Cost-Effectiveness of Preventing Depression Among At-Risk Youths: Postintervention and 2-Year Follow-Up
Abstract
Objective:
Youth depression can be prevented, yet few programs are offered. Decision makers lack cost information. This study evaluated the cost-effectiveness of a cognitive-behavioral prevention program (CBP) versus usual care.
Methods:
A cost-effectiveness analysis was conducted with data from a randomized controlled trial of 316 youths, ages 13–17, randomly assigned to CBP or usual care. Youths were at risk of depression because of a prior depressive disorder or subthreshold depressive symptoms, or both, and had parents with a prior or current depressive disorder. Outcomes included depression-free days (DFDs), quality-adjusted life years (QALYs), and costs.
Results:
Nine months after baseline assessment, youths in CBP experienced 12 more DFDs (p=.020) and .018 more QALYs (p=.007), compared with youths in usual care, with an incremental cost-effectiveness ratio (ICER) of $24,558 per QALY. For youths whose parents were not depressed at baseline, CBP youths had 26 more DFDs (p=.001), compared with those in usual care (ICER=$10,498 per QALY). At 33 months postbaseline, youths in CBP had 40 more DFDs (p=.05) (ICER=$12,787 per QALY). At 33 months, CBP youths whose parents were not depressed at baseline had 91 more DFDs (p=.001) (ICER=$13,620 per QALY). For youths with a currently depressed parent at baseline, CBP was not significantly more effective than usual care at either 9 or 33 months, and costs were higher.
Conclusions:
CBP produced significantly better outcomes than usual care and was particularly cost-effective for youths whose parents were not depressed at baseline. Depression prevention programs could improve youths’ health at a reasonable cost; services to treat depressed parents may also be warranted.
Depression among youths is a leading cause of disability (1, 2), including social and academic impairment, and is associated with increased rates of teen pregnancy, substance abuse, and suicide (1, 3). Factors linked to increased depression risk include a prior depressive episode, current subthreshold depressive symptoms (4, 5), and a family history of mood disorders. Approximately 10% to 20% of children live with a depressed parent (6, 7), and these children are three times more likely to develop depression and other psychopathology, compared with children of parents without a depression history (4, 5).
Although evidence-based treatments for youth depression exist (8, 9), only about 25% of depressed youths receive treatment; fewer than half of those treated achieve full remission (10). By the time depressed youths come to clinical attention, their symptoms are often serious. As severity and chronicity increase, treatment responsiveness decreases and costs grow (11, 12). Thus efforts to prevent depression are needed.
Youth depression can be prevented (13, 14), yet few at-risk youths have access to prevention services (14). One barrier has been a lack of information about the costs of depression prevention programs. Our study evaluated the cost-effectiveness of a cognitive-behavioral depression prevention program (CBP) for at-risk youths (15). The multisite randomized clinical trial built on the results of a single-site study (16), extending these findings to a larger, more diverse sample. Previous studies indicated a robust prevention effect (15), maintained for 2 years (17). The study reported here examined whether these significant clinical effects were cost-effective. We conducted cost-effectiveness analyses (CEAs) of CBP relative to usual care from a societal perspective for two time periods: immediately following the intervention (9 months postrandomization) and 2 years later (33 months postrandomization).
HIGHLIGHTS
| • | This study evaluated the cost-effectiveness of a cognitive-behavioral prevention (CBP) program versus usual care in preventing depression among at-risk youths. | ||||
| • | Youths in the CBP program had significantly more depression-free days and quality-adjusted life years (QALYs) at 9 months and 2 years postintervention. | ||||
| • | The program was cost-effective, particularly for youths whose parents were not depressed at baseline. For all participants, the cost per QALY was $24,558 at 9 months and $12,787 at 2 years. | ||||
Methods
Participants
Participants were 316 youths, ages 13–17 (mean±SD=14.8±1.4). Eligibility criteria were having current subsyndromal depressive symptoms (≥20 on the Center for Epidemiological Studies-Depression scale (18), a prior depressive episode in full remission for at least 2 months, or both. All youths had a parent with current or prior depression. Eighty percent of youths had at least one prior depressive episode; the mean number of prior episodes did not differ significantly by group (CBP=1.4±1.0; usual care=1.3±0.8). Youths were excluded if they currently were taking a therapeutic dosage of an antidepressant, they had received more than eight sessions of CBT for depression, or they or their biological parent had a diagnosis of bipolar I disorder or schizophrenia. Participants were recruited at Vanderbilt University, Nashville, Tennessee; University of Pittsburgh, Pittsburgh; Kaiser Permanente Northwest, Portland, Oregon; and Judge Baker Children’s Center and Children’s Hospital, Boston. Institutional review boards at each site approved the study. Parents and youths provided written informed consent and assent, respectively. Study recruitment began in August 2003 and ran through February 2006.
