Nearly all participants assigned to the experimental arm (N=35 of 37, 95%) commenced treatment by sending at least one text message. Those who engaged recorded a mean±SD of 41.3±17.1 days with any text exchanges between the patient and interventionist. This figure represents approximately 69% of the days in which texting could have occurred. Patients sent 4.0±3.9 daily messages and received a mean of 3.6±1.5 daily messages from the interventionist. Over the study period, participants sent 174.7±164.5 text messages and received 157.9±111.6.
Acceptability
Participants reported the mobile interventionist approach to be acceptable. A majority reported that they were satisfied with the intervention (N=29 of 32, 91%), that it made them feel better (N=30 of 32, 94%), and that they would recommend it to a friend (N=27 of 31, 87%). Posttreatment satisfaction ratings were similar across arms (mobile interventionist, 30.1±6.1; treatment as usual, 28.2±6.2) with no significant difference between the two (t=0.55, df=11.5, p=0.59) (Figure 1).
Clinical Effects
Postintervention (3-month) outcomes controlling for baseline values reflected medium effect sizes in the severity of persecutory ideation (GPTS d=–0.61) and depression (BDI-2 d=–0.63) and small effects indicating greater illness management (IMRS d=0.23) and recovery (RAS d=0.24) in the mobile interventionist group (Table 2). PSYRATS and BAI scores were more stable over time for both conditions, and between-condition effects at 3 months were smaller for these measures (PSYRATS d=0.06; BAI d=−0.17). Between-group effects were nonsignificant, as expected due to sample size. Some clinical outcomes worsened for the treatment-as-usual condition from baseline to 3 months, while the mobile interventionist group remained more stable (Figure 2). Follow-up (6-month) assessments revealed that these gains were not maintained after the intervention was discontinued.
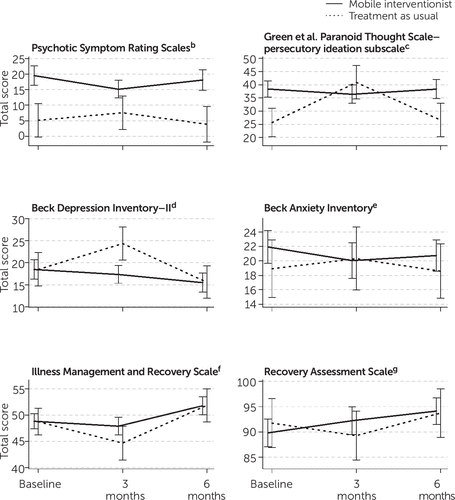
FIGURE 2. Clinical outcome measures among patients receiving mobile interventionist treatment (N=37) and treatment as usual (N=12)a
aVertical lines indicate standard error.
bScores range from 0 to 68, with higher scores indicating more severe symptomatology.
cScores range from 16 to 80, with higher scores indicating more severe symptomatology.
dScores range from 0 to 63, with higher scores indicating more severe symptomatology.
eScores range from 0 to 63, with higher scores indicating more severe symptomatology.
fScores range from 15 to 75, with higher scores indicating better illness management.
gScores range from 24 to 120, with higher scores indicating better recovery.
Discussion
This article reports on the feasibility, acceptability, and preliminary effectiveness of integrating clinical texting into ACT services for individuals with serious mental illness. Results demonstrate feasibility (95% of participants commenced the intervention, exchanging messages on 69% of possible days, averaging four texts per day), acceptability (91% reported satisfaction), and safety (no adverse events were reported). The mobile interventionist approach produced greater clinical stability or improvements relative to treatment as usual, including medium reductions in depression (d=–0.59) and persecutory ideation (d=–0.61) and small improvements in illness management (d=0.31) and self-reported recovery (d=0.23). The advantage for the texting condition diminished at 6-month follow-up, suggesting that access to the mobile interventionist must persist to maintain treatment effects.
