Interventions to Improve Metabolic Risk Screening Among Adult Patients Taking Antipsychotic Medication: A Systematic Review
Abstract
Objective:
Antipsychotic use is associated with elevated cardiometabolic risk. Guidelines for metabolic risk screening of individuals taking antipsychotics have been issued, but with little uptake into clinical practice. This review systematically assessed interventions that address this guideline-to-practice gap and described their quality, improvement strategies, and effect on screening rates.
Methods:
Studies of interventions that addressed metabolic risk screening of adult patients taking antipsychotics, published from inception to July 2018, were selected from MEDLINE, Embase, PsycINFO, CINAHL, and Cochrane Reviews databases. Information was extracted on study characteristics; improvement strategies at the provider, patient, and system levels; and screening rates in the intervention and comparison groups.
Results:
The review included 30 complex interventions that used between one and nine unique improvement strategies. Social influence to shift provider and health organization culture to encourage metabolic risk screening was a common strategy, as were clinical prompts and monitoring tools to capture provider attention. Most studies were deemed at high risk of bias. Relative to comparison groups, the interventions were associated with an increase in median screening rates for glucose (28% to 65%), lipids (22% to 61%), weight (19% to 67%), and blood pressure (22% to 80%).
Conclusions:
This knowledge synthesis points to shortcomings of current interventions to improve antipsychotic metabolic risk screening, both in quality and in outcomes. Findings may be used to inform the design of future programs. Additional interventions are needed to address the current guideline-to-practice gap, in which approximately one-third of patients are unscreened for metabolic risk.
HIGHLIGHTS
Clinical uptake of antipsychotic metabolic risk screening guidelines has been limited, and interventions to improve risk screening have been implemented.
This systematic review identified 30 interventions that targeted antipsychotic metabolic risk screening and described the strategies used to improve risk screening at the provider, patient, and system levels, as well as the interventions’ effects.
Social influence to shift provider and health organization culture to encourage metabolic risk screening was a common strategy, as were clinical prompts and monitoring tools to capture provider attention.
Most interventions were successful and achieved screening of approximately two-thirds of patients; however, methodological limitations prevented firm conclusions regarding the true effect of the interventions.
In recent years, greater attention has been given to general medical comorbidities among individuals with severe mental illness, specifically metabolic and cardiovascular diseases (1, 2). This population is at risk of developing general medical problems for a myriad of reasons, including limited access to primary care (3), illness-related difficulty in acquiring adequate medical care (4, 5), social disadvantage (6), poor health behaviors (7), and propensity for metabolic dysregulation that may be inherent to psychotic disorders (8). Antipsychotic medication use, which is the mainstay of pharmacotherapy in this group, further increases cardiometabolic risk (9, 10).
Therefore, metabolic abnormalities are prevalent among individuals with severe mental illness. One review found that one in three individuals with schizophrenia and more than half of those receiving clozapine treatment were affected by the metabolic syndrome (11), a cluster of metabolic abnormalities linked with two- to threefold risk of cardiovascular mortality (12). In response to the high prevalence of cardiometabolic risk, several health authorities have published recommendations for risk screening. U.S. providers follow the guidance of the American Diabetes Association and American Psychiatric Association consensus paper, which recommends regular monitoring of specific metabolic measures for those prescribed antipsychotics, including weight, waist circumference, blood pressure (BP), fasting blood glucose, and a lipid profile (13). In the United Kingdom, the National Institute for Health and Clinical Excellence schizophrenia guidelines call for yearly physical health assessment, which includes a similar metabolic profile screen (14).
Despite the known elevated cardiometabolic risk among individuals with severe mental illness receiving antipsychotic medication and the presence of clear recommendations for risk screening, evidence suggests that many patients remain unscreened (15, 16). Mitchell et al. (17) conducted a review and meta-analysis of the effect of guideline publication on metabolic risk screening and found partial uptake of guidelines, with only 56% of patients being screened for glucose and 29% for lipids. In response to these findings, interventions that aim to improve metabolic risk screening have been implemented into practice; however, the literature describing these interventions has been only selectively reviewed. One review was limited to a single database and did not assess the effect of the interventions on metabolic risk screening rates or the quality of evidence of the included studies (18). Another review was comprehensive but included interventions that target metabolic risk screening among only a small subgroup of young people with psychosis (19). Neither of these reviews provided a detailed characterization of the improvement strategies that were used in the interventions.
Against this background, we aimed to systematically review interventions that aim to improve metabolic risk screening of adults taking antipsychotic medication. We also aimed to characterize the improvement strategies used by the interventions, describe the intervention effect on screening rates, and comment on their methodological rigor. The goal of this review was to summarize the existing literature to help inform the development of future programs that aim to optimize antipsychotic metabolic risk screening.
Methods
Overview
We conducted a systematic review of interventions that target metabolic risk screening of adult patients taking antipsychotic medication with the objective of improving screening rates to the level recommended by clinical guidelines. We aimed to categorize and describe the various improvement strategies used by the interventions and assess intervention quality and overall efficacy in increasing metabolic screening rates. This review was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20).
Search Strategy
Articles published in print or online from inception to July 11, 2018, were identified through electronic searches by using medical subject headings (MeSH) and keywords of MEDLINE (including Epub ahead of print, in-process, and other nonindexed citations), Embase, PsycINFO, CINAHL, and Cochrane Reviews. The search strategy was developed in collaboration with an information specialist (see online supplement for further details). Search terms related to antipsychotics included the MeSH terms and keywords “antipsychotic” or any known antipsychotic medication (e.g., clozapine). Metabolic monitoring search terms included “monitor,” “measure,” “track,” “metabolic,” “glucose,” “weight,” “blood pressure,” or “lipid.” Search terms for antipsychotics and search terms for metabolic monitoring were combined using the “AND” function. Bibliographies of articles and review articles were hand searched to identify primary articles that may have been missed in the initial search. Only published, peer-reviewed articles available in English were included. Two authors (O.C.M. and E.N.W.) independently completed the title-abstract search, followed by a full-text screen against a priori inclusion criteria. Any disagreements between the two were resolved by discussion and consensus.
Criteria for Study Selection
Inclusion criteria.
We included interventions that targeted metabolic risk screening among adult patients (ages ≥18) taking antipsychotic medication. Eligible interventions targeted screening practices of at least one of four metabolic measures: obesity-weight (body mass index [BMI] or waist circumference), glucose (blood glucose or HbA1C), lipids (total cholesterol, LDL cholesterol, HDL cholesterol, or triglycerides), and BP (systolic BP, diastolic BP, or both). Studies were not restricted to a minimum time duration for an intervention or to a diagnosis of a specific mental illness or type of antipsychotic medication exposure. Studies were required to have an experimental design in which the intervention was implemented into clinical practice, because studies that examined the effect of passive uptake of monitoring guidelines on metabolic screening rates have been reviewed elsewhere and were excluded from this review (17). When studies reported metabolic screening rates at multiple time points in the intervention group (e.g., 1 month postintervention or 3 months postintervention), we considered the latest time point available as the outcome for the review. In cases in which monitoring rates were reported for initiation of antipsychotic medications and ongoing use (maintenance), only the latter was considered. Studies of any design and clinical setting (inpatient or outpatient) were included.
We use the term “intervention group” for the experimental group in controlled designs and for the postintervention group in uncontrolled pre-post designs. Similarly, “comparison group” is used for the control group in controlled designs and for the preintervention group in uncontrolled pre-post designs. Electronic correspondence was sent to authors of articles that described eligible interventions but lacked reporting of either sample size or intervention or comparison group screening rates in order to make the review as exhaustive as possible following the recommendations for “best evidence synthesis” (21).
Exclusion criteria.
