Risk Factors for Utilization of Acute Care Services for Lithium Toxicity
Abstract
Objective:
This study evaluated risk factors for utilization of acute care services (ACS) (hospitalization or emergency department or urgent care visit) for lithium toxicity and the prevalence of lithium toxicity in a large, ambulatory population.
Methods:
A nested case-control study compared lithium users with ACS utilization for lithium toxicity (case group) to lithium users without toxicity (control group) by using data from Kaiser Permanente Colorado for patients with at least one lithium prescription purchase. Patients in the case group were matched 1:5 with patients in the control group who had purchased lithium within 39 days of the ACS encounter. Possible lithium toxicity, identified by lithium level or diagnosis, was confirmed by chart review. Multivariable, conditional logistic regression analysis was used to identify patient and prescription characteristics associated with ACS utilization for lithium toxicity. The prevalence of lithium toxicity was determined.
Results:
Of 3,115 individuals who took lithium, 70 experienced lithium toxicity, with or without ACS utilization, for a prevalence of 2.2%. Identified risk factors for ACS utilization for lithium toxicity included a newly initiated potentially interacting medication (odds ratio [OR]=30.30, 95% confidence interval [CI]=2.32–394.95), a higher number of treated chronic diseases (OR=1.28, CI=1.12–1.45), older age (OR=1.05, CI=1.02–1.09), and higher total daily lithium dose (OR=1.00, CI=1.00–1.00).
Conclusions:
Newly initiated, potentially interacting medications are a major preventable driver of ACS use for lithium toxicity, whereas age, chronic disease, and total daily lithium dose are small but significant factors. Clinicians should use extra caution when initiating a potentially interacting medication.
Lithium is recommended as a first-line option for treatment of bipolar disorder and refractory unipolar depression (1–3). However, its use is limited by the necessity of close therapeutic drug monitoring to prevent toxicity and by concerns about long-term risks, including development of chronic kidney disease (4–6). Lithium toxicity is commonly classified into three categories: acute toxicity, which occurs after recent initiation of lithium; acute on chronic toxicity, which occurs after an excessive amount of lithium is ingested by a chronic user of lithium; and chronic toxicity, which occurs after long-term use with no increased acute ingestion (7,8). Severe lithium toxicity typically ensues at serum lithium levels ≥1.5 mEq/L, but toxicity can also occur at levels considered therapeutic (7).
Among cohorts of patients hospitalized for lithium toxicity, risk factors for lithium toxicity include older age and factors that lead to disturbances in renal blood flow and water-salt homeostasis, such as fever, diarrhea or vomiting, and use of nonsteroidal anti-inflammatory drugs (NSAIDs), diuretics, and renin-angiotensin-aldosterone system (RAAS) inhibitors (8–11). Female sex has also been associated with a higher risk of lithium toxicity (9). Severe symptoms, such as neurotoxicity, may be more common in cases of chronic toxicity compared with acute toxicity, suggesting that duration of lithium use may also be a factor in the development of toxicity (8). Population-based risk factor assessment in the general ambulatory population has been limited to univariate analysis of the effects of individual factors on toxicity, defined administratively by hospital diagnosis code or a lithium level >1.5 mmol/L (4,12). Factors that have been studied include age, drug-drug interactions, and renal function (4,12).
The population-based prevalence of lithium toxicity associated with current dosing and monitoring guidelines is not well characterized. Over decades of lithium treatment, health systems have developed strategies to reduce population risk of lithium toxicity, including conservative dosing strategies, monitoring of lithium concentration and renal function, drug-drug interaction alerts, patient education, and clinical decision support tools (2,13,14). One prior population-based study estimated that 3.9% of patients age 66 or older using lithium were hospitalized for lithium toxicity over a nine-year period (12). A second population-based study determined that 7% of all ambulatory patients using lithium experienced a lithium level of ≥1.5 mmol/L, an incidence rate of one per 100 for every year of treatment (4).