Multiple studies have demonstrated the feasibility of texting among people with serious mental illness (33–35). The mobile interventionist approach demonstrates that after a brief community-based training, mental health workers can provide ongoing psychosocial interventions via messaging. This personalized, adaptive, and team-integrated approach is a novel use of texting in this population. The model blends key elements of person-delivered care and mobile health strategies to maximize treatment engagement, potency, and reach. But unlike in standard in-person care that involves seeing clients sequentially, a mobile interventionist can engage with multiple patients in parallel (e.g., several separate exchanges per hour) and asynchronously, potentially expanding reach and efficiency of care.
This study had several limitations. Analyses were not powered to detect statistically significant differences between groups. Study conditions were imbalanced to obtain feasibility and study protocol acceptability data by allocating more participants to the experimental intervention. Although results of this study are promising, larger fully powered trials are required to address questions related to intervention effectiveness. All participants in the trial owned a mobile device with a data plan. Results may have differed if samples had included individuals who did not own a phone, a small subpopulation (10–12). Finally, it is possible that additional daily contact with another person is what produced positive patient outcomes. Future research employing a comparison arm with simpler supportive messaging may help elucidate the potent elements of the intervention.
Conclusions
ACT is an established model of care (23), but illness management and other psychosocial functioning outcomes have not been fully achieved by ACT alone (36, 37). Augmenting ACT with a mobile interventionist is feasible and may amplify treatment effects, a high priority for ACT funders, administrators, and practitioners (38). The findings of this study are encouraging given the relative ease of training ACT staff to serve as interventionists and supervising them, the low burden placed on both patients and practitioners over the intervention period, and the simplicity of the technology used. The technological resources employed in the intervention—any type of mobile phone, nationwide mobile-cellular infrastructure, standard data plans—are already widely accessible, which bodes well for the potential scalability of the approach. If future research replicates our findings in larger samples supporting the clinical utility of the intervention, the treatment could be disseminated broadly and rapidly. Finally, this study was completed before the global COVID-19 pandemic, which led to sweeping social distancing practices and, in many instances, systemic collapses in capacity to deliver standard mental health services. In an era when in-person patient-provider contact may be discontinued, evidence-based texting interventions can serve a crucial role in supporting continuity of care.
1 : A technology-assisted life of recovery from psychosis. NPJ Schizophr 2019; 5:15Crossref, Medline, Google Scholar
2 : Technology in mental health: creating new knowledge and inventing the future of services. Psychiatr Serv 2017; 68:107–108Link, Google Scholar
3 : The future is in our hands: the role of mobile phones in the prevention and management of mental disorders. Aust N Z J Psychiatry 2013; 47:111–113Crossref, Medline, Google Scholar
4 : Unmet need for mental health care in schizophrenia: an overview of literature and new data from a first-admission study. Schizophr Bull 2009; 35:679–695Crossref, Medline, Google Scholar
5 : Implementing evidence-based practices for people with schizophrenia. Schizophr Bull 2009; 35:704–713Crossref, Medline, Google Scholar
6 : How stigma interferes with mental health care. Am Psychol 2004; 59:614–625Crossref, Medline, Google Scholar
7 : Illness management and recovery: a review of the literature. Psychiatr Serv 2014; 65:171–179Link, Google Scholar
8 : Mobile technologies in the study, assessment, and treatment of schizophrenia. Schizophr Bull 2012; 38:384–385Crossref, Medline, Google Scholar
9 Measuring the Information Society Report, 2018. Geneva, , 2018Google Scholar
10 : Mobile technologies among people with serious mental illness: opportunities for future services. Adm Policy Ment Health Ment Health Serv Res 2013; 40:340–343Crossref, Medline, Google Scholar