Studies describing interventions that do not target adult populations were excluded, as were studies examining metabolic screening of individuals taking psychotropic medications other than antipsychotics (for example, mood stabilizers and selective serotonin reuptake inhibitors), because guideline recommendations are specific for antipsychotics. Studies that described metabolic screening rates as self-reported by physicians were excluded.
Data Extraction and Classification
Data were extracted on the basis of criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (22), and data extraction was completed by two authors (O.C.M and L.R.L). Information on the study populations, including demographic factors, psychiatric diagnosis and type of antipsychotic medication, study setting, study design, and rates of metabolic screening in the intervention and the comparison groups, were extracted from the included studies. Strategies used by the intervention to improve metabolic risk screening (i.e., improvement strategies) at the levels of the patient, provider, and system were extracted, because these were found to influence the uptake of clinical guidelines (23).
Outcome Measures
Primary outcome measures included metabolic screening rates for obesity-weight (BMI or waist circumference), glucose (blood glucose or HbA1C), lipids (total cholesterol, LDL cholesterol, HDL cholesterol, or triglycerides), and BP (systolic BP, diastolic BP, or both) and the proportion of patients screened for these four metabolic measures (obesity, glucose, lipids, and BP) in the intervention and comparison groups. Secondary outcome measures included the type and number of improvement strategies used in each intervention to influence monitoring rates at the provider, patient, and system levels.
Quality Assessment
To appraise the internal validity of the studies, each study was examined for risk of bias by using the Effective Public Health Practice Tool (EPHPP) (24). Specifically, the EPHPP assesses the following six domains: sample selection, study design, identification and treatment of confounders, blinding of outcome assessors and participants, reliability and validity of data collection methods, and withdrawals and dropouts. Each domain is rated according to a standardized guide and given a rating of 1, strong; 2, moderate; or 3, weak. Once all domains have been assessed, the study is rated according to the number of domains with a weak rating: strong (no weak ratings), moderate (one weak rating), or weak (two or more weak ratings). Quality assessment was conducted by two authors (O.C.M. and L.R.L.), with discrepancies resolved by consensus.
Data Analysis
Study characteristics and quality appraisal were summarized in tables and the online supplement, respectively. Quantitative data regarding screening rates for four metabolic indices (obesity, glucose, lipid measures, and BP measures) and the proportion of patients screened for four metabolic measures (obesity, glucose, lipids, and BP) were summarized and depicted. For each metabolic measure, the median, minimum, and maximum screening rates for the intervention and the comparison groups were calculated by using Microsoft Excel. We aimed to assess the quantitative data by using standard meta-analytic methods to produce odds ratios to estimate the effect of the intervention on metabolic screening rates when a minimum of three clinically homogeneous studies were found. Qualitative data regarding the strategies used by the intervention to improve screening rates were organized into three a priori categories according to their level of action at the provider, patient, and system levels. The lead author (O.C.M.) initially identified the improvement strategies, and in an iterative process, the research team met several times to analyze and identify these strategies in the studies by using a consensus model (25). We anchored our findings within the Theoretical Domains Framework (TDF), which is commonly used to describe theoretical constructs associated with change in health care provider behavior (26).
Results
Characteristics of Included Studies
The database search led to identification of 7,596 unique citations, and the reference lists of relevant articles and review papers yielded an additional 11 citations that had not been retrieved by the database search. (The online supplement includes a flowchart of the search process and study selection according to PRISMA guidelines (20).) Overall, 166 full-text articles were reviewed in detail, with 30 meeting inclusion criteria. However, the DelMonte et al. (27) and Lee et al. (28) publications were considered as one study because the latter is a long-term follow-up of the former.
The characteristics of the 29 included studies are summarized in Table 1 (27, 29–56). All of the included studies were conducted in mental health care systems, and most (N=23) were conducted in outpatient settings. Over two-thirds of the included studies (N=21) were conducted in the United States or the United Kingdom. A study design of clinical audit cycle with an assessment of preintervention screening rates compared with postintervention screening rates was reported in 23 studies, four reported an interrupted time series, and two were randomized controlled trials (RCTs). Five and seven studies, respectively, were reported to be quality improvement and implementation programs. All 29 included studies were published between 2007 and 2018, and more than half of all publications (N=15) were published in 2015 or later.
| Study | Country | Setting | Populationb | Intervention | Design/intervention duration | Sample size (comparison/intervention group) | Synopsis of findings |
|---|---|---|---|---|---|---|---|
| Abdallah et al., 2016 (29) | UK | MH outpatient, community | Schizophrenia; any oral antipsychotic and long-acting depot treatment in MH; exclusion of clozapine | Intervention targeted the primary care–MH care interface; MH clinicians liaised with GPs via letters to improve metabolic screening; patients empowered to seek general medical care with their GPs; care home staff participated in monitoring of weight and BP. | Prospective audit with 10 weeks of follow-up/10 weeks | 95/33 | Improvement was noted in GPs’ performance of monitoring; care homes took on responsibility to monitor weight and BP; optimal monitoring achieved for glucose. |
| Barnes et al., 2008 (31) | UK | MH outpatient, community | SMI; psychotic spectrum disorders, 82%/84%; mood disorders, 13%/12%; SGA, 62%/65%; FGA, 36%/33% | QIP that included a benchmarked audit of baseline monitoring delivered to providers; educational activities for providers; lifestyle management pack for providers and patients; physical health check reminder card given to patients | Retrospective audit with 1 year of follow-up after intervention/1 year | 1,966/1,516 | Proportion of patients screened for four metabolic measures increased from 11% to 23%. |
| Barnes et al., 2015 (30) | UK | MH outpatient, community | SMI; psychotic spectrum disorders, 72%; mood disorders, 13%; any antipsychotic | QIP that included a benchmarked audit of baseline monitoring delivered to providers; educational activities for providers; lifestyle management pack for providers and patients; physical health check reminder card given to patients | Retrospective audits in 6 years; up to 6 years of follow-up after intervention/1 year | 1,966/1,591 | Proportion of patients screened for four metabolic measures increased from 11% to 34%; over 60% of patients were screened by the MH providers. |
| Cotes et al., 2015 (32) | USA | MH outpatient, community | SMI; any antipsychotic | QIP with provider and leadership education; education of patients and family; local leaders given results of benchmarked monitoring audits | Retrospective audit with up to 22 months of follow-up after intervention/22 months | 230/265 | The 10 MH centers audited varied widely in intervention uptake; despite local increases in MM, no significant change was seen at the state level. |
| DelMonte et al., 2012 (27); Lee et al., 2016 (28)c | USA | MH inpatient | SMI; mood disorders, 51%/51%; psychotic spectrum disorders, 36%/36%; SGA only | Clinicians ordering SGA for inpatients received a “pop-up” reminder in EMR to order glucose and lipid testing; a dedicated clinician (“champion”) supported provider behavior change. | Retrospective audit with up to 4 years of follow-up after intervention/4 years | 171/129 | Implementation of a “pop-up” alert in an inpatient EMR increased the proportion of patients with both glucose and lipid testing from 13% to 48% at 6 months and 51% at 4 years. |