In a comparison of health care utilization by patients with bipolar disorder treated for acute mania with lithium or valproate, lithium was associated with a higher number of hospital visits (15). It is unknown whether these visits were due to lithium toxicity, but lithium toxicity may be associated with utilization of higher-level health care resources, such as hospitalization, emergency department visits, or urgent care visits. Only one previous population-based study assessed utilization of higher levels of care for lithium toxicity (12). The study was limited to elderly hospitalized patients and may not be generalizable. Factors that drive the need for higher levels of care for lithium toxicity among ambulatory patients are not well understood. Further development of clinical programs targeting population-based risk factors for utilization of acute care services (ACS) for lithium toxicity may improve care.
The purpose of this study was to describe the population risk for lithium toxicity and determine risk factors associated with utilization of ACS for lithium toxicity in a large, integrated health care delivery system. Factors that drive utilization of higher levels of service for lithium toxicity should influence clinical programs to improve lithium safety and outcomes.
Methods
Study Design and Setting
This nested case-control study compared characteristics of lithium users who utilized ACS (hospitalization, emergency department visits, or urgent care visits) for lithium toxicity (case group) with those of lithium users who did not experience lithium toxicity or utilize ACS (control group). This study was conducted at Kaiser Permanente Colorado (KPCO), a nonprofit, integrated health care delivery system serving approximately 680,000 members across 29 medical offices in Colorado. Member hospitalization and emergency department visits occur at contracted facilities where KPCO providers have privileges and document care into the KPCO electronic health record (EHR). Annual laboratory monitoring is the standard of practice during chronic lithium therapy. Additionally, drug-drug interaction decision support tools have been implemented at the point of both prescribing and dispensing. This study was approved by the KPCO Institutional Review Board. A waiver of informed consent was obtained and followed all principles in the Declaration of Helsinki.
Study Population
All KPCO patients who purchased at least one prescription for lithium between January 1, 2010, and December 31, 2014, and who had been a KPCO member for at least 365 days prior to the initial date of the ACS encounter for lithium toxicity (index date) were eligible for inclusion. Lithium toxicity at the ACS encounter was identified by an ICD-9 code for lithium toxicity (969.8 or 985.9); a lithium level of ≥1.5 mEq/L or 1.2–1.49 mEq/L; or a lithium level <1.2 mEq/L or missing associated with an ACS encounter containing an ICD-9 code suggesting signs or symptoms of lithium toxicity. [A list of diagnosis codes considered possibly associated with lithium toxicity is available as an online supplement to this article.]
All instances of lithium toxicity were confirmed by an independent review of the EHR by a study investigator (LJH) and a research assistant, with disagreements resolved by a third study investigator (KMG). Lithium toxicity was determined during chart review by new onset or intensification of at least two of the following criteria: nausea, vomiting, or diarrhea; tremors; other neurotoxicity (ataxia, seizures, slurred speech, nystagmus, coma, or psychosis); symptoms of diabetes insipidus; acute kidney injury; or cardiac dysrhythmia. Confirmation of lithium toxicity also required that patients have a lithium supply on hand at the time of lithium toxicity. Lithium supply on hand was defined as having purchased a lithium prescription prior to the date of toxicity with a days’ supply that carried over to the index date or would carry over if multiplied by 1.3 (to account for imperfect medication adherence).
Patients in the case group were lithium users who utilized ACS for lithium toxicity. For all patients in this group, symptoms of lithium toxicity were confirmed as evident on presentation and as part of the patient’s rationale for seeking care. The index date was defined as the initial date of the ACS encounter for lithium toxicity. If more than one eligible ACS event was identified during the study period, only the first event was included in the analysis. All cases of lithium toxicity were verified by review of the EHR, and those considered an intentional overdose with documented intent for harm were excluded.
Patients in the control group were lithium users who met study inclusion criteria but who did not have an ACS encounter for lithium toxicity during the study period. Patients in the case group were matched 1:5 to patients in the control group on the basis of date of lithium prescription purchase to ensure that the patients in the control group had a day’s supply of lithium on hand at the time of the index event. Therefore, patients in the case group were matched with patients in the control group who had purchased a lithium prescription within 39 days prior to the index date of the ACS encounter. Patients in the control group were assigned the same index date as their matched case.
