Mobile Health (mHealth) Versus Clinic-Based Group Intervention for People With Serious Mental Illness: A Randomized Controlled Trial
Abstract
Objective:
mHealth approaches that use mobile phones to deliver interventions can help improve access to care for people with serious mental illness. The goal was to evaluate how mHealth performs against more traditional treatment.
Methods:
A three-month randomized controlled trial was conducted of a smartphone-delivered intervention (FOCUS) versus a clinic-based group intervention (Wellness Recovery Action Plan [WRAP]). Participants were 163 clients, mostly from racial minority groups and with long-term, serious mental illness (schizophrenia or schizoaffective disorder, 49%; bipolar disorder, 28%; and major depressive disorder, 23%). Outcomes were engagement throughout the intervention; satisfaction posttreatment (three months); and improvement in clinical symptoms, recovery, and quality of life (assessed at baseline, posttreatment, and six months).
Results:
Participants assigned to FOCUS were more likely than those assigned to WRAP to commence treatment (90% versus 58%) and remain fully engaged in eight weeks of care (56% versus 40%). Satisfaction ratings were comparably high for both interventions. Participants in both groups improved significantly and did not differ in clinical outcomes, including general psychopathology and depression. Significant improvements in recovery were seen for the WRAP group posttreatment, and significant improvements in recovery and quality of life were seen for the FOCUS group at six months.
Conclusions:
Both interventions produced significant gains among clients with serious and persistent mental illnesses who were mostly from racial minority groups. The mHealth intervention showed superior patient engagement and produced patient satisfaction and clinical and recovery outcomes that were comparable to those from a widely used clinic-based group intervention for illness management.
Serious mental illnesses affect approximately 4% of the population (1). Functional impairments related to serious mental illness interfere with life activities, such as work, independent living, and self-care (2). Individuals with serious mental illness typically experience periods of illness exacerbation characterized by greater impairment interspersed with periods of partial or complete remission (3,4). With appropriate supports, people with serious mental illness can lead rewarding and productive lives, even in the context of ongoing symptoms (5,6).
Self-management interventions that increase and lengthen the periods in which people with serious mental illness remain healthier are popular and increasingly offered at clinics (7–12). Self-management interventions can help people adhere to treatment regimens, reduce the severity and distress associated with symptoms, avoid hospitalization, increase self-esteem, and improve perceived recovery (13). However, the barriers associated with clinic-based care may limit the benefits of these interventions. When individuals experience symptom exacerbations (arguably, when they need illness management support the most), they may avoid going to a clinic or interacting with others, perhaps because of the clinic’s distance from their residence or hours of operation (14,15), the stigma associated with seeking care (16), or dissatisfaction with services (17–19).
Mobile health (mHealth) approaches that use mobile phones in support of health care can help overcome some of the barriers associated with clinic-based care. Mobile phones are ubiquitous, even among people with serious mental illness, who often have limited access to resources (20–22). Research across continents has shown that the majority of adults with serious mental illness are interested in using their mobile phones as instruments for self-management (23). Early mHealth efforts have produced promising outcomes in terms of feasibility, acceptability, and preliminary efficacy in this population (24–29). Whether mHealth interventions can serve as stand-alone treatments, effectively engage individuals with serious mental illness in remote care, and produce clinical outcomes that are comparable to those of clinic-based interventions is unknown. As more mHealth and clinic-based interventions are made accessible in real-world practice, patients and their providers will have more options to choose from when deciding on their preferred model of care. Direct comparison of the strengths and weaknesses of existing interventions for a designated clinical problem is at the core of comparative effectiveness research.
Our objective was to compare smartphone-delivered mHealth to a clinic-based, group self-management intervention for people with serious mental illness. We evaluated differences between treatment groups in patient engagement, satisfaction, and clinical outcomes. To our knowledge, this article reports on the first comparative effectiveness trial with a head-to-head comparison of mHealth and a clinic-based intervention for people with serious mental illness.
Methods
We conducted an assessor-blind, two-arm, randomized controlled trial between June 2015 and September 2017 in partnership with Thresholds, a large agency that provides services to people with serious mental illness living in the midwestern United Sates. The study was approved by the Institutional Review Boards of the University of Washington and Dartmouth College and monitored by an independent safety monitoring board at Dartmouth’s Department of Psychiatry. All study participants completed informed consent. Individuals were randomly assigned (1:1 ratio) into one of two treatment arms: an mHealth intervention (FOCUS) or a clinic-based group intervention (Wellness Recovery Action Plan [WRAP]). Interventions were deployed for a period of 12 weeks, using cycles of eight cohorts of participants assigned to individual FOCUS or group-based WRAP over parallel periods. We conducted assessments at baseline (zero months), posttrial (three months), and follow-up (six months). Participants were not monetarily incentivized to engage in interventions but were compensated for completing assessments ($30 per assessment). [A CONSORT diagram of the study is available in an online supplement to this article.]