Youths were randomly assigned to CBP or usual care. CBP consisted of eight weekly, 90-minute sessions and six monthly continuation sessions led by a mental health professional. CBP taught cognitive restructuring and problem-solving skills and additional skills during continuation sessions. All youths in CBP or usual care could seek mental health services postrandomization. Thus youths were assigned to either usual care alone or usual care plus CBP (CBP, hereafter). Additional details about the study design are reported elsewhere (15).
Clinical Outcomes
At baseline, independent evaluators assessed youths’ current and lifetime depressive disorders and other diagnoses (for example, substance use disorders) with the Schedule for Affective Disorders and Schizophrenia for School-Age Children (19). Current depressive symptoms and severity were evaluated with the 17-item Children’s Depression Rating Scale–Revised (CDRS-R) (20) and the Longitudinal Interval Follow-up Evaluation (LIFE) (21) at 3-, 9-, 21-, and 33-month follow-ups. The LIFE provides a continuous assessment of symptoms and impairment since the previous assessment, rated on a 6-point Depression Symptom Rating (DSR) scale. The primary clinical outcome was a probable or definite episode of depression (that is, DSR ≥4) for at least 2 weeks. Parents’ current and past depressive episodes were evaluated with the Structured Clinical Interview for DSM-IV (22).
Following other CEAs of depression interventions, we created a measure of depression-free days (DFDs) (11, 23–25) by using CDRS-R scores from all follow-up points to categorize DFDs, days with some depression but not meeting full criteria, and days in a depressive episode. To calculate depressive symptoms for each day over the follow-up period, we used linear weighting to interpolate between the nondepressed and fully depressed thresholds and assign an estimated depression value to each day in the interval; days with fewer depression symptoms were weighted less. DFDs were the total number of days in the interval minus days with depressive symptoms. This approach captured the effects of the intervention, including both days with some depressive symptoms and days in a full depressive episode (24, 25). DFDs based on weekly DSR scores yielded similar results. [Additional details are provided in an online supplement to this article.]
We transformed DFDs into quality-adjusted life years (QALYs) with utility weights derived from empirical studies using large populations that allow stable estimation of the impact of depression on quality of life (24, 26). Specifically, each day in the interval received a QALY weight. A DFD with no depressive symptoms was assigned a utility weight of 1.0 (full health). Days in a full depressive episode were estimated to have a lower weight, reflecting a decrease in quality of life during a depressive episode. Depression has been associated with a decrease in quality of life of 0.2–0.6 (24, 26). We used 0.4 as the decrease in utility weight for the base-case analysis, such that a day in a full depressive episode would be assigned a weight of 0.6. We also explored a range of weights in sensitivity analyses.
Costs
Accounting records provided costs for payroll, facilities, and overhead. CBP leaders estimated time to complete tasks and use of equipment, space, and supplies. We included costs of CBP sessions, time leaders spent with individuals by phone or in person, supervision, training, and materials. We excluded research-specific costs (for example, randomization).
We administered the Child and Adolescent Services Assessment (CASA) (27) to gather data on services received outside the study by youths in both CBP and usual care. The CASA captures a wide range of services, including inpatient mental health, counseling, medication management, crisis services, and mental health counseling in nonhealth settings (for example, schools). We applied unit costs developed from the Truven Health MarketScan Commercial Claims Database for medical services; for social services, we used unit costs developed for other studies (11, 25). At baseline, youths and their parents separately reported on services that the youth received in the previous 3 months. At each follow-up, we assessed youths’ service use since the previous evaluation. Table 1 lists the mental health and other services that youths in CBP or usual care used, the percentage of youths receiving each service, and the frequency (mean) of services used.