Study Outcomes
The primary outcome was risk factors associated with ACS utilization for lithium toxicity. This was determined by comparing characteristics between the subset of patients with lithium toxicity who received ACS services and the matched control group. Eight potential risk factors for lithium toxicity were defined for inclusion in a multivariable analysis: duration of lithium use, age, initiation of a potentially interacting medication, gender, chronic disease score (CDS), estimated glomerular filtration rate (eGFR) or ratio of blood urea nitrogen (BUN) to serum creatinine (SCr), and total prescribed daily lithium dose (16). Secondary outcomes included the prevalence of lithium toxicity (with and without ACS utilization) among lithium users in the ambulatory KPCO population.
Data Collection
Lithium users were identified administratively by querying KPCO pharmacy databases, and possible lithium toxicity was identified and confirmed by chart review, as described above. Analyses involving lithium levels included the level measured most proximal to and within five days of the index date. Assessment of other laboratory values (BUN and/or SCr) included the values reported most proximal to and within one year of the index date. Any purchases for lithium salts up to 14 months prior to the index date were used to calculate length of lithium use, and average daily dose was determined based on the instructions for the prescription most proximal to the index date.
Common psychiatric diagnoses associated with lithium use were identified by ICD-9 codes assigned within 180 days prior to the index date. The presence of a drug interaction with lithium was defined as the purchase of a potentially interacting medication during the 28 days prior to the index date and preceded by a period of 180 days in which there had been no purchases for a medication from the same class (12). Potentially interacting medications were those that posed a high risk of affecting lithium clearance: thiazide diuretics, loop diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and oral NSAIDs except aspirin or sulindac (17). A patient’s CDS was calculated on the basis of purchase history for all medications within 180 days prior to the index date. Possible scores range from 0 to 35, with higher scores indicating a higher number of treated chronic diseases and greater hospitalization risk (16).
Data Analysis
Patient and prescription characteristics were reported as means and standard deviations for continuous data and as proportions for categorical data. To differentiate acute and chronic toxicity, duration of lithium use before the index date was categorized as less than three months, three months to one year, and continuous use for more than one year. The eGFR was calculated by using the Modification of Diet in Renal Disease Equation (18). The eGFR ranges from 0 mL/min/1.73 m2 to >90 mL/min/1.73 m2, where higher values indicate better renal function; eGFR values >90 mL/min/1.73 m2 suggest normal or high renal function, and values between 60 to 89 mL/min/1.73 m2 suggest mildly reduced renal function (19). ACS utilization was characterized as hospitalization if the patient initially presented to the emergency department and was subsequently hospitalized.
Differences in baseline characteristics between patients in the case and control groups were assessed by using univariate conditional logistic regression to account for all five patients in the control group who were matched to a patient in the case group. The alpha was set at .05. Multivariable conditional logistic regression modeling of utilization of ACS for lithium toxicity was performed to identify factors significantly associated with ACS utilization. Risk factors with high percentages of missing data (eGFR and BUN-to-SCr ratio) were excluded from the multivariable logistic regression model. Risk factors in the model were reported with an odds ratio (OR) and 95% confidence interval (CI), with alpha set at .05. All data extraction and analyses were performed with SAS, version 9.4.
Prevalence of lithium toxicity during the study period, regardless of ACS utilization, was calculated by dividing the number of patients identified as having unintentional lithium toxicity by the number of patients exposed to lithium during the study period.
Results
A total of 3,115 patients were exposed to lithium during the study period, and 79 had confirmed lithium toxicity. Nine patients were excluded because of an intentional overdose with documented intent for self-harm, leaving 70 patients with unintentional lithium toxicity, 50 of whom utilized ACS services (the case group). The overall prevalence of lithium toxicity was 2.2%, whereas the prevalence of lithium toxicity associated with ACS utilization was 1.6%.