Participants
Participants were identified by using the electronic health record and then recruited by 20 clinical teams at three centers. Clinical staff approached candidates to describe the project and provide informational handouts with a contact number for the research team. Interested clients called study staff to learn more and undergo a brief phone screening. Suitable candidates were invited to attend a more comprehensive in-person evaluation meeting. Inclusion criteria were chart diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, or major depressive disorder; age ≥18 years; and a rating ≤3 on one of three items constituting the domination by symptoms factor from the Recovery Assessment Scale (RAS) (30,31), indicating a need for the type of resources both interventions may offer. Exclusion criteria were hearing, vision, or motor impairment affecting operation of a smartphone (determined by using a demonstration device); less than fifth-grade English reading ability (determined with the reading section of the WRAT-4 [32]); and exposure to WRAP or FOCUS in the past three years.
Randomization and Blinding
The study statistician created a computer-generated randomization list. Individual intervention allocations were placed in sequentially numbered envelopes containing instructions to contact an mHealth support specialist (for FOCUS) or a group facilitator (for WRAP) to schedule an appointment. Study assessors were excluded from study meetings in which procedures that would jeopardize blinding were discussed. Participants were instructed not to disclose their treatment allocation during assessments.
Interventions
FOCUS (25,33,34) is a multimodal, smartphone-delivered intervention for people with serious mental illness that includes three components: FOCUS application (app), clinician dashboard, and mHealth support specialist. The system includes preprogrammed daily self-assessment prompts and on-demand functions that can be accessed 24 hours a day. Self-management content targets five broad domains: voices (coping with auditory hallucinations via cognitive restructuring, distraction, and guided hypothesis testing), mood (managing depression and anxiety via behavioral activation, relaxation techniques, and supportive content), sleep (sleep hygiene, relaxation, and health and wellness psychoeducation), social functioning (cognitive restructuring of persecutory ideation, anger management, activity scheduling, and skills training), and medication (behavioral tailoring, reminders, and psychoeducation). Content can be accessed as either brief video or audio clips or sequences of digital screens with written material coupled with images (34). FOCUS users’ responses to daily self-assessments are displayed on a digital dashboard. Participants received brief weekly calls from an mHealth support specialist who assisted them in all technical and clinical aspects of the intervention (35). [A complete description of the interventions is available in the online supplement.]
WRAP (12) is a widely used (36) group self-management intervention led by trained facilitators with lived experience of mental illness. Sessions follow a sequenced curriculum, and specific group discussion topics and examples draw from the personal experiences of the participants and cofacilitators. The model emphasizes individuals’ equipping themselves with “personal wellness tools,” each focusing on recovery concepts (for example, hope, personal responsibility, and self-advocacy), language (for example, person-first recovery language), development of a WRAP (for example, establishing a daily maintenance plan and identifying and responding to triggers and early warning signs), and encouraging positive thinking (for example, changing negative thoughts to positive thoughts, building self-esteem, suicide prevention, and journaling). Facilitators incorporate these tools into a written plan, which includes daily maintenance, identification of triggers and methods to avoid them, identification of warning signs and response options, and a crisis management plan.
FOCUS and WRAP are similar in that both are recovery oriented, use an array of empowerment and self-management techniques, and involve similar intervention periods; empirical findings suggest that both interventions are engaging and beneficial to people with serious mental illness (25,34,37–40). The differences between these approaches represent core distinctions between mHealth and clinic-based models of care (that is, accessed in one’s own environment versus administered in a center, largely automated versus person delivered, and on demand versus scheduled).
Measures
Engagement.
We considered participants as commencing treatment if they used FOCUS once or attended one WRAP session. We calculated engagement in treatment for each participant weekly by using his or her FOCUS use data (logged by the software) or WRAP session attendance (logged by WRAP facilitators). Participants in the FOCUS arm were considered engaged if they used the app on at least five of seven days a week (that is, approximately 70%). A FOCUS “use” event is recorded as such only if, following a prompt, participants elect to engage in a clinical status assessment or if they self-initiate one of the FOCUS on-demand tools. Participants in the WRAP arm were considered engaged if they attended at least 60 minutes of the scheduled 90-minute group session (that is, approximately 70%) or completed a makeup session in the same week.
Satisfaction.