| Any use (%) | Frequency of use | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 months | 33 months | 9 months | 33 months | |||||||||
| CBP | Usual care | CBP | Usual care | |||||||||
| Service | CBP | Usual care | CBP | Usual care | M | SD | M | SD | M | SD | M | SD |
| Inpatient mental health days | 1.4 | 1.3 | 3.4 | 3.4 | 46.5 | 58.7 | 11.0 | 9.9 | 6.6 | 76.6 | 15.2 | 15.0 |
| Inpatient alcohol or drug days | 1.4 | 0 | .7 | 0 | 24.0 | 28.3 | na | na | 25.5 | — | na | na |
| Counseling or medication management visits | 30.7 | 27.4 | 47.3 | 43.8 | 12.0 | 17.9 | 9.1 | 14.3 | 16.0 | 22.9 | 15.2 | 22.0 |
| Day hospital days | 0 | 0 | .7 | 2.1 | na | na | na | na | 107.0 | — | 11.7 | 9.5 |
| Alcohol or drug treatment visits | .7 | .6 | 4.1 | 2.1 | 13.0 | — | 33.0 | — | 8.0 | 5.1 | 31.0 | 26.1 |
| Crisis services | 2.1 | .6 | 2.7 | 2.7 | 31.7 | 37.9 | 2.0 | — | 24.3 | 34.3 | 4.3 | 4.0 |
| Medical doctor visits | 7.1 | 11.5 | 15.8 | 15.8 | 2.1 | 1.5 | 1.8 | 1.1 | 2.6 | 1.9 | 2.8 | 4.8 |
| Emergency department visits | 2.1 | 1.3 | 3.4 | 2.1 | 1.0 | 0 | 2.5 | 2.1 | 1.0 | 0 | 2.0 | 1.7 |
| Days of antidepressant medication | 5.1 | 5.1 | 15.8 | 12.7 | 120.9 | 77.8 | 126.0 | 86.5 | 145.0 | 194.5 | 168.8 | 214.4 |
| Days of stimulant medication | 3.2 | 1.9 | 8.9 | 6.4 | 105.6 | 74.2 | 61.0 | 30.0 | 202.1 | 257.0 | 535.9 | 806.6 |
| Days of other psychotropic medication | 0 | 1.3 | .6 | 1.9 | na | na | 92.0 | 86.3 | 121.0 | — | 223.7 | 236.1 |
| Any school services | 22.1 | 22.9 | 32.9 | 34.2 | 23.4 | 52.5 | 44.9 | 105.2 | 33.5 | 91.9 | 38.6 | 99.2 |
| Juvenile correction contact | .7 | 3.2 | 4.1 | 11.6 | 1.0 | — | 5.2 | 7.3 | 6.2 | 4.5 | 7.6 | 9.3 |
TABLE 1. Unadjusted service use among youths at risk of depression, by randomization condition (cognitive-behavioral depression prevention program [CBP] or usual care) and follow-up point
Following recommended guidelines (28), we estimated family costs for the time parents spent in taking youths to services. To value parent time, we created profiles based on published research on parent time spent in services, travel to services, and waiting (11, 25).
Statistical Analyses
We estimated incremental cost-effectiveness ratios (ICERs) from a health and public services perspective for 9- and 33-month postintake evaluations, computing the ratio in each instance as the mean cost difference between CBP and usual care divided by the mean difference in clinical outcome (DFD or QALY). In the base-case analysis, outcomes and costs beyond 12 months were discounted at a rate of 3%. Costs were adjusted for inflation. We used the net benefit regression method (29) with ordinary least-squares regression to examine cost-effectiveness; we confirmed the robustness of parametric tests by using nonparametric bootstrapping (30) with a single model with 1,000 replications and the bias-corrected and accelerated method (31). We performed hypothesis tests and estimated adjusted differences between groups by using ordinary least-squares regression models with bootstrap interval estimates. We adjusted all analyses for baseline CDRS-R scores, baseline costs, age, sex, race-ethnicity, and socioeconomic status.
Following recent work on highly influential observations (32), we examined all outliers, considering whether any observation met the strictest criteria for possible removal. Criteria for removal were observations above the 99th percentile for total costs and whose removal from analyses would influence parameter estimates by a factor of 1.25; both criteria well exceeded the suggested threshold for removal of .15 (32). On the basis of these criteria, in each analysis a highly influential observation was removed from the base-case analyses. We also conducted subanalyses with the outlier included to test for robustness.