| Fischler et al., 2016 (33) | Canada | MH inpatient | Schizophrenia, schizoaffective disorder; any antipsychotic | An implementation study to improve adherence with schizophrenia CPG; CDSS ordered complete MM when antipsychotics were prescribed; clinicians and leaders received benchmarked feedback on their performance. | Prospective audit with up to 12 months of follow-up after intervention/12 months | 192/184 | Proportion of patients screened for four metabolic measures increased from 36% to 56%. |
| Gallagher et al., 2013 (34) | Ireland | MH outpatient, community | SMI; schizophrenia, 48%/50%; mood disorders, 33%/30%; SGA, 90%/93%; FGA, 20%/15%; exclusion of clozapine | Patients on antipsychotics were identified by a registry and summoned to a health screening and promotion clinic to conduct MM; patients were educated about the need for monitoring; liaison with GPs for treatment of metabolic abnormalities; patients’ records were supplemented with a designated MM sheet. | Prospective audit with up to 3 months of follow-up after intervention/2 clinic days during 3 months | 40/40 | Attendance rates for health promotion clinic were over 70%; all four metabolic measures were screened in over 75% of patients. |
| Gill et al., 2016 (35) | Ireland | MH outpatient, community | SMI; any long-term depot antipsychotic | Patients were invited by letter to attend a semiannual health monitoring clinic staffed by psychiatrists, nurses, and an administrator; clinic conducted MM; liaison with GPs for treatment of metabolic abnormalities; patients’ records were supplemented with a designated MM sheet. | Prospective interrupted time-series with 1 year of follow-up after intervention/1 year | 23/23 | Attendance rates for health promotion clinic were over 90%; screening for glucose and lipids increased from 9% to 61% following the intervention. |
| Gonzalez et al., 2010 (36) | UK | MH outpatient | SMI; any antipsychotic, excluding clozapine | MM practice was audited and results were communicated to senior physicians; brief educational sessions were delivered to physicians; patients’ records were supplemented with a designated MM sheet. | Retrospective audit with 1 year of follow-up after intervention/1 year | 126/106 | Intervention achieved increases in MM rates; MM sheet was implemented in nearly half the charts. |
| Green et al., 2018 (37) | UK | MH inpatient | SMI; any antipsychotic | QIP that was overseen by an implementation science expert; input from clinicians and patients before and during the program; monitoring tool was created; education given to staff and patients | Retrospective baseline audit of 10 months; prospective audit with 15 months of follow-up/15 months | 247/318 | Improvements were observed in MM for BMI and BP; QIP education and codesign with patients was well received by the clinical team. |
| Gumber et al., 2010 (38) | UK | MH outpatient | SMI; SGA only | Patients taking antipsychotics were identified by a registry and referred to a metabolic clinic for MM; GPs received the results of the monitoring; physicians received benchmarked audit and educational activities; patients’ records were supplemented with a designated MM sheet. | Prospective audit with up to 12 months of follow-up after intervention/ongoing clinic with 1–2 months of implementation and promotion of clinic | 54/110 | High monitoring rates in the comparison group obscured possible effects of the intervention; no changes in monitoring were observed. |
| Hinds et al., 2015 (39) | USA | MH outpatient, academic | SMI; SGA only; excluding known diagnosis of diabetes | Pharmacist-led initiative to promote glucose monitoring; EMR database used to identify gaps in glucose testing; electronic alert sent to treating physician to conduct MM | Prospective audit with up to 3 months of follow-up after intervention/3 months | 104/86 | A 10% absolute increase in screening rates of a glucose measure was observed. |
| Hor et al., 2016 (40) | Malaysia | MH outpatient, general hospital | SMI; psychotic spectrum disorders, 75%; mood disorders, 15%; any antipsychotic | MM protocol was developed and endorsed by clinical leaders; patients’ records were supplemented with a designated MM sheet; protocol assigned responsibility for MM to specific team members; barriers to monitoring were assessed. | Prospective audit with up to 1 week of follow-up after intervention/1 week | 300/32 | Anthropometric measures saw a greater increase than blood testing; waist circumference was measured less often because of cultural and religious barriers. |
| Kioko et al., 2016 (41) | USA | MH outpatient, community | SMI; SGA only | QIP with implementation of a paper MM tool; clinical staff educated on metabolic health | Prospective audit with up to 3 weeks of follow-up after intervention/3 weeks | 50/50 | Intervention group was associated with increases in monitoring for glucose and lipids, relative to comparison group. |
| Kirchner et al., 2016 (42) | USA | MH inpatient and outpatient, VA | Schizophrenia, schizoaffective disorder; any antipsychotic | QIP that was overseen by an implementation science expert; clinicians received educational materials and electronic reminders in EMRs; audit and feedback of monitoring rates were given to clinicians and managers; patients in need of MM were identified on a weekly basis by the EMR, and this was communicated to the metabolic champion. | Prospective audit with up to 6 months of follow-up after intervention/6 months | 17/15 | Intervention was associated with increases in monitoring for weight, glucose, and lipids, relative to comparison group. |
| Kreyenbuhl et al., 2016 (43) | USA | MH outpatient, VA | SMI; mood disorders, 58%; psychotic spectrum disorders, 30%; SGA only; excluding known diagnosis of dementia | Intervention targeted patients as agents of change; patients received personalized feedback on their MM and education on metabolic health via tablet devices in waiting rooms; patients were empowered to discuss metabolic health with their provider; the comparison group received generic printed materials on metabolic health without personalized information. | Randomized controlled trial of 1-year duration | 119/120 | No changes in monitoring were observed; high monitoring rates in the comparison group obscured possible effects of the intervention. |
| Lai et al., 2015 (44) | Taiwan | MH outpatient, psychiatric hospital | Schizophrenia; SGA only | EMRs were supplemented with an electronic prompt for physicians to conduct MM. | Retrospective interrupted time-series; 2 years before and 2 years after intervention/2 years | 38/37 | Intervention increased rates of patient visits adherent with monitoring guidelines, among those prescribed high-risk (clozapine or olanzapine) versus intermediate-risk SGAs. |
| Latoo et al., 2015 (45) | UK | MH outpatient, community | EIP; any antipsychotic | QIP that was overseen by a multidisciplinary clinical team and patients; barriers to MM were assessed; a monitoring tool and clinical prompts were introduced; collaboration with GPs increased, and specialized physical health clinics were introduced. | Retrospective audit with 6 months of follow-up after intervention/6 months | 55/52 | Screening for all four metabolic measures increased from 7% to 40% after the intervention. |
| Lui et al., 2016 (46) | USA | MH inpatient | SMI; any antipsychotic | Implementation of a mandatory admission electronic order set that included all four metabolic measures | Retrospective audit with 6 months of follow-up after intervention/6 months | 9,100/1,499 | Screening for all four metabolic measures increased from 2% to 100% after the intervention. |
| Nicol et al., 2011 (47) | USA | MH outpatient, community and academic | SMI; SGA only | A registry of patients treated by SGA was created; charts of patients due for monitoring were flagged; screening was encouraged by leadership; providers received benchmarked audits; intervention was biphasic, with the aim of gradually improving screening practices over time. | Prospective interrupted time-series with up to 3 years of follow-up after intervention/3 years | 7,300/2,000 | An increase was observed in the screening rate for glucose from 46% at baseline to 67% and 90% after 1 and 3 years, respectively. |
| Osborn et al., 2010 (48) | UK | MH outpatient | SMI; psychotic spectrum disorders, 59%/50%; mood disorders, 22%/23%; SGA, 68%/61%; FGA, 19%/11%; unmedicated, 13%/28% | Nurse-led intervention at the primary care–MH care interface; nurse liaised with primary care and later MH providers to conduct MM; nurse to conduct MM if this was not done by either provider; comparison group was treatment as usual; both groups received an education pack on MM and metabolic health directed at providers and patients. | Cluster randomized controlled trial of 6 months’ duration | 59/62 | Increased rates of MM in intervention and to a lesser extent in comparison group; most MM conducted by nurse and primary care services and not MH providers. |