Among the patients in the case group, 25 (50%) were hospitalized, 21 (42%) presented to the emergency department without hospitalization, and four (8%) presented to an urgent care facility. Five patients were dispensed a potentially interacting medication with lithium within 28 days of developing toxicity (lisinopril, N=2; losartan, N=1; indomethacin, N=1; and furosemide, N=1), compared with one control patient, who was prescribed ibuprofen.
The 50 patients in the case group were matched to 250 patients without lithium toxicity (control group) (Figure 1). The majority of both groups of patients were white and non-Hispanic. There were no significant differences in race or Hispanic ethnicity between the two groups (Table 1). Compared with the control group, patients in the case group were older (p<.001); had differing duration of lithium use (p=.071); were more likely to use potentially interacting medications (p=.003); had a higher daily lithium dose (p=.017), a higher CDS (p<.001), and a lower eGFR (p=.003); and were more likely to have diagnoses of bipolar disorder (p=.003) and schizoaffective disorder (p=.001) (Table 1).
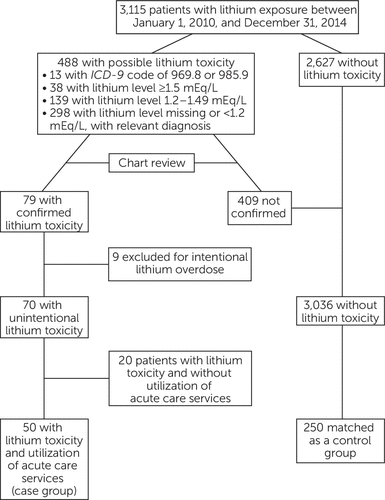
FIGURE 1. Disposition of patients with lithium exposure in a nested case-control study of risk factors associated with use of acute care services for lithium toxicitya
aAcute care services were defined as inpatient hospitalizations, emergency department visits, and urgent care visits.
| Case group (N=50) | Control group (N=250) | Wald | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | χ2b | p |
| Age (M±SD) | 53.6±15.7 | 40.7±15.3 | 21.77 | <.001 | ||
| Female sex | 32 | 64 | 147 | 59 | .27 | .602 |
| Race | .60 | .742 | ||||
| White | 39 | 78 | 193 | 77 | ||
| Unknown or undeclared | 5 | 10 | 33 | 13 | ||
| Otherc | 6 | 12 | 24 | 10 | ||
| Hispanic ethnicity | 2.34 | .310 | ||||
| Yes | 4 | 8 | 29 | 12 | ||
| Unknown or undeclared | 2 | 4 | 24 | 10 | ||
| Psychiatric diagnosis | ||||||
| Bipolar disorder | 43 | 86 | 161 | 64 | 8.93 | .003 |
| Major depressive disorder | 9 | 18 | 52 | 21 | .20 | .653 |
| Schizoaffective disorder | 8 | 16 | 7 | 3 | 15.28 | .001 |
| Duration of lithium use | 5.30 | .071 | ||||
| <3 months | 8 | 16 | 73 | 29 | ||
| 3 months to <1 year | 7 | 14 | 44 | 18 | ||
| ≥1 year | 35 | 70 | 133 | 53 | ||
| Potential drug-drug interaction | 5 | 10 | 1 | .4 | 8.63 | .003 |
| Total daily lithium dose (M±SD mg) | 993.0±443.2 | 845.3±374.7 | 5.69 | .017 | ||
| Chronic Disease Score (M±SD)d | 4.4±3.3 | 1.8±2.5 | 26.46 | <.001 | ||
| eGFR (M±SD mL/min/1.73 m2)e | 69.6±19.0 | 82.9±22.7 | 8.74 | .003 | ||
| BUN-to-SCr ratio (M±SD)f | 13.3±5.1 | 14.5±4.3 | 2.05 | .152 | ||
TABLE 1. Characteristics of patients who received acute care services for lithium toxicity (case group) and a matched control groupa
Both eGFR and the BUN-to-SCr ratio were excluded from the multivariable model because of a high percentage of missing laboratory data among patients in the control group. Risk factors significantly associated with an increased likelihood of ACS utilization for lithium toxicity were presence of a new, potentially interacting medication (OR=30.30; χ2=6.78, p=.009), higher CDS (OR=1.28; χ2=13.82, p<.001), older age (OR=1.05; χ2=10.92, p<.001), and higher total daily lithium dose (OR=1.00; χ2=8.20, p=.004) (Table 2).