We measured satisfaction as the sum of five self-report items completed during the three-month, postintervention assessment. Participants rated the following statements with a 7-point rating scale (1, strongly disagree, to 7, strongly agree): I am satisfied with the treatment program, the treatment program helped me feel better, the treatment program was not interactive enough (reverse scored), I enjoyed the treatment program, and I would recommend the treatment program to a friend.
Clinical outcomes.
Our primary clinical outcome was general psychopathology, which is most appropriate for the different clinical groups represented in the sample of people with serious mental illness. General psychopathology was measured with the Symptom Checklist–9 (SCL-9) (41), a brief version of the Symptom Checklist–90R (42) that captures several domains of mental health (for example, anxiety, somatization, hostility, paranoid thinking, and psychoticism) and provides a single global rating of severity (43,44). Secondary clinical outcomes included depression, psychosis, recovery, and quality of life. Depression was assessed with the Beck Depression Inventory–Second Edition (BDI-II) (45), which includes 21 items rated on a 4-point scale, summed for a total depression severity score. Psychosis was assessed with the Psychotic Symptom Rating Scales (PSYRATS) (46). PSYRATS includes dimensions of auditory hallucinations (for example, frequency, duration, loudness, and distress) and delusions (for example, preoccupation, conviction, and disruption). Recovery was assessed with the RAS (30,31), a 24-item measure assessing five recovery factors with a 5-point Likert scale: personal confidence and hope, willingness to ask for help, goal and success orientation, reliance on others, and domination by symptoms. Quality of life was assessed as the total of six items focusing on one’s personal evaluation of one’s life, self, family, time spent with family, time spent with others, and participation in activities. Participants respond on a 7-point delighted-terrible scale. Clinical outcome measures were administered by assessors who were trained and supervised by licensed clinical psychologists with extensive experience in their administration among individuals with serious mental illness. Challenges in administration or scoring were discussed and resolved during weekly project team meetings.
Sample Size
We designed the study to detect a medium effect (defined as f=.24) in difference between groups in change in clinical outcome from baseline to three months, with 80% power (α=.05). This power is achieved with 72 participants per group. To allow for 10% attrition, 80 participants were randomly assigned to each group.
Analytic Approach
We used an intent-to-treat analysis that included all randomly assigned individuals. For treatment comparisons among clinical outcomes, we used mixed-effects models, including treatment condition, time of assessment, and an interaction term for treatment condition × time. Linear mixed models (47) were fit for all outcomes except PSYRATS, which was modeled via nonlinear Poisson hurdle mixed model, which estimates a logistic model for probability of a count >0 (likelihood of experiencing symptoms) as well as a Poisson model for mean symptom ratings if any symptoms were experienced (48). PSYRATS was modeled in this way because of the skewed nature and zero-inflation observed for this outcome (for example, 64% of individuals had a score of 0 at baseline), which made linear models inappropriate for this outcome. We evaluated engagement by using chi-square tests, and treatment satisfaction was assessed with t tests. [The online supplement includes a complete description of the analyses.]
Results
The study enrolled 163 participants, whose mean age was 49. Most participants were male (N=96, 59%) and African American (N=106, 65%). Diagnoses were as follows: schizophrenia or schizoaffective disorder, 49% (N=80); bipolar disorder, 28% (N=46); and major depressive disorder, 23% (N=37). Participants differed between treatment groups only in that significantly more participants randomly assigned to FOCUS had previously used a smartphone (73% versus 57%, χ2=4.81, df=1, p=.03). Table 1 summarizes participant characteristics by group.
| WRAP (N=81) | FOCUS (N=82) | |||
|---|---|---|---|---|
| Characteristic | N | % | N | % |
| Age (M±SD) | 49±9.8 | 49±10.1 | ||
| Male | 47 | 58 | 49 | 60 |
| Previously used smartphone | 46 | 57 | 60 | 73 |
| Race | ||||
| White | 22 | 28 | 22 | 27 |
| African American | 53 | 66 | 53 | 65 |
| Other or more than 1 race | 5 | 6 | 7 | 9 |
| Education | ||||
| High school or less | 48 | 59 | 51 | 63 |
| More than high school | 33 | 41 | 31 | 38 |
| Diagnosis | ||||
| Schizophrenia or schizoaffective disorder | 42 | 52 | 38 | 46 |
| Bipolar disorder | 25 | 31 | 21 | 26 |
| Major depressive disorder | 14 | 17 | 23 | 28 |
| Lifetime psychiatric hospitalizations | ||||
| 0 | 4 | 5 | 5 | 6 |
| 1–5 | 25 | 32 | 34 | 41 |
| 6–10 | 18 | 23 | 15 | 18 |
| 11–15 | 10 | 13 | 12 | 15 |
| 16–20 | 5 | 6 | 4 | 5 |
| ≥20 | 17 | 22 | 12 | 15 |
TABLE 1. Baseline characteristics of participants in Wellness Recovery Action Plan (WRAP) and FOCUS
Engagement
Following randomization, 90% (N=74) of participants assigned to FOCUS commenced use of the mHealth app, and 58% (N=47) of those assigned to WRAP attended at least one group session (χ2=22.11, df=1, p<.001) (Figure 1). Averaging across all participants assigned to FOCUS, participants used the app on 5.4±2.4 days in the first week of the intervention, on 4.6±2.7 days in the third week, on 4.3±2.7 days in the sixth week, on 3.9±2.7 days in the ninth week, and on 3.8±2.9 days in the last week. In the first week of WRAP, 48% (N=39) of assigned participants attended the weekly meeting; 42% (N=34) attended in the third and sixth weeks, 36% (N=29) attended in the ninth week, and 28% (N=23) attended in the last week. FOCUS group participants were more likely than WRAP participants to fully engage in treatment for at least eight weeks (56% versus 40%) (χ2=4.50, df=1, p=.03) (Figure 1). The groups did not differ in the proportions of participants fully engaging in all weeks of treatment.