| Ramanuj, 2013 (49) | UK | MH inpatient | SMI; psychotic spectrum disorders, 39%/32%; mood disorders, 33%/40%; any antipsychotic | Physicians received a benchmarked audit of MM in their service and educational activities; clinical areas were augmented with visual posters promoting MM. | Prospective audit with up to 13 months of follow-up after intervention/13 months | 16/10 | Intervention group saw increases (60%) in monitoring for lipids, relative to comparison group (25%). |
| Runcie et al., 2007 (50) | UK | MH inpatient | SMI; any antipsychotic | Local MM protocol was developed and endorsed by clinical leaders; protocol was disseminated by letters to all physicians. | Retrospective audit with 3 months of follow-up after intervention/3 months | 51/61 | Wards with a dedicated metabolic clinician showed improved monitoring; intervention did not lead to increases in monitoring overall; however, local influence of metabolic champion was noted. |
| Thompson et al., 2011 (51) | Australia | MH outpatient, community | EIP; SGA only | Physicians received education and a benchmarked audit of MM practice; local MM protocol was developed and visually displayed in clinical areas; patients’ records were supplemented with a designated MM sheet. | Prospective audit with up to 30 months of follow-up after intervention/6 months | 106/86 | Screening for all four metabolic measures increased in the intervention versus the comparison group from 22% to 81%. |
| Tully et al., 2012 (52) | Ireland | MH outpatient, community | SMI; clozapine treatment | Local MM protocol was developed; patients’ records were supplemented with a designated MM sheet; ongoing educational sessions for clinicians were conducted. | Prospective audit with up to 1 year of follow-up after intervention/1 year | 84/74 | Glucose and lipids screening in the intervention group increased to 65% and 70%, respectively; the monitoring protocol was implemented in 92% of the charts. |
| Vasudev and Martindale, 2010 (53) | UK | MH outpatient, community | EIP; any antipsychotic; unmedicated, 9%/11% | Intervention targeted the primary care–MH care interface; EIP clinicians liaised with GP via letters to facilitate physical check-ups; EIP patients were included in SMI registry of primary care; EIP clinicians received an educational workshop. | Prospective audit with up to 6 months of follow-up after intervention/6 months | 66/76 | The number of patients for whom cardiometabolic risk screening was completed by primary care providers increased from 20% to 58% after the intervention. |
| Velligan et al., 2013 (54) | USA | MH outpatient, community | SMI; SGA only | QIP that included an implementation working group that oversaw educational interventions and monitoring procedures; patients’ records were supplemented with a designated MM sheet. | Prospective controlled interrupted time-series with up to 2 years of follow-up after intervention/2 years | 100/50/ | Rates of screening for anthropometric measures increased from 0% to 80% in the intervention clinic and remained lower than 10% at the control clinics. |
| Wiechers et al., 2012 (55) | USA | MH outpatient, academic | SMI; mood disorders, 72%; psychotic spectrum disorders, 33%; any antipsychotic | QIP in a resident-led clinic; physicians received educational sessions; barriers for MM were assessed in focus groups; patients received instruction on fasting lab testing; EMR was enhanced with designated fields to record MM. | Prospective audit with up to 1 year of follow-up after intervention/1 year | 140/131 | Screening for all four metabolic measures increased in the intervention (31%) versus the comparison group, (1%). |
| Wilson et al., 2014 (56) | Australia | MH outpatient, psychiatric hospital | SMI; clozapine treatment | 2 months in a year were chosen as “physical health months” and were promoted visually and educationally to both physicians and patients by clinical directors; during this time, all patients were to be monitored; patients’ records were supplemented with a designated MM sheet. | Prospective audit with up to 1 year of follow-up after intervention/2 months plus 2 months | 107/232 | Screening rates for four metabolic measures exceeded 85% in the intervention group. |
TABLE 1. Characteristics of 29 studies of interventions to improve metabolic risk screening of adults taking antipsychoticsa
Quality Assessment of Included Studies
Overall, 16 studies were rated as weak (29, 31, 33, 37, 38, 40–42, 46, 48, 50–53, 55, 56), and 13 studies were rated as moderate (27, 30, 32, 34–36, 39, 43–45, 47, 49, 54) (see online supplement for a summary of the EPHPP (24) assessment). No studies were given the rating of strong. For 13 of the studies assigned a weak rating (29, 33, 37, 38, 40–42, 46, 50–53, 55), the rating stemmed from their uncontrolled audit cycle design, comparing preintervention screening rates to postintervention screening rates and the lack of adjustment for confounding factors known to influence monitoring rates, such as age and medical comorbidities (15).
Categorization and Description of Improvement Strategies
The included studies described complex interventions that used multiple strategies that aimed to improve metabolic risk screening. Providers were the target of four improvement strategies, patients were targeted by two strategies, and the health system was targeted by seven strategies. Osborn et al. (48) conducted an RCT with two active arms; therefore, our review categorized 30 interventions, which were published in 29 studies as summarized in Table 2 (27, 29–56). The number of improvement strategies utilized in the studies ranged from one to nine, with a median of five.
| Provider | Patient | System | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | N of improvement strategies | Education | Personalized audit and feedback | Clinical promptsa | MM toolb | Education | Empowerment | Leadership support | Patient identification | Collaboration with primary care | Clinical champion for monitoring | Practice audit and feedback | CDSSc | Barrier assessment |
| Abdallah et al., 2016 (29) | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Barnes et al., 2008 (31) | 7 | ✓ | ✓ | ✓ (v) | ✓ | ✓ | ✓ | ✓ | ||||||
| Barnes et al., 2015 (30) | 6 | ✓ | ✓ | ✓ (v) | ✓ | ✓ | ✓ | |||||||
| Cotes et al., 2015 (32) | 4 | ✓ | ✓ | ✓ | ✓ | |||||||||
| DelMonte et al., 2012 (27); Lee et al., 2016 (28) | 3 | ✓ | ✓ (e) | ✓ | ||||||||||
| Fischler et al., 2016 (33) | 9 | ✓ | ✓ | ✓ (e) | ✓ (e) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Gallagher et al., 2013 (34) | 6 | ✓ (p) | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Gill et al., 2016 (35) | 7 | ✓ (p) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Gonzalez et al., 2010 (36) | 4 | ✓ | ✓ | ✓ (p) | ✓ | |||||||||
| Green et al., 2018 (37) | 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Gumber et al., 2010 (38) | 5 | ✓ | ✓ | ✓ (p) | ✓ | ✓ | ||||||||
| Hinds et al., 2015 (39) | 3 | ✓ (e) | ✓ | ✓ | ||||||||||
| Hor et al., 2016 (40) | 4 | ✓ (p) | ✓ | ✓ | ✓ | |||||||||
| Kioko et al., 2016 (41) | 3 | ✓ | ✓ (p) | ✓ | ||||||||||
| Kirchner et al., 2016 (42) | 8 | ✓ | ✓ | ✓ (e) | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Kreyenbuhl et al., 2016 (43) | 2 | ✓ | ✓ | |||||||||||
| Lai et al., 2015 (44) | 2 | ✓ (e) | ✓ | |||||||||||
| Latoo et al., 2015 (45) | 9 | ✓ | ✓ | ✓ (e) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Lui et al., 2016 (46) | 1 | ✓ | ||||||||||||
| Nicol et al., 2011 (47) | 6 | ✓ | ✓ | ✓ (p) | ✓ | ✓ | ✓ | |||||||
| Osborn et al., 2010 (48) Full | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Osborn et al., 2010 (48) Partial | 2 | ✓ | ✓ | |||||||||||
| Ramanuj, 2013 (49) | 5 | ✓ | ✓ | ✓ (v) | ✓ | ✓ | ||||||||
| Runcie et al., 2007 (50) | 3 | ✓ | ✓ | ✓ | ||||||||||
| Thompson et al., 2011 (51) | 6 | ✓ | ✓ | ✓ (v, p) | ✓ | ✓ | ✓ | |||||||
| Tully et al., 2012 (52) | 3 | ✓ | ✓ (p) | ✓ | ||||||||||
| Vasudev and Martindale, 2010 (53) | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Velligan et al., 2013 (54) | 8 | ✓ | ✓ | ✓ (p) | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Wiechers et al., 2012 (55) | 5 | ✓ | ✓ (e) | ✓ | ✓ | ✓ | ||||||||
| Wilson et al., 2014 (56) | 8 | ✓ | ✓ | ✓ (p, v) | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
TABLE 2. Strategies for improving metabolic screening rates for persons taking antipsychotics reported in 29 studies, by provider, patient, and system levels
Improvement strategies targeting providers.