To represent uncertainty in estimates, we first used bootstrap observations to estimate a 95% confidence interval (CI) around the average ICERs. We then created a scatterplot of bootstrapped cost-and-effect pairs to construct a cost-effectiveness plane (33) divided into four quadrants, centered at zero (Figure 1). If the majority of the cost-effect observations are located in the SE quadrant (less costly/more effective), then the CBP intervention is “dominant” over the alternative. Similarly, if most observations are in the NW quadrant (more costly/less effective), then the intervention is “dominated” by the alternative.
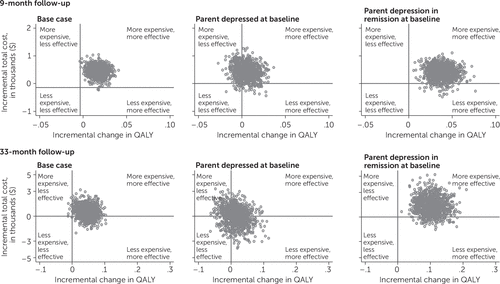
FIGURE 1. Cost-effectiveness planes for a cognitive-behavioral depression prevention program for the base-case analysis and for youths with or without a parent who was depressed at baseline, by follow-up pointa
aQALY, quality-adjusted life year.
Sensitivity Analyses
We conducted sensitivity analyses to examine the robustness of the results. We examined how results might change with different utility weights and with the inclusion of the highly influential observation. On the basis of the literature, we ran a model limited to outpatient costs because utilization of higher levels of care (for example, inpatient) is rare and likely not affected by a short-term intervention (34).
Missing Data
Of the 316 youths in the sample, approximately 85% (N=269) completed the 33-month assessment, 69% (N=218) completed all evaluations, and 98% (N=310) completed at least one follow-up assessment. Sample retention did not differ by group or site (15), demographic characteristics, entry characteristics, or depression measures (15). We imputed missing data by using Stata multiple imputation with chained equations (35, 36). We included baseline demographic characteristics and all nonmissing values of costs or outcomes at all time points in the imputation models. We created five imputation data sets and combined estimates so that standard errors reflected the variability introduced by the imputation process.
Results
Baseline characteristics and detailed group comparisons are reported elsewhere (15, 17). The sample included 131 males and 185 females, and the mean±SD age of the sample was 14.8±1.5. The race-ethnicity data were as follows: white, N=254; Hispanic, N=21. At baseline, no significant differences were found in any demographic or clinical characteristics between CBP youths and those in usual care.
Clinical Outcomes
The percentage of youths who experienced depressive episodes was smaller in the CBP group than in usual care over 9 months (21% versus 33%, p=0.03) (15) and over 33 months (37% versus 48%, p=0.04) (17). Compared with youths in usual care, at 9 months youths in CBP had 12 more DFDs (p=0.02) and .018 more QALYs (p=0.02). Two years later (that is, at 33 months), youths in CBP had 40 additional DFDs (p=0.05) and .045 more QALYs (p=0.05), compared with those in usual care. DFDs increased over time for both groups (p<0.001), but the proportion of DFDs for youths in CBP was significantly greater at each follow-up point.
Service Use and Costs
Table 1 presents descriptive estimates of services not related to the study, by treatment group. The pattern of service use was similar at both 9 and 33 months. Some differences were noted in the group means for specific services. On average (mean±SD), the cost of the CBP intervention, separate from use of nonstudy services, was $591±$286.
Cost-Effectiveness Analysis
Table 2 presents the ICERs, a measure of the estimated cost per DFD and QALY. At 9 months, the estimated cost was $35 per DFD and $24,558 per QALY. At 33 months, the cost was $15 per DFD and $12,787 per QALY.
| Outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical outcome | ||||||||||
| Cost ($) | DFD | QALY | Cost per DFD ($) | Cost per QALY ($) | ||||||
| Follow-up point and variable | M | SEb | M | SEb | M | SEb | M | 95% CIc | M | 95% CIc |
| 9 months | ||||||||||
| Base-case analysis (N=315) | ||||||||||
| CBP | 1,189 | 177 | 231.1 | 4.3 | .853 | .006 | ||||
| Usual care | 753 | 174 | 218.8 | 4.3 | .835 | .006 | ||||
| Difference (CBP–usual care)d | 436* | 184 | 12.3* | 4.9 | .018* | .007 | ||||
| ICER | 35 | 1 to 154 | 24,558 | 663 to 106,839 | ||||||
| Subgroup analysis | ||||||||||
| Parental depression, current | ||||||||||
| CBP | 1,326 | 281 | 229.6 | 6.4 | .850 | .009 | ||||
| Usual care | 892 | 271 | 226.1 | 6.6 | .845 | .009 | ||||
| Difference (CBP–usual care)d | 433 | 266 | 3.5 | 6.5 | .005 | .009 | ||||
| ICER | —e | —e | —e | —e | ||||||
| Parental depression, not current | ||||||||||
| CBP | 1,008 | 232 | 235.4 | 6.0 | .859 | .009 | ||||
| Usual care | 611 | 232 | 209.2 | 5.9 | .821 | .009 | ||||
| Difference (CBP–usual care) | 397 | 244 | 26.2** | 7.5 | .038** | .011 | ||||
| ICER | 15 | –6 to 43 | 10,498 | –3,793 to 29,965 | ||||||
| 33 months | ||||||||||
| Base-case analysis (N=315) | ||||||||||
| CBP | 3,879 | 667 | 887.6 | 17.8 | 2.586 | .020 | ||||
| Usual care | 3,305 | 667 | 846.7 | 17.6 | 2.541 | .020 | ||||
| Difference (CBP–usual care)d | 574 | 738 | 40.4* | 17.9 | .045* | .020 | ||||
| ICER | 14 | –28 to 133 | 12,787 | –24,991 to 119,958 | ||||||
| Subgroup analysis | ||||||||||
| Parental depression, current | ||||||||||
| CBP | 3,894 | 989 | 872.6 | 25.3 | 2.570 | .028 | ||||
| Usual care | 3,867 | 992 | 858.9 | 26.2 | 2.554 | .029 | ||||
| Difference (CBP–usual care) | 28 | 1,109 | 13.7 | 25.5 | .015 | .028 | ||||
| ICER | —e | —e | —e | —e | ||||||
| Parental depression, not current | ||||||||||
| CBP | 4,037 | 951 | 912.3 | 24.1 | 2.614 | .027 | ||||
| Usual care | 2,656 | 933 | 821.0 | 22.5 | 2.512 | .025 | ||||
| Difference (CBP–usual care) | 1,381 | 1,060 | 91.3** | 27.2 | .101** | .030 | ||||
| ICER | 15 | –5 to 53 | 13,620 | –4,111 to 47,563 | ||||||
TABLE 2. Differences in outcomes and ICERs associated with randomization condition (cognitive-behavioral depression prevention program [CBP] or usual care), by follow-up pointa
Figure 1 presents the cost-effectiveness planes for the base-case analysis and the subgroup analyses. Each point in the scatterplots represents a cost-clinical outcome pair for a single bootstrap replication. Most observations indicated that CBP had better outcomes and higher costs, 98% of observations at 9 months and 77% of observations at 33 months. In addition, at 33 months, 23% of observations had better outcomes and lower costs.
Figure 2 presents the cost-effectiveness acceptability curves for QALYs at 9 and 33 months for the base-case analysis and subgroup analyses. The curve represents the probability that CBP was cost-effective compared with usual care over a range of dollar amounts that a decision maker might pay for an additional outcome (for example, a QALY). For example, if a decision maker can pay $50,000 per QALY, the probability that CBP is cost-effective compared with usual care at 9 months is 85%, and at 33 months it is 89%.