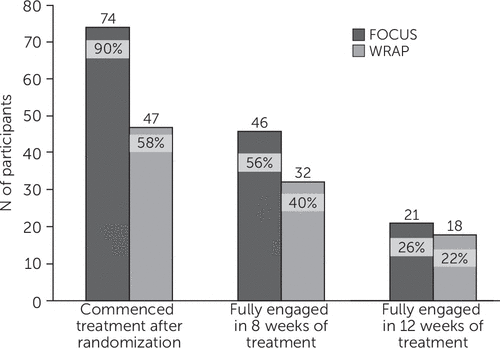
FIGURE 1. Percentage of patients fully engaged in Wellness Recovery Action Plan (WRAP) and FOCUS, by stage of intervention
Satisfaction
Mean posttreatment satisfaction ratings were similar between groups: overall ratings of 25.7±3.8 for FOCUS and 25.5±3.6 for WRAP (t=–.31, df=1, p=.76). Figure 2 shows average responses for the individual satisfaction items. No adverse events were reported for participants in either intervention arm.
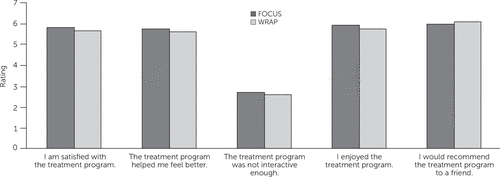
FIGURE 2. Mean satisfaction ratings after treatment among participants in Wellness Recovery Action Plan (WRAP) and FOCUSa
aResponses range from 1, strongly disagree, to 7, strongly agree.
Clinical Outcomes
Treatment groups did not differ in change from baseline to three months postintervention on primary and secondary clinical outcomes. Primary analyses found no significant differences in clinical outcomes between diagnostic groups. Exploratory analyses found within-group changes. Table 2 summarizes the mean scores for clinical outcomes. Improvement between baseline and three months (end of treatment) in the primary clinical outcome, general psychopathology as measured by the SCL-9, was seen for FOCUS (mean±SE of the estimated mean difference=−2.73±.75, t=−3.64, df=289, p<.001) and WRAP (−2.14±.76, t=−2.84, df=289, p=.005). Similar improvements in SCL-9 scores were seen between baseline and six months for FOCUS (−2.51±.75, t=−3.33, df=289, p=.001) and for WRAP (−1.93±.77, t=−2.51, df=289, p=.01). Improvement between baseline and three months in BDI-II scores was seen for FOCUS (−2.76±1.09, t=−2.54, df=289, p=.01) and WRAP (−2.33±1.10, t=−2.13, df=289, p=.03). Similar improvements in BDI-II were seen between baseline and six months for FOCUS (−4.21±1.09, t=−3.85, df=289, p<.001) and for WRAP (−3.74±1.12, t=−3.34, df=289, p<.001). Improvements between baseline and three months in RAS scores were seen for WRAP (2.44±1.10, t=2.21, df=288, p=.03). Between baseline and six months, improvements in RAS scores were seen for FOCUS (4.56±1.10, t=4.16, df=289, p<.001) and for WRAP (2.86±1.12, t=2.55, df=289, p=.01). No significant within-group differences in PSYRATS or quality-of-life scores were noted between baseline and three months. Between baseline and six months, significant improvements were seen in quality of life among the FOCUS participants (1.58±.62, t=2.55, df=289, p=.01).