11 : Use of mobile phones, computers and internet among clients of an inner-city community psychiatric clinic. J Psychiatr Pract 2014; 20:94–103Crossref, Medline, Google Scholar
12 : Mobile phone ownership and endorsement of “mHealth” among people with psychosis: a meta-analysis of cross-sectional studies. Schizophr Bull 2016; 42:448–455Crossref, Medline, Google Scholar
13 : Mobile text messaging for health: a systematic review of reviews. Annu Rev Public Health 2015; 36:393–415Crossref, Medline, Google Scholar
14 : A survey of online and mobile technology use at peer support agencies. Psychiatr Q 2018; 89:539–548Crossref, Medline, Google Scholar
15 : Use of mobile and computer devices to support recovery in people with serious mental illness: survey study. JMIR Ment Health 2019; 6:
16 : The use of text messaging to improve clinical engagement for individuals with psychosis: systematic review. JMIR Ment Health 2020; 7:
17 : Remote “hovering” with individuals with psychotic disorders and substance use: feasibility, engagement, and therapeutic alliance with a text-messaging mobile interventionist. J Dual Diagn 2014; 10:197–203Crossref, Medline, Google Scholar
18 : Wide Range Achievement Test, 4th ed (WRAT-4) Professional Manual. Lutz, FL, , 2004Google Scholar
19 Assertive Community Treatment (ACT) Evidence Based Practices (EBP) Kit. Rockville, MD, Substance Abuse and Mental Health Service Administration, 2008. https://store.samhsa.gov/product/Assertive-Community-Treatment-ACT-Evidence-Based-Practices-EBP-KIT/sma08-4344Google Scholar
20 Stein LI, Santos AB: Assertive Community Treatment of Persons With Severe Mental Illness. New York, W.W. Norton, 1998Google Scholar
21 : The effectiveness of assertive community treatment for homeless populations with severe mental illness: a meta-analysis. Am J Psychiatry 2007; 164:393–399Link, Google Scholar
22 : Experimental studies of the program of assertive community treatment (pact): a meta-analysis. J Disabil Policy Stud 1999; 10:53–89Crossref, Google Scholar
23 , Mueser KT, et al: Assertive community treatment for people with severe mental illness. Disease Management and Health Outcomes 2001; 9:141–159Crossref, Google Scholar
24 : Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull 2014; 40:1244–1253Crossref, Medline, Google Scholar
25 : Beck Depression Inventory II. San Antonio, TX, , 1996Google Scholar
26 : Beck Anxiety Inventory. San Antonio, TX, , 1990Google Scholar
27 : Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med 1999; 29:879–889Crossref, Medline, Google Scholar
28 : Measuring ideas of persecution and social reference: the Green et al Paranoid Thought Scales (GPTS). Psychol Med 2008; 38:101–111Crossref, Medline, Google Scholar
29 : Illness management and recovery (IMR) scales; in Measuring the Promise: A Compendium of Recovery Measures. Edited by Campbell-Orde T, Chamberlin J, Carpenter J, . Cambridge, MA, , 2005Google Scholar
30 : Recovery as a psychological construct. Community Ment Health J 1999; 35:231–239Crossref, Medline, Google Scholar
31 : Examining the factor structure of the recovery assessment scale. Schizophr Bull 2004; 30:1035–1041Crossref, Medline, Google Scholar
32 : Missing data in alcohol clinical trials: a comparison of methods. Alcohol Clin Exp Res 2013; 37:2152–2160Crossref, Medline, Google Scholar
33 : A comparison of telephone and texting interventions for persons with schizophrenia spectrum disorders. Issues Ment Health Nurs 2014; 35:323–329Crossref, Medline, Google Scholar
34 : ITAREPS: information technology aided relapse prevention programme in schizophrenia. Schizophr Res 2008; 98:312–317Crossref, Medline, Google Scholar
35 : Mobile assessment and treatment for schizophrenia (MATS): a pilot trial of an interactive text-messaging intervention for medication adherence, socialization, and auditory hallucinations. Schizophr Bull 2012; 38:414–425Crossref, Medline, Google Scholar
36 : Use of intensive case management to reduce time in hospital in people with severe mental illness: systematic review and meta-regression. BMJ 2007; 335:336Crossref, Medline, Google Scholar
37 : A meta-analysis of the effectiveness of mental health case management over 20 years. Psychiatr Serv 2000; 51:1410–1421Link, Google Scholar
38 Olmstead v LC, 527 US 581Google Scholar