Adequate knowledge and skills regarding metabolic risk screening are key to changing providers’ practice. Concordantly, 23 interventions had an educational component that aimed to enhance providers’ knowledge regarding the importance of metabolic risk screening (27, 29–33, 36–38, 41, 42, 45, 47–56). There was large variation in the intensity and type of educational material delivered to providers, which included both in-person training sessions (ranging from a single 30-minute session [49] to an interactive series [51]) and passive dissemination of information (17, 18). Memory and attention processes of providers need to be influenced for monitoring to occur; therefore, prompts were integrated into routine clinical practice in 21 interventions and were paper based, electronic, or visual (posters) (27, 30, 31, 33–36, 38–42, 44, 45, 47, 49, 51, 52, 54–56). Most commonly, a paper sheet designated for metabolic monitoring was added into patients’ charts and served as a clinical reminder for monitoring in adherence with guidelines as well as a form for recording results (34–36, 38, 40, 41, 47, 51, 52, 54, 56). Electronic prompts for monitoring within an electronic medical record (EMR) appeared when physicians entered a prescription for antipsychotics or when monitoring was due (27, 33, 39, 42, 44, 45, 55), and large posters were displayed in clinical areas with information regarding local monitoring protocols (30, 31, 49, 51, 56). Establishing new social norms that favor metabolic risk screening was attempted by 13 interventions. In this instance, interventions presented providers with a personalized audit of their metabolic monitoring performance benchmarked with rates of monitoring by providers in similar mental health services (30, 31, 33, 36–38, 42, 45, 47, 49, 51, 54, 56).
Improvement strategies targeting patients.
Providing education to patients regarding the need and importance of metabolic risk screening was reported in 12 interventions (29–34, 37, 43, 48, 55, 56), with educational content being delivered both face to face and via paper-based materials. In four interventions, a component of a “lifestyle pack” was added to the educational materials, which included advice regarding physical activity, smoking cessation, and a healthy diet (30, 31, 34, 56). Patients were empowered to seek metabolic risk screening from their health care providers and given practical tools that could assist them in preparing for fasting blood tests (53, 55). The intervention conducted by Kreyenbuhl et al. (43) consisted solely of improvement strategies targeting patients. These strategies included patient education, a personalized feedback of one’s metabolic risk screening status, and an empowerment strategy that urged patients to ask their providers to conduct metabolic risk screening. In some studies, the intervention was codesigned by health care staff and patients to ensure that the proposed intervention was patient centered (37, 45).
Improvement strategies targeting systemic issues.
The majority of the interventions (N=23) included improvement strategies that operated at the system level. Most notably, a number of strategies aimed to create an organizational culture that promotes the practice of metabolic risk screening through social influences. A “metabolic champion,” usually a health care provider on the clinical team in charge of promoting metabolic risk screening in adherence with guidelines, was designated in 11 of the interventions (11, 15, 17, 19, 21, 23, 26–28, 30, 36), and 13 interventions included leadership support in which clinical or administrative service leaders actively supported clinicians’ adherence to monitoring guidelines (32, 33, 35, 37, 40, 42, 44, 45, 47, 50, 53, 54, 56). Leadership involvement included the endorsement of a local monitoring protocol (40), clinical site visits, and newsletters sent to clinicians to encourage metabolic risk screening (47). To further facilitate leadership support, seven studies reported that leaders had access to benchmarked performance audits for metabolic risk screening within their services (30–33, 42, 45, 47). Similarly, mental health organizations that prioritized metabolic risk screening utilized a clinical decision support system to allow for adequate metabolic risk screening upon inpatient admission (33, 46).
Some interventions targeted the environments in which providers work, and as a consequence, an assessment of local barriers to and facilitators of performing metabolic risk screening was reported in 15 interventions (29, 31, 33, 35, 37, 40, 42, 45, 48, 49, 51, 53–56). These evaluations subsequently informed actions, such as the provision of training on how to conduct anthropometric measurements (40, 49) and ensuring that clinicians’ offices contained measuring equipment (51). Promoting collaboration between mental health services and primary care services was a strategy used by four interventions (29, 34, 48, 53) and aimed to increase the supports available for providers to interpret screening results and intervene when appropriate. Finally, nine interventions employed a systematic method to identify patients who are in need of metabolic risk screening as an initial step in improving screening rates (34, 35, 38, 39, 42, 45, 47, 53, 54). These included searching paper and electronic charts to identify patients in a given setting who lack metabolic screening and flagging those charts for intervention (39, 47).
Intervention Effects on Metabolic Risk Screening
There was wide clinical heterogeneity of the included studies; the 30 identified interventions used between one and nine unique strategies to increase metabolic screening rates. Considerable variation was also noted within each strategy across studies (i.e., provider education and metabolic champion). Moreover, studies differed in baseline monitoring rates (e.g., glucose monitoring rates at baseline varied from 0% to 92%) and the target metabolic measure and the timing of postintervention screening rates (1 week to 6 years). Therefore, a meta-analysis of the results was not feasible.
The quantitative results of 28 interventions (27, 29–42, 45–56) are presented in a descriptive manner in Table 3 and depicted in Figure 1. Positive results were reported for 21 of the 30 interventions, in which significant increases to screening rates of all targeted metabolic measures had been observed (27, 29–31, 33–37, 39, 44–48, 51, 53–56). Mixed results were reported for five interventions, with at least one metabolic measure showing an increase in screening rates in the intervention group (40–42, 49, 52). A null effect on screening rates was observed for four interventions (32, 38, 43, 50).
| Proportion screened (%) | |||||||
|---|---|---|---|---|---|---|---|
| N of | Comparison group | Intervention group | |||||
| Measure | studies | Mdn | Min | Max | Mdn | Min | Max |
| Glucose | 21 | 28 | 0 | 92 | 65 | 13 | 100 |
| Lipids | 18 | 22 | 5 | 99 | 61 | 27 | 97 |
| Body mass index, weight | 16 | 19 | 0 | 99 | 67 | 34 | 100 |
| Waist circumference | 6 | 2 | 1 | 99 | 87 | 7 | 100 |
| Blood pressure | 15 | 22 | 3 | 100 | 80 | 38 | 99 |
| All four measuresa | 8 | 11 | 0 | 36 | 57 | 23 | 100 |
TABLE 3. Intervention effects on metabolic screening rates for 28 of the 30 interventions described in the studies review
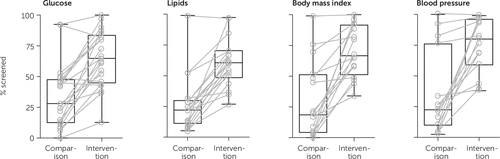
FIGURE 1. Median rates of metabolic risk screening in intervention and comparison groups, by type of screeninga
aResults are from 28 interventions described in Table 3.
Intervention effects on glucose and lipids screening.
Glucose was the most prevalent metabolic measure screened in the studies and was targeted by 21 interventions (27, 29–32, 34–36, 38–42, 47–50, 52, 55, 56) (Table 3). Median screening rates for glucose increased from 28% in the comparison groups to 65% in the intervention groups. Similarly, lipids were screened in 18 interventions (27, 29–32, 34–36, 38, 40–42, 48, 49, 52, 55, 56). Median screening rates for lipids increased from 22% in the comparison groups to 61% in the intervention groups.
Intervention effects on anthropometric and physical measurements.