| Baseline | 3 months (end of treatment) | 6-month follow-up | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FOCUS | WRAP | FOCUS | WRAP | FOCUS | WRAP | |||||||||||||
| Measure | N | M | SD | N | M | SD | N | M | SD | N | M | SD | N | M | SD | N | M | SD |
| SCL-9a | 82 | 12.71 | 7.24 | 81 | 11.93 | 8.06 | 75 | 10.0b | 6.52 | 74 | 9.53b | 7.33 | 74 | 10.38c | 8.08 | 70 | 9.99c | 7.76 |
| BDI-IId | 82 | 22.00 | 11.20 | 81 | 19.53 | 12.09 | 75 | 19.08b | 12.57 | 74 | 16.80b | 11.66 | 74 | 17.85c | 12.79 | 70 | 16.03c | 11.61 |
| PSYRATSe | ||||||||||||||||||
| Score >0 | 82 | 31 | 38 | 81 | 28 | 35 | 75 | 25 | 33 | 74 | 18 | 24 | 74 | 19 | 26 | 69 | 11 | 16 |
| Among those with score >0 | 31 | 21.6 | 12.1 | 28 | 23.0 | 13.9 | 25 | 30.6 | 12.1 | 18 | 25.0 | 15.1 | 19 | 27.7 | 14.0 | 11 | 29.5 | 14.0 |
| RASf | 82 | 90.34 | 13.33 | 81 | 91.72 | 13.23 | 75 | 92.39 | 11.91 | 73 | 94.89b | 15.89 | 74 | 94.59 c,g | 13.02 | 70 | 94.40c | 13.74 |
| Quality of lifeh | 81 | 26.54 | 7.10 | 82 | 26.69 | 7.46 | 75 | 26.87 | 7.44 | 74 | 27.80 | 7.48 | 74 | 28.11c | 7.30 | 70 | 27.60 | 7.91 |
TABLE 2. Clinical outcomes over time among participants in Wellness Recovery Action Plan (WRAP) and FOCUS (intent-to-treat sample)
Treatment groups did not differ on changes from three months postintervention to the six-month follow-up. Within treatment group, improvement in RAS scores was seen for FOCUS (2.74±1.11, t=2.46, df=288, p=.01) (Table 2).
FOCUS Effects
In the FOCUS group, education and treatment engagement were significantly associated with secondary clinical outcomes. Having more than a high school education was associated with larger increases in RAS scores (mean±SE of the estimated mean difference from baseline to three months=4.31±2.15, t=1.71, df=145, p=.05), as was more weeks of engagement (five or more days per week of FOCUS use) (.57±.28, t=2.07, df=133, p=.04). Age, gender, race, past use of a smartphone, and number of hospitalizations were not associated with mHealth intervention outcomes.
Discussion
The FOCUS mHealth intervention produced clinical outcomes and patient satisfaction ratings that were comparable to those of WRAP, an evidence-based, self-management intervention. No adverse events were reported for participants in either intervention arm. FOCUS had significantly higher treatment commencement rates after random assignment (90%), compared with WRAP (58%). These findings suggest that FOCUS was easier to initiate or more accessible. Significantly more FOCUS participants fully completed eight or more weeks of treatment (56%), compared with WRAP (40%). Groups did not differ significantly in the percentage of participants who fully completed 12 weeks of treatment. Taken together, participants were exposed to treatment content more often and over longer intervention periods (dose) via FOCUS than via clinic-based WRAP.
Satisfaction with treatment did not differ across groups. Participants provided high satisfaction ratings for FOCUS and WRAP, and participants in each approach reported that it was enjoyable and interactive and helped them feel better. We draw several conclusions. First, people with serious mental illness can be satisfied with mHealth treatments that are largely automated, involving weekly remote check-in calls but minimal in-person contact. Second, although barriers related to clinic-based care might have affected treatment commencement and engagement in WRAP, such barriers did not negatively affect participants’ overall impressions of the intervention. Participants in each arm were not exposed to the other treatment, and thus their satisfaction ratings were not grounded in familiarity with an alternative. A cross-over design in which participants experienced both interventions would enable more direct comparison of satisfaction.
Changes in primary (general psychopathology) and secondary (depression, psychosis, recovery, and quality of life) clinical outcomes did not differ by intervention. No differences were seen between groups in retention of gains three months after the conclusion of the intervention (at six-month follow-up). Exploratory analyses within treatment arms showed significant and comparable reductions in psychopathology and depression in both treatment groups. Significant improvements in recovery were seen in the WRAP group at the end of treatment (three months) and in the FOCUS group at six-month follow-up.