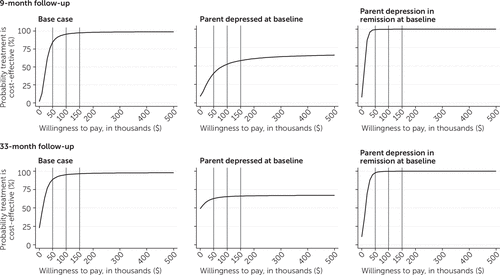
FIGURE 2. Cost-effectiveness acceptability curves for a cognitive-behavioral depression prevention program for the base-case analysis and for youths with or without a parent who was depressed at baseline, by follow-up point
Table 2 also presents data on the subgroup analyses. Elsewhere, we reported that time to onset of depressive episodes varied depending on whether the parent was currently depressed at baseline (15). Therefore, we examined CEAs by subgroup following the net benefit regression method (29). CBP had a higher net benefit for youths whose parents were not depressed at baseline (Table 2). At 9 months, youths whose parents were not depressed at baseline had estimated ICERs of $15 per DFD or $10,498 per QALY. At 33 months, estimated ICERs were $15 per DFD, or $13,620 per QALY. For youths whose parents were in a depressive episode at baseline, CBP had higher cost with no more effective outcomes than usual care at both 9 and 33 months.
Sensitivity analyses and secondary analyses examined whether CEA estimates were different when various parameters were used [see online supplement]. Results indicated some variation in cost per QALY, with a range of $10,229 to $53,383 per QALY.
Discussion
Despite evidence that programs for preventing depression among youths are effective (37), such interventions generally are neither offered nor covered by health insurance (38). One barrier has been a lack of information about the costs of such programs. We found that CBP was relatively low cost, at $591 per youth, and cost-effective by most standards (39). Results of our base-case analyses fell below suggested cost per QALY levels, and cost-effectiveness persisted over 2 years (39).
To date, only two studies—an analysis of a school-based program from Australia (40) and a single-site, randomized trial of an earlier version of CBP (16, 24)—have examined the cost of preventing depression among youths, and they found a lower cost per QALY than the base-case results reported here. Our study, however, compared CBP to usual care, which likely made our estimates more conservative than the school-based study that compared CBP to no intervention (40). Results for the better-performing CBP subgroup were similar to results of an earlier variant of CBP—$9,275 per QALY in the previous study (24) versus $10,498 per QALY in our study. In addition, our base-case results of $24,558 per QALY at 9 months and $12,787 per QALY at 33 months are substantially lower than reports of the cost per QALY for treating youth depression. Using inflation-adjusted results from recent depression treatment trials, the cost per QALY is in the range of at least $37,000 to $96,000 per QALY (11, 25, 41).
Elsewhere, we reported that youths whose parents were depressed at baseline had a poorer response to CBP than did those whose parents were not currently depressed (15). Our primary CEA included all youths in the study—those whose parents were and were not currently depressed. CBP was cost-effective, although this varied by parents’ current depression status. For youths whose parents were depressed, the program was not more effective than usual care and was modestly more expensive; thus it was not cost-effective. In contrast, for youths whose parents were not depressed, CEA results were $10,498 per QALY at 9 months and $13,620 at 33 months; these results meet even the most conservative standards for determining cost-effectiveness (39).
There may be value in combining parent and youth interventions or offering a sequenced approach, although there are likely challenges to coordinating care; some depressed parents may not participate in a program. Additional approaches may be necessary for youths whose parents are actively depressed, including individual interventions or family-based depression prevention programs (42, 43).
Approximately 30% of participants had some missing data. We did not include measures of some important depression-associated costs for youths, and we did not have data on school performance or other education costs. Although the CASA evaluates a range of services, including school counseling, it does not assess special education services. Similar to the negative workplace outcomes found for depressed adults, depression among youths is associated with negative educational outcomes (for example, poorer school performance and lower graduation rates) (44, 45). Our analyses did not include the impact of CBP on these outcomes. Improvements in depression likely lead to improved educational outcomes; therefore, our results may be conservative.
The study did not assess the cost of identifying youths who might benefit from CBP. Recent policies encouraging regular depression screening for youths and adults (46, 47) may help to identify youths who could benefit. Research regarding the costs and effectiveness of alternative identification and engagement approaches for prevention is an important next step.
Results were maintained over 2 years and may extend beyond this. The costs of CBP occurred immediately, but the benefits accrued over time. We presented both short- and longer-term analyses. Health systems may be most concerned about the immediate budgetary impact, whereas longer-term benefits may accrue to other entities or to society. The 2-year assessment revealed that the CBP program did not significantly increase costs over time.