Waist circumference screening rates were reported in six studies, with median screening rates increasing from 2% in the comparison groups to 87% in the intervention groups (32, 34, 35, 38, 40, 52). BMI/weight screening rates were reported in 16 studies, with median screening rates increasing from 19% in the comparison groups to 67% in the intervention groups (29–32, 34, 35, 37, 38, 40–42, 48, 50, 55, 56). BP screening rates were reported in 15 studies, with median screening rates increasing from 22% in the comparison groups to 80% in the intervention groups (29–32, 34, 35, 37, 38, 40, 41, 48, 52, 55, 56).
Additional summary of intervention effects.
Eight studies reported the intervention effects by presenting the change in the proportion of patients who were screened for all four metabolic measures (obesity, glucose, lipids, and BP) (30, 31, 33, 45, 46, 51, 53, 54), and the median screening rates for the four measures increased from 11% in the comparison groups to 57% in the intervention groups.
In two studies, outcomes of the intervention were not presented as screening rates. In one, Kreyenbuhl et al. (43) conducted an RCT in which the outcome was the proportion of days in which patients were adherent with monitoring guidelines. All metabolic parameters were appropriately monitored for over 75% of the days in both intervention and comparison groups, and no beneficial effect was observed in the intervention group. Similarly, Lai et al. (44) described the effect of the intervention as a proportion of “qualified patient visits” (QPVs), in which patients were in concordance with monitoring guidelines within 6 months of the visit date. A sevenfold increase in the percentage of QPVs was found among the patients taking clozapine or olanzapine, compared with those taking first-generation antipsychotics. The types of measures used in these two studies precluded the inclusion of the studies in the intervention summaries provided in Table 3 and Figure 1.
Discussion
This review conducted a comprehensive knowledge synthesis of interventions that target metabolic risk screening of adults taking antipsychotic medication. Using a theoretical framework (26), the review identified improvement strategies used by the interventions at the levels of the provider, patient, and system, highlighting that organizational commitment to monitoring is a key issue. Common methodological limitations were observed in most interventions, including uncontrolled design and lack of adjustment for confounders, which reduced our ability to draw firm conclusions regarding the true effect of the interventions. Findings suggest that interventions were overall successful in increasing screening rates; however, up to a third of patients remained unscreened.
This review identified 30 unique interventions that were carried out in mental health services and targeted at improving metabolic risk screening of adults taking antipsychotics. Given that antipsychotic metabolic screening falls short of recommended guidelines (15, 17), we reported on programs that addressed this guideline-to-practice gap. Interventions varied widely on the “continuum of quality improvement” (57). That is, some interventions were local staff initiatives involving a small unit (34, 50) while others were resource-intensive organizationwide programs (32, 56). Over time, it was evident that some programs advanced from local practice improvement initiatives to large-scale theory-driven implementation science programs (29, 33, 37, 42, 45, 47, 54). Some included external experts and advisors who worked longitudinally in collaboration with clinicians, managers, and patients to achieve predefined measurable improvements in metabolic risk screening. Inherently, implementation programs are contextualized to existing resources, processes, and structure, which limits their transferability between organizations (26). Nevertheless, interventions reviewed here shared certain similarities in objectives, tools, and improvement strategies that have been used to narrow guideline-to-practice gaps in other domains of clinical practice (58). This review suggests that use of improvement strategies is beneficial in reducing the guideline-to-practice gap for metabolic risk screening of patients who take antipsychotics; however, additional reports are needed to support the knowledge base.
The quality of the studies included in this review was found to be weak (N=16) or moderate (N=13), and no studies were rated as strong. Quality improvement programs are gaining momentum in health care and approximately 1,000 quality improvement studies are published each year (59). Nevertheless, scholars are divided about whether findings from quality improvement programs hold similar scientific rigor as other methods of inquiry, such as RCTs (60). Given that quality improvement programs are often multifaceted and implemented into intricate systems, it is challenging to ascertain the effect of an intervention with traditional highly controlled designs. This is evident in the studies reviewed here; over three-quarters of them chose a clinical audit cycle to identify the effect of the intervention, which contributed to their lower quality ratings. Recently, burgeoning methodological advancements are likely to increase the scientific rigor of quality improvement programs to a degree that allows us to infer causality (61)—for example, use of interrupted time-series analyses, cluster RCTs, and theory-driven improvement strategies (60, 61). These advances are timely and should be incorporated into future interventions that aim to improve antipsychotic risk screening in mental health services.
Using a theoretical framework (26), this review identified the improvement strategies used by the interventions to influence metabolic risk screening at the provider, patient, and system levels. For providers, considerable efforts were made to incorporate screening into practice by influencing memory and attention processes with prompts and monitoring tools. Physician reminders, both paper and electronic, have been known to influence physician behavior and thus are frequently used in medical practice (62). Similarly, nearly all interventions included provider education regarding metabolic risk and recommended antipsychotic monitoring; however, most would not be considered as continuing medical education (CME) activities. A recent review found that CME had a profound effect on physician performance (63); therefore, CME activities have the potential to further improve antipsychotic monitoring (64).
Patients were empowered to seek metabolic risk screening from their health care providers (43, 53) by provision of both written materials and verbal encouragement. These approaches are in line with contemporary trends positing that individuals with severe mental illness benefit from acquiring self-management skills to better manage their health and health care, as reported in the Health and Recovery Peer program (65). Future research is needed to examine the effect of peer-support self-management programs on reducing cardiometabolic risk and improving screening among individuals with severe mental illness.
Interventions attempted to change metabolic risk screening by using social influences to alter providers’ behavior. Social influence is a construct targeting interpersonal processes that are known to change providers’ thoughts, feelings, and behaviors (26). Interventions reported commitment at the mental health system level, where leaders and managers took an active part in promoting physical health (32, 42, 54), thus creating an organizational culture that encouraged metabolic risk screening. For example, managers and leaders who had access to data comparing the metabolic risk screening rates in their organization or unit with those in similar services could discuss this with their clinical teams. Similarly, the appointment of a clinician-leader for metabolic monitoring (i.e., metabolic champion) helped facilitate change to clinicians’ professional role to one that includes general medical issues (27, 37). The environmental context, in terms of resources and capability to conduct metabolic risk screening, was targeted by some interventions. Some conducted a barrier assessment and used the results to inform the intervention, because practical issues, such as lack of measurement equipment in clinic rooms, often prevent monitoring (40, 42). Others used available external resources, such as primary care providers in a shared care model, to promote metabolic risk screening (29, 48). This was seen in studies originating from the United Kingdom, where primary and mental health care services are geographically aligned. Such an approach may be difficult to replicate in the United States because of fragmentation of the two systems. Future research is needed to test whether integration of primary care activities into mental health care services would improve metabolic risk screening and management in that setting.
Most interventions were successful, as evidenced by the increase in median metabolic screening rates for glucose (28% to 65%), lipids (22% to 61%), weight (19% to 67%), and BP (22% to 80%) (Table 3 and Figure 1). However, after implementation of the intervention, up to one-third of patients still remained unscreened. This is consistent with other reports that describe suboptimal medical care for diabetes, hypertension, and ischemic heart disease and suboptimal preventive medicine among people with severe mental illness (66, 67). Anthropometric measurements were easier to obtain, compared with blood tests, especially when this task was delegated to nonmedical providers, such as administrative staff and home care workers (29, 40). Blood tests for glucose and lipids pose a burden for individuals, mostly because they require fasting samples but also because of access issues in services that do not offer on-site testing. Overcoming these barriers can be achieved by the use of point-of-care (POC) testing. Conducting a POC random glucose measurement, along with weight and BP assessments, during a routine mental health outpatient visit has been used successfully to screen for metabolic risk (68).
Studies differed substantially in the metabolic measure targeted for improvement—while some targeted a single measure (e.g., glucose only), others targeted four (glucose, lipids, weight, and BP). Individuals with severe mental illness are at risk of abnormalities in all metabolic measures; therefore, future antipsychotic surveillance programs should aim to improve monitoring that is concordant with published guidelines.