Among FOCUS participants, a higher level of education and greater treatment engagement (that is, weeks of daily smartphone app use) were both linked to greater recovery postintervention. Notably, age, gender, race, having previous experience with smartphones, and number of previous psychiatric hospitalizations (an indicator of baseline illness severity) were not associated with clinical outcomes.
This study had notable strengths. To our knowledge, it is the first randomized controlled trial examining the effects of a smartphone intervention involving individuals with schizophrenia spectrum disorders. The comparator intervention (WRAP) is an active evidence-based treatment. Both interventions were introduced at the study site at the same time, ensuring equal levels of enthusiasm among study staff and clinical personnel. Additional methodological strengths included use of psychometrically sound outcomes measures, random assignment, maintenance of assessor blindness throughout the study, and deployment of interventions as parallel cohorts to control for historical effects. Both interventions were delivered with guidance from treatment experts; the mHealth support specialist was trained and supervised via phone by the lead FOCUS developer (DBZ). WRAP facilitators were trained by the Copeland Center for Wellness and Recovery (the premiere WRAP training and education center) and supervised on site by a certified advanced-level WRAP facilitator.
The study had several limitations. We did not include a treatment-as-usual comparator arm. We thus cannot conclude that the clinical outcomes reported in the study are a direct result of the interventions deployed rather than of the passage of time or of artifacts related to involvement in research. FOCUS participants received a smartphone with an active data plan, which may be less likely in standard care. However, once they were randomly assigned to the mHealth arm, participants’ access to the device and data were not contingent upon their use of the intervention app. This noncontingent access suggests that ongoing engagement in FOCUS was not for secondary gains. In addition to receiving new interventions in the context of research, participants continued to receive various services from the community agency, which may have influenced the results. The measures of engagement and satisfaction were developed for this study and have not been validated in previous research. Because the study was powered to detect differences in the full sample between treatment groups, exploratory analyses had reduced power, and thus the results should be interpreted cautiously.
The findings support the notion that mHealth can play an important role in 21st century mental health care (49,50). Contemporary mobile phone “smart” functionalities enable these devices to serve as much more than static information repositories (51). Audio and video media players, graphic displays, interactive capabilities, bidirectional calling and texting, and Internet connectivity create new opportunities to engage patients with both automated resources and human supports. The portability of smartphones enables patients to take them wherever they go. FOCUS users in this study could read, hear, or view self-management skills, suggestions, and demonstrations that were relevant to the challenges they encountered as they went about their daily life. Instead of having to retain and recall clinicians’ suggestions, they accessed their “pocket therapist” on demand—an experience akin to a friend checking in on them (34). FOCUS users’ daily self-assessments were relayed to their mHealth support specialist, who reviewed the data to better understand what they were experiencing. This information was brought up in their weekly check-in calls, which likely strengthened the feeling that a caring individual was paying attention to their status (35). The combination of automated functions and live remote human support facilitated a therapeutic model unlike any they had encountered. For some, this proved to be engaging and helpful.
Conclusions
The FOCUS model is one of several promising mHealth approaches. Additional paradigms include using mHealth applications to augment clinic-based services to improve their efficacy (52,53) and leveraging multimodal smartphone embedded sensors (for example, GPS, accelerometers, and microphone) to unobtrusively collect information about patients’ behavior, context, and functioning (54,55). These mobile data can be shared with clinicians to enhance their patient-monitoring capabilities and inform more tailored in-person care (56,57).
Evidence from mHealth research will accumulate over the upcoming years, including additional promising results from randomized controlled trials. As more mHealth interventions prove to be engaging and clinically useful to patients with serious mental illness, enthusiasm for their use in clinical practice will grow (15). In the future, it will be important to ensure that these mHealth technologies are deployed ethically and responsibly (58).