We also did not include a separate measure of quality of life that directly assessed QALYs. Rather, we relied on indirect methods for translating DFDs into QALYs (23–25), and these measures used utility weights reported in the literature for depressed adults. Adult weights may not be an accurate proxy for youth weights, but no empirical weights yet exist for youths (48). The weights used here measured decreases in quality of life associated with depression but not other psychopathology or impairment, although presumably these would have been distributed randomly across treatment groups. Finally, for service utilization we used nationally representative unit costs, which may not reflect site costs.
Conclusions
Although depression among youths can be prevented (13), dissemination of programs depends on their costs and benefits. This study demonstrated that it is possible to prevent depression in at-risk youths in a cost-effective manner. However, future economic research is needed to replicate these results and to estimate the costs of identifying and screening youths. In addition, providing mental health services to currently depressed parents, either concurrently or prior to offering their children a prevention program, may be warranted.
Unfortunately, few health systems currently provide any mental health prevention services. Decision makers may not understand the benefits of prevention and thus may be more inclined to pay for treatment than for prevention. However, there is growing awareness of the relation of depression to long-term health (49), and prevention could reduce the overall clinical and financial impact of depression. Disseminating effective depression prevention programs could improve both short- and longer-term health of youths, and at a reasonable cost.
1 : Depressive disorders in childhood and adolescence; in Rutter’s Child and Adolescent Psychiatry. Edited by Rutter M, Bishop D, Pine D, . Oxford, England, Blackwell, 2008Crossref, Google Scholar
2 Maternal, Newborn, Child and Adolescent Health. Geneva, World Health Organization, 2016. http://www.who.int/maternal_child_adolescent/epidemiology/adolescence/en. Accessed May 18, 2016Google Scholar
3 : Estimating the economic burden of depression in children and adolescents. Am J Prev Med 2006; 31(suppl 1):S143–S151Crossref, Medline, Google Scholar
4 : Developmental risk of depression: experience matters. Child Adolesc Psychiatr Clin N Am 2012; 21:261–278Crossref, Medline, Google Scholar
5 : Depressed adolescents grown up. JAMA 1999; 281:1707–1713Crossref, Medline, Google Scholar
6 : Incidence of maternal and paternal depression in primary care: a cohort study using a primary care database. Arch Pediatr Adolesc Med 2010; 164:1038–1044Crossref, Medline, Google Scholar
7 : Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention. Washington, DC, National Academy of Sciences Press, 2009Google Scholar
8 : Child and adolescent depression intervention overview: what works, for whom and how well? Child Adolesc Psychiatr Clin N Am 2012; 21:299–312Crossref, Medline, Google Scholar
9 : Effects of psychotherapy for depression in children and adolescents: a meta-analysis. Psychol Bull 2006; 132:132–149Crossref, Medline, Google Scholar
10 : Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS). J Am Acad Child Adolesc Psychiatry 2006; 45:1404–1411Crossref, Medline, Google Scholar
11 : Incremental cost-effectiveness of combined therapy vs medication only for youth with selective serotonin reuptake inhibitor-resistant depression: treatment of SSRI-resistant depression in adolescents trial findings. Arch Gen Psychiatry 2011; 68:253–262Crossref, Medline, Google Scholar
12 : The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 2015; 76:155–162Crossref, Medline, Google Scholar
13 : Psychological and educational interventions for preventing depression in children and adolescents. Cochrane Database Syst Rev 2011; CD003380Medline, Google Scholar
14 : Reducing the global burden of depression: population-level analysis of intervention cost-effectiveness in 14 world regions. Br J Psychiatry 2004; 184:393–403Crossref, Medline, Google Scholar
15 : Prevention of depression in at-risk adolescents: a randomized controlled trial. JAMA 2009; 301:2215–2224Crossref, Medline, Google Scholar
16 : A randomized trial of a group cognitive intervention for preventing depression in adolescent offspring of depressed parents. Arch Gen Psychiatry 2001; 58:1127–1134Crossref, Medline, Google Scholar
17 : Prevention of depression in at-risk adolescents: longer-term effects. JAMA Psychiatry 2013; 70:1161–1170Crossref, Medline, Google Scholar