Most of the interventions focused on improving metabolic risk screening, and only a few reported linking screening programs with management of identified risk (38, 45, 46, 51, 56, 68). This is of concern because far too often metabolic risk factors are left untreated. For example, at the time of patient enrollment into the Clinical Antipsychotic Trials of Intervention Effectiveness schizophrenia trial, no-treatment rates were 30% for diabetes, 62% for hypertension, and 88% for dyslipidemia (69). Psychiatrists acknowledge the importance of conducting metabolic risk screening; however, most reported difficulty in arranging medical follow-up for identified risk, and only about a third were willing to prescribe medications such as statins or metformin (70). Thus a clear rationale exists for linking metabolic risk screening with management of identified risk by local resources that are available to mental health services. Close collaboration with primary care services is recommended in the guidelines (71). When primary care services are not available, delegation of responsibility for metabolic risk screening to clinical pharmacists, who frequently work alongside psychiatrists, has been used successfully (27, 39, 68). The role of clinical pharmacists offers advantages beyond screening. This includes pharmacotherapy recommendations for treatment of identified risk and patient education.
Similarly, identification and management of tobacco use, although not directly recommended in antipsychotic monitoring guidelines, should be routinely conducted alongside metabolic risk screening programs, given its strong link with cardiovascular risk and metabolic dysregulation (72). Indeed, nearly a third of all interventions screened for tobacco use, which is a prerequisite for linking patients with smoking cessation programs, preferably those adapted to meet the needs of individuals with severe mental illness (73, 74).
This review, which offered a knowledge synthesis of intervention studies that aimed to improve metabolic risk screening of patients taking antipsychotic medication, had some limitations. Because of the heterogeneity of the included interventions in terms of design, duration, outcomes, and improvement strategies, we were not able to combine the effects of the interventions in meta-analyses or assess for publication bias. This is in line with Cochrane guidance, which discourages use of meta-analyses in heterogeneous studies (22). The fact that many interventions used multiple improvement strategies in an uncontrolled design puts the study results at high risk of bias. This limited our ability to draw firm conclusions regarding the true effect or the superiority of one type of intervention over another. A strength of this review was its attempt to describe the improvement strategies of the intervention at the levels of the provider, patient, and system. To establish the credibility of this process we followed expert guidance (75), which included anchoring our findings within a theoretical framework (26), meeting several times to discuss the results, and working iteratively to analyze the findings.
Conclusions
Interventions to increase metabolic risk screening among individuals taking antipsychotic medications include multiple improvement strategies that are shared by other programs in clinical areas that address an evidence-to-practice gap (57, 58). Although interventions were successful in improving median screening rates, up to one-third of patients remained unscreened. Improvement strategies that are key to the success of metabolic risk screening programs create a culture within the organization that prioritizes general medical care by means of education, leadership support, clinician leadership, and metabolic risk screening audit and feedback. Current knowledge is based on studies that lack scientific rigor, which plagues many quality improvement programs. However, timely advances in theoretical understanding of implementation problems (26) and methodological issues (60) offer guidance for the future design of scientifically sound programs. These advances include the use of interrupted time-series analyses and cluster RCTs.
Treatment of identified metabolic risk is of utmost importance and should be addressed by all risk screening programs in consideration of the availability of local resources, including but not limited to primary care providers (29, 45), allied health clinicians in mental health services (27, 48), and psychiatrists and aided by a robust clinical decision support system (76). Mental health organizations are urged to provide adequate monitoring and management of metabolic risk of patients taking antipsychotic medication, and the knowledge synthesis presented here can inform the design and evaluation of future programs in this area.
1 : Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care 2011; 49:599–604Crossref, Medline, Google Scholar
2 : Physical health care in persons with severe mental illness: a public health and ethical priority. World Psychiatry 2009; 8:1–2Crossref, Medline, Google Scholar
3 : Barriers and facilitators to primary care for people with mental health and/or substance use issues: a qualitative study. BMC Fam Pract 2015; 16:135Crossref, Medline, Google Scholar
4 : Perceived barriers to medical care and mental health care among veterans with serious mental illness. Psychiatr Serv 2008; 59:921–924Link, Google Scholar
5 : Understanding engagement with a physical health service: a qualitative study of patients with severe mental illness. Can J Psychiatry (Epub ahead of print, July 14, 2019)Crossref, Medline, Google Scholar
6 : Psychosis, socioeconomic disadvantage, and health service use in South Australia: findings from the Second Australian National Survey of Psychosis. Front Public Health 2015; 3:259Crossref, Medline, Google Scholar
7 : Association of psychiatric illness and obesity, physical inactivity, and smoking among a national sample of veterans. Psychosomatics 2011; 52:230–236Crossref, Medline, Google Scholar
8 : Is psychosis a multisystem disorder? A meta-review of central nervous system, immune, cardiometabolic, and endocrine alterations in first-episode psychosis and perspective on potential models. Mol Psychiatry 2019; 24:776–794Crossref, Medline, Google Scholar
9 : Cardiovascular and cerebrovascular risk factors and events associated with second-generation antipsychotic compared to antidepressant use in a non-elderly adult sample: results from a claims-based inception cohort study. World Psychiatry 2015; 14:56–63Crossref, Medline, Google Scholar
10 : Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011; 8:114–126Crossref, Medline, Google Scholar
11 : Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders: a systematic review and meta-analysis. Schizophr Bull 2013; 39:306–318Crossref, Medline, Google Scholar
12 : The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002; 288:2709–2716Crossref, Medline, Google Scholar
13 : Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 27:596–601Crossref, Medline, Google Scholar
14 Psychosis and Schizophrenia in Adults: Treatment and Management. NICE Clinical Guidelines, no 178. London, National Institute for Health and Care Excellence¸ 2014Google Scholar
15 : Metabolic testing for adults in a state Medicaid program receiving antipsychotics: remaining barriers to achieving population health prevention goals. JAMA Psychiatry 2016; 73:721–730Crossref, Medline, Google Scholar
16 : Identifying clinically questionable psychotropic prescribing practices for Medicaid recipients in New York State. Psychiatr Serv 2009; 60:1595–1602Link, Google Scholar
17 : Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 2012; 42:125–147Crossref, Medline, Google Scholar
18 : Strategies to implement physical health monitoring in people affected by severe mental illness: a literature review and introduction to the Italian adaptation of the positive cardiometabolic health algorithm. Psychopathology 2015; 21:269–280Google Scholar
19 : Improving metabolic monitoring rate for young people aged 35 and younger taking antipsychotic medications to treat a psychosis: a literature review. Arch Psychiatr Nurs 2017; 31:624–633Crossref, Medline, Google Scholar
20 : Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535Crossref, Medline, Google Scholar
21 : Best evidence synthesis: an intelligent alternative to meta-analysis. J Clin Epidemiol 1995; 48:9–18Crossref, Medline, Google Scholar
22 : Cochrane Handbook for Systematic Reviews of Interventions. New York, Wiley, 2011Google Scholar
23 : Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak 2008; 8:38Crossref, Medline, Google Scholar
24 : A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004; 1:176–184Crossref, Medline, Google Scholar