1 Key Substance Use and Mental Health Indicators in the United States: Results From the 2015 National Survey on Drug Use and Health. SMA 16-4984, NSDUH Series H-51. Rockville, MD, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2016Google Scholar
2 Substance Abuse and Mental Health Services Administration, Center for Mental Health Services: Final notice. Federal Register 58: 29422–29425, 1993Google Scholar
3 : Periods of recovery in deficit syndrome schizophrenia: a 20-year multi-follow-up longitudinal study. Schizophrenia Bulletin 36:788–799, 2010Crossref, Medline, Google Scholar
4 : Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. Journal of Affective Disorders 108:49–58, 2008Crossref, Medline, Google Scholar
5 : Remission and recovery in schizophrenia: practitioner and patient perspectives. Schizophrenia Bulletin 34:5–8, 2008Crossref, Medline, Google Scholar
6 : Recovery: the lived experience of rehabilitation. Psychosocial Rehabilitation Journal 11:11–19, 1988Crossref, Google Scholar
7 : Introduction to the special issue on illness self-management. Psychiatric Rehabilitation Journal 36:229–230, 2013Crossref, Medline, Google Scholar
8 : Pathways to Recovery: A Strengths Recovery Self-Help Workbook. Lawrence, KS, University of Kansas School of Social Welfare, 2002Google Scholar
9 : The Recovery Workbook: Practical Coping and Empowerment Strategies for People With Psychiatric Disability. Leader’s Guide. Boston, Boston University, Sargent College of Health and Rehabilitation Sciences, Center for Psychiatric Rehabilitation, 2000Google Scholar
10 : Building Recovery of Individual Dreams and Goals Through Education and Support: A Peer-Taught Curriculum on Recovery From Mental Illness. Chicago, University of Illinois, 2006Google Scholar
11 Illness Management and Recovery: Practitioner Guides and Handouts. Rockville, MD, Substance Abuse and Mental Health Services Administration, 2009Google Scholar
12 : Wellness Recovery Action Plan and Peer Support: Personal, Group and Program Development. Brattleboro, VT, Peach Press, 2004Google Scholar
13 : Reviewing illness self-management programs: a selection guide for consumers, practitioners, and administrators. Psychiatric Services 66:1180–1193, 2015Link, Google Scholar
14 : Disengagement from mental health treatment among individuals with schizophrenia and strategies for facilitating connections to care: a review of the literature. Schizophrenia Bulletin 35:696–703, 2009Crossref, Medline, Google Scholar
15 : Technology in mental health: creating new knowledge and inventing the future of services. Psychiatric Services 68:107–108, 2017Link, Google Scholar
16 : How stigma interferes with mental health care. American Psychologist 59:614–625, 2004Crossref, Medline, Google Scholar
17 : Unmet need for mental health care in schizophrenia: an overview of literature and new data from a first-admission study. Schizophrenia Bulletin 35:679–695, 2009Crossref, Medline, Google Scholar
18 : Implementing evidence-based practices for people with schizophrenia. Schizophrenia Bulletin 35:704–713, 2009Crossref, Medline, Google Scholar
19 : Illness management and recovery in community practice. Psychiatric Rehabilitation Journal 39:343–351, 2016Crossref, Medline, Google Scholar
20 : Mobile technologies among people with serious mental illness: opportunities for future services. Administration and Policy in Mental Health and Mental Health Services Research 40:340–343, 2013Crossref, Medline, Google Scholar
21 : Patient smartphone ownership and interest in mobile apps to monitor symptoms of mental health conditions: a survey in four geographically distinct psychiatric clinics. JMIR Mental Health 1:e5, 2014Crossref, Medline, Google Scholar
22 : How people with serious mental illness use smartphones, mobile apps, and social media. Psychiatric Rehabilitation Journal 39:364–367, 2016Crossref, Medline, Google Scholar
23 : Mobile phone ownership and endorsement of “mHealth” among people with psychosis: a meta-analysis of cross-sectional studies. Schizophrenia Bulletin 42:448–455, 2016Crossref, Medline, Google Scholar
24 : Mobile Assessment and Treatment for Schizophrenia (MATS): a pilot trial of an interactive text-messaging intervention for medication adherence, socialization, and auditory hallucinations. Schizophrenia Bulletin 38:414–425, 2012Crossref, Medline, Google Scholar
25 : Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophrenia Bulletin 40:1244–1253, 2014Crossref, Medline, Google Scholar
26 : The efficacy of SMS text messages to compensate for the effects of cognitive impairments in schizophrenia. British Journal of Clinical Psychology 49:259–274, 2010Crossref, Medline, Google Scholar
27 : Feedback on SMS reminders to encourage adherence among patients taking antipsychotic medication: a cross-sectional survey nested within a randomised trial. BMJ Open 5:e008574, 2015Crossref, Medline, Google Scholar
28 : First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. Journal of Clinical Psychiatry 74:e533–e540, 2013Crossref, Medline, Google Scholar