18 : The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. J Youth Adolesc 1991; 20:149–166Crossref, Medline, Google Scholar
19 : Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
20 : Children’s Depression Rating Scale–Revised. Psychopharmacol Bull 1984; 21:979–989Google Scholar
21 : The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry 1987; 44:540–548Crossref, Medline, Google Scholar
22 : Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (SCID-I/P) Version 2.0. New York, New York State Psychiatric Institute, Biometrics Research Dept, 1997Google Scholar
23 : Cost-effectiveness of treatments for major depression in primary care practice. Arch Gen Psychiatry 1998; 55:645–651Crossref, Medline, Google Scholar
24 : Cost-effectiveness of an intervention to prevent depression in at-risk teens. Arch Gen Psychiatry 2005; 62:1241–1248Crossref, Medline, Google Scholar
25 : Cost-effectiveness of treatments for adolescent depression: results from TADS. Am J Psychiatry 2008; 165:588–596Link, Google Scholar
26 : How bad is depression? Preference score estimates from depressed patients and the general population. Health Serv Res 2009; 44:1406–1423Crossref, Medline, Google Scholar
27 : Reliability of self-reported service use: test-retest consistency of children’s responses to the Child and Adolescent Services Assessment (CASA). J Child Fam Stud 1994; 3:307–325Crossref, Google Scholar
28 : Cost-Effectiveness in Health and Medicine. New York, Oxford University Press, 1996Google Scholar
29 : Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002; 11:415–430Crossref, Medline, Google Scholar
30 : An Introduction to the Bootstrap. London, Chapman and Hall, 1993Crossref, Google Scholar
31 : How should cost data in pragmatic randomised trials be analysed? BMJ 2000; 320:1197–1200Crossref, Medline, Google Scholar
32 : Impact of alternative approaches to assess outlying and influential observations on health care costs. Springerplus 2013; 2:614Crossref, Medline, Google Scholar
33 : A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005; 187:106–108Crossref, Medline, Google Scholar
34 : Incremental benefit and cost of telephone care management and telephone psychotherapy for depression in primary care. Arch Gen Psychiatry 2009; 66:1081–1089Crossref, Medline, Google Scholar
35 : Can one assess whether missing data are missing at random in medical studies? Stat Methods Med Res 2006; 15:213–234Crossref, Medline, Google Scholar
36 : Multiple imputation of missing values. Stata J 2004; 4:227–241Crossref, Google Scholar
37 : Major depression can be prevented. Am Psychol 2012; 67:285–295Crossref, Medline, Google Scholar
38 Prevention of Mental Disorders: Effective Interventions and Policy Options. Geneva, World Health Organization, 2004Google Scholar
39 : Using threshold values for cost per quality-adjusted life-year gained in healthcare decisions. Int J Technol Assess Health Care 2011; 27:71–76Crossref, Medline, Google Scholar
40 : The population cost-effectiveness of interventions designed to prevent childhood depression. Pediatrics 2012; 129:e723–e730Crossref, Medline, Google Scholar
41 : Cost-effectiveness of selective serotonin reuptake inhibitors and routine specialist care with and without cognitive behavioural therapy in adolescents with major depression. Br J Psychiatry 2007; 191:521–527Crossref, Medline, Google Scholar
42 : Brief psychotherapy for maternal depression: impact on mothers and children. J Am Acad Child Adoles Psychiatry 2016; 55:495–503Crossref, Medline, Google Scholar
43 : Family group cognitive-behavioral preventive intervention for families of depressed parents: 18- and 24-month outcomes. J Consult Clin Psychol 2011; 79:488–499Crossref, Medline, Google Scholar
44 : Lost human capital from early-onset chronic depression. Am J Psychiatry 2000; 157:940–947Link, Google Scholar
45 : Child mental health and human capital accumulation: the case of ADHD revisited. J Health Econ 2008; 27:794–800Crossref, Medline, Google Scholar
46 : Screening for Depression in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD, Agency for Healthcare Research and Quality, 2016Google Scholar
47 ; Screening for depression in children and adolescents: US Preventive Services Task Force recommendation statement. Ann Intern Med 2016; 164:360–366Crossref, Medline, Google Scholar
48 : Evaluating health-related quality-of-life studies in paediatric populations: some conceptual, methodological and developmental considerations and recent applications. Pharmacoeconomics 2005; 23:659–685Crossref, Medline, Google Scholar
49 Obama Health Care Summary. Washington, DC, ObamaCare Facts, 2014. http://obamacarefacts.com/obamahealthcare-summary/. Accessed June 1, 2016Google Scholar