25 : Determining validity in qualitative inquiry. Theory Pract 2000; 39:124–130Crossref, Google Scholar
26 : A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci 2017; 12:77Crossref, Medline, Google Scholar
27 : Evaluation of a computer-based intervention to enhance metabolic monitoring in psychiatry inpatients treated with second-generation antipsychotics. J Clin Pharm Ther 2012; 37:668–673Crossref, Medline, Google Scholar
28 : Persistence of metabolic monitoring for psychiatry inpatients treated with second-generation antipsychotics utilizing a computer-based intervention. J Clin Pharm Ther 2016; 41:209–213Crossref, Medline, Google Scholar
29 : Improving physical health monitoring for patients with chronic mental health problems who receive antipsychotic medications. BMJ Qual Improv Rep 2016; 5:5Crossref, Google Scholar
30 : Screening for the metabolic side effects of antipsychotic medication: findings of a 6-year quality improvement programme in the UK. BMJ Open 2015; 5:
31 : Screening for the metabolic syndrome in community psychiatric patients prescribed antipsychotics: a quality improvement programme. Acta Psychiatr Scand 2008; 118:26–33Crossref, Medline, Google Scholar
32 : Antipsychotic cardiometabolic side effect monitoring in a state community mental health system. Community Ment Health J 2015; 51:685–694Crossref, Medline, Google Scholar
33 : Implementation of a clinical practice guideline for schizophrenia in a specialist mental health center: an observational study. BMC Health Serv Res 2016; 16(a):372Crossref, Medline, Google Scholar
34 : A health screening and promotion clinic to improve metabolic monitoring for patients prescribed antipsychotic medication. Ir J Psychol Med 2013; 30:113–118Crossref, Medline, Google Scholar
35 : Establishing a physical health monitoring service for patients on depot antipsychotic medication. Ir J Psychol Med (Epub Nov 7, 2016Crossref, Google Scholar
36 : Improving physical health monitoring for out-patients on antipsychotic medication. Psychiatrist 2010; 34:91–94Crossref, Google Scholar
37 : Implementing guidelines on physical health in the acute mental health setting: a quality improvement approach. Int J Ment Health Syst 2018; 12:1Crossref, Medline, Google Scholar
38 : Monitoring the metabolic side-effects of atypical antipsychotics. Psychiatrist 2010; 34:390–395Crossref, Google Scholar
39 : Screening for diabetes in patients receiving second-generation atypical antipsychotics. Am J Health Syst Pharm 2015; 72(suppl 2):S70–S73Crossref, Medline, Google Scholar
40 : Improving metabolic monitoring in patients maintained on antipsychotics in Penang, Malaysia. Australas Psychiatry 2016; 24:67–71Crossref, Medline, Google Scholar
41 : Improving metabolic syndrome screening on patients on second generation antipsychotic medication. Arch Psychiatr Nurs 2016; 30:671–677Crossref, Medline, Google Scholar
42 : Implementation science supports core clinical competencies: an overview and clinical example. Prim Care Companion CNS Disord 2016; 18:18Crossref, Google Scholar
43 : A randomized controlled trial of a patient-centered approach to improve screening for the metabolic side effects of antipsychotic medications. Community Ment Health J 2016; 53:163–175Crossref, Medline, Google Scholar
44 : The effectiveness of a computer reminder system for laboratory monitoring of metabolic syndrome in schizophrenic outpatients using second-generation antipsychotics. Pharmacopsychiatry 2015; 48:25–29Medline, Google Scholar
45 : Physical health of people with severe mental illness: don’t just screen . . . intervene! Br J Med Pract 2015; 8(3):a821Google Scholar
46 : Is metabolic syndrome on the radar? Improving real-time detection of metabolic syndrome and physician response by computerized scan of the electronic medical record. Prim Care Companion CNS Disord 2016; 18:18Google Scholar
47 : Implementation of a glucose screening program based on diffusion of innovation theory methods. Psychiatr Serv 2011; 62:12–14Link, Google Scholar
48 : Impact of a nurse-led intervention to improve screening for cardiovascular risk factors in people with severe mental illnesses: phase-two cluster randomised feasibility trial of community mental health teams. BMC Health Serv Res 2010; 10:61Crossref, Medline, Google Scholar
49 : Improving blood and ECG monitoring among patients prescribed regular antipsychotic medications. Ment Health Fam Med 2013; 10:29–36Medline, Google Scholar
50 : Monitoring weight and blood glucose in in-patients: how helpful is a protocol? Psychiatr Bull 2007; 31:88–91Crossref, Google Scholar
51 : Targeted intervention to improve monitoring of antipsychotic-induced weight gain and metabolic disturbance in first episode psychosis. Aust N Z J Psychiatry 2011; 45:740–748Crossref, Medline, Google Scholar
52 : Audit of monitoring of the parameters of metabolic syndrome in patients on clozapine. Psychiatrist 2012; 36:466–469Crossref, Google Scholar
53 : Physical healthcare of people with severe mental illness: everybody’s business! Ment Health Fam Med 2010; 7:115–122Medline, Google Scholar
54 : A case control study of the implementation of change model versus passive dissemination of practice guidelines for compliance in monitoring for metabolic syndrome. Community Ment Health J 2013; 49:141–149Crossref, Medline, Google Scholar
55 : Impact of a metabolic screening bundle on rates of screening for metabolic syndrome in a psychiatry resident outpatient clinic. Acad Psychiatry 2012; 36:118–121Crossref, Medline, Google Scholar
56 : Monitoring and management of metabolic abnormalities: mixed-method evaluation of a successful intervention. Australas Psychiatry 2014; 22:248–253Crossref, Medline, Google Scholar
57 : Defining quality improvement in public health. J Public Health Manag Pract 2010; 16:5–7Crossref, Medline, Google Scholar
58 : A Systematic Narrative Review of Quality Improvement Models in Health Care. Glasgow, National Health Service Quality Improvement Scotland, 2009Google Scholar
59 : Health care quality improvement publication trends. Am J Med Qual 2014; 29:403–407Crossref, Medline, Google Scholar
60 : Do we really need scholarly quality improvement? JAMA Pediatr 2019; 173:413–414Crossref, Medline, Google Scholar
61 : How to attribute causality in quality improvement: lessons from epidemiology. BMJ Qual Saf 2017; 26:933–937Crossref, Medline, Google Scholar
62 : Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inform Assoc 2008; 15:311–320Crossref, Medline, Google Scholar
63 : Changing physician behavior: what works? Am J Manag Care 2015; 21:75–84Medline, Google Scholar
64 : Clinician education improves lipid monitoring in patients taking second-generation antipsychotic agents, nationally and locally. Health Outcomes Res Med 2012; 3:e121–e37Crossref, Google Scholar
65 : Peer-led self-management of general medical conditions for patients with serious mental illnesses: a randomized trial. Psychiatr Serv 2018; 69:529–535Link, Google Scholar
66 : Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry 2010; 32:519–543Crossref, Medline, Google Scholar
67 : Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry 2009; 194:491–499Crossref, Medline, Google Scholar
68 : Assessment of a point-of-care metabolic risk screening program in outpatients receiving antipsychotic agents. Pharmacotherapy 2009; 29:975–987Crossref, Medline, Google Scholar
69 : Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006; 86:15–22Crossref, Medline, Google Scholar
70 : Roles in and barriers to metabolic screening for people taking antipsychotic medications: a survey of psychiatrists. Schizophr Res 2013; 143:395–396Crossref, Medline, Google Scholar
71 : Physical illness in patients with severe mental disorders: II. barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry 2011; 10:138–151Crossref, Medline, Google Scholar
72 : Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation 2005; 112:862–869Crossref, Medline, Google Scholar
73 : An electronic decision support system to motivate people with severe mental illnesses to quit smoking. Psychiatr Serv 2011; 62:360–366Link, Google Scholar
74 : Smoking cessation for people with severe mental illness (SCIMITAR+): a pragmatic randomised controlled trial. Lancet Psychiatry 2019; 6:379–390Crossref, Medline, Google Scholar
75 : Rigor or reliability and validity in qualitative research: perspectives, strategies, reconceptualization, and recommendations. Dimens Crit Care Nurs 2017; 36:253–263Crossref, Medline, Google Scholar
76 : Utilization of the Behavior Change Wheel framework to develop a model to improve cardiometabolic screening for people with severe mental illness. Implement Sci 2017; 12:134Crossref, Medline, Google Scholar