29 : Smartphone data as objective measures of bipolar disorder symptoms. Psychiatry Research 217:124–127, 2014Crossref, Medline, Google Scholar
30 : Recovery as a psychological construct. Community Mental Health Journal 35:231–239, 1999Crossref, Medline, Google Scholar
31 : Examining the factor structure of the Recovery Assessment Scale. Schizophrenia Bulletin 30:1035–1041, 2004Crossref, Medline, Google Scholar
32 : Wide Range Achievement Test, Fourth Edition (WRAT–4) Professional Manual. Lutz, FL, Psychological Assessment Resources, Inc, 2004Google Scholar
33 : Development and usability testing of FOCUS: a smartphone system for self-management of schizophrenia. Psychiatric Rehabilitation Journal 36:289–296, 2013Crossref, Medline, Google Scholar
34 : Video-based mobile health interventions for people with schizophrenia: bringing the “pocket therapist” to life. Psychiatric Rehabilitation Journal 41:39–45, 2018Crossref, Medline, Google Scholar
35 : Augmenting mHealth with human support: notes from community care of people with serious mental illnesses. Psychiatric Rehabilitation Journal 40:336–338, 2017Crossref, Medline, Google Scholar
36 : The rediscovery of recovery: open to all. Advances in Psychiatric Treatment 10:37–48, 2004Crossref, Google Scholar
37 : mHealth for schizophrenia: patient engagement with a mobile phone intervention following hospital discharge. JMIR Mental Health 3:e34, 2016Crossref, Medline, Google Scholar
38 : Results of a randomized controlled trial of mental illness self-management using Wellness Recovery Action Planning. Schizophrenia Bulletin 38:881–891, 2012Crossref, Medline, Google Scholar
39 : A randomized controlled trial of effects of Wellness Recovery Action Planning on depression, anxiety, and recovery. Psychiatric Services 63:541–547, 2012Link, Google Scholar
40 : Developing the evidence base for peer-led services: changes among participants following Wellness Recovery Action Planning (WRAP) education in two statewide initiatives. Psychiatric Rehabilitation Journal 34:113–120, 2010Crossref, Medline, Google Scholar
41 : Construction and statistical test of a short form of the SCL-90-R [in German]. Zeitschrift für Klinische Psychologie, Psychiatrie und Psychotherapie 49:115–124, 2001Google Scholar
42 : Symptom Checklist‐90‐Revised. New York, Wiley, 2010Google Scholar
43 : Comparative psychometric analyses of the SCL-90-R and its short versions in patients with affective disorders. BMC Psychiatry 13:104, 2013Crossref, Medline, Google Scholar
44 : Comparison of eleven short versions of the Symptom Checklist 90-Revised (SCL-90-R) for use in the assessment of general psychopathology. Journal of Psychopathology and Behavioral Assessment 32:246–254, 2010Crossref, Google Scholar
45 : Beck Depression Inventory–II. San Antonio, TX, Psychological Corp, 1996Google Scholar
46 : Scales to measure dimensions of hallucinations and delusions: the Psychotic Symptom Rating Scales (PSYRATS). Psychological Medicine 29:879–889, 1999Crossref, Medline, Google Scholar
47 : Applied Longitudinal Analysis. New York, Wiley, 2012Google Scholar
48 : A tutorial on count regression and zero-altered count models for longitudinal substance use data. Psychology of Addictive Behaviors 27:166–177, 2013Crossref, Medline, Google Scholar
49 : The application of mHealth to mental health: opportunities and challenges. Lancet Psychiatry 2:942–948, 2015Crossref, Medline, Google Scholar
50 : The future is in our hands: the role of mobile phones in the prevention and management of mental disorders. Australian and New Zealand Journal of Psychiatry 47:111–113, 2013Crossref, Medline, Google Scholar
51 : Using contemporary technologies in the assessment and treatment of serious mental illness. American Journal of Psychiatric Rehabilitation 15:357–376, 2012Crossref, Google Scholar
52 : Augmenting psychoeducation with a mobile intervention for bipolar disorder: a randomized controlled trial. Journal of Affective Disorders 174:23–30, 2015Crossref, Medline, Google Scholar
53 : Patient-driven innovation for mobile mental health technology: case report of symptom tracking in schizophrenia. JMIR Mental Health 4:e27, 2017Crossref, Medline, Google Scholar
54 : Using smartphones to collect behavioral data in psychological science: opportunities, practical considerations, and challenges. Perspectives on Psychological Science 11:838–854, 2016Crossref, Medline, Google Scholar
55 : Personal sensing: understanding mental health using ubiquitous sensors and machine learning. Annual Review of Clinical Psychology 13:23–47, 2017Crossref, Medline, Google Scholar
56 : CrossCheck: integrating self-report, behavioral sensing, and smartphone use to identify digital indicators of psychotic relapse. Psychiatric Rehabilitation Journal 40:266–275, 2017Crossref, Medline, Google Scholar
57 : Development and evaluation of a smartphone-based measure of social rhythms for bipolar disorder. Assessment 23:472–483, 2016Crossref, Medline, Google Scholar
58 : The ethical use of mobile health technology in clinical psychiatry. Journal of Nervous and Mental Disease 205:4–8, 2017Crossref, Medline, Google Scholar