| Risk factor | OR | 95% CI | Wald χ2b | p |
|---|---|---|---|---|
| Age | 1.05 | 1.02–1.09 | 10.92 | <.001 |
| Sex (reference: male) | 1.61 | .68–3.78 | 1.17 | .279 |
| Duration of lithium use (reference: >1 year) | ||||
| <3 months | .81 | .27–2.42 | .14 | .705 |
| 3 months to 1 year | 1.17 | .39–3.54 | .08 | .777 |
| Potential drug-drug interaction (reference: no) | 30.30 | 2.32–394.95 | 6.78 | .009 |
| Chronic Disease Score | 1.28 | 1.12–1.45 | 13.82 | <.001 |
| Total daily lithium dose | 1.00 | 1.00–1.00 | 8.20 | .004 |
TABLE 2. Risk factors for utilization of acute care services for lithium toxicitya
Discussion
This unique nested case-control study assessed risk factors for requiring higher levels of care for lithium toxicity in a broad ambulatory population, highlighting preventable and actionable factors. Overall, the risk factors we identified as associated with use of ACS for lithium toxicity were similar to risk factors previously identified for lithium toxicity alone (4,6,11,12,20). Utilization of ACS for lithium toxicity was associated with 30-fold higher odds of initiation of a possibly interacting medication and slightly but significantly increased odds of a higher CDS, older age, and higher total daily lithium dose. Collectively, our findings emphasize potentially interacting medications as an important target for population-based strategies to mitigate risk of ACS utilization for lithium toxicity.
The most commonly initiated interacting medications were RAAS modulators, including lisinopril or losartan, and NSAIDs. Although the relationship between potentially interacting medications and lithium toxicity is not a new finding, this study highlights that potential interactions between lithium and other medications may be a major driver of ACS use for lithium toxicity (11,12). Because we considered only potentially interacting medications that had been introduced in the past 28 days, the results reinforce the importance of efforts to immediately identify and adjust for interacting medications.
Using decision support software at point of prescribing and dispensing is a common tool to flag drug-drug interactions. Unfortunately, override rates are as high as 90% (21). Many providers cite problems with alert fatigue or with low usefulness, which may negatively affect alert effectiveness (22). In fact, an alert fired for all potentially interacting medications identified in this study, but it was overridden by the prescriber. Improved clinical decision support tools may help prevent lithium toxicity events that are secondary to drug interactions (22,23). For example, launching an automatic order for appropriate lithium monitoring (for example, monitoring lithium levels between five and seven days after starting a potentially interacting medication) may be useful. Understanding provider behaviors concerning potentially high-risk drug interactions within the health system may lead to other opportunities.
Finally, patient education and empowerment continue to be imperative. Many patients on chronic lithium therapy, particularly older adults, lack appropriate knowledge about the medication (24). In this study, several instances of lithium toxicity occurred because of patient confusion about differing dosage forms (for example, taking a 300-mg capsule instead of a 150-mg capsule) or because a patient attempted to “catch up” on lithium after missing a few doses. Patient-directed lithium and disease state education has been associated with decreased symptoms, improved quality of life, and more regular medication use (14).
There are notable differences between risk factors for ACS utilization for lithium toxicity identified in this study compared with previous studies of risk factors for lithium toxicity alone. Previous studies identified female patients as being at higher risk of toxicity (9,25). A higher proportion of female patients utilized ACS for lithium toxicity; however, this finding was not statistically significant. Our findings may differ from previous studies because of inconsistencies in the definition of lithium toxicity (9,25). Duration of lithium use as categorized in our study was not significantly associated with increased odds of ACS utilization for lithium toxicity. This finding contrasts with previous studies, which found an association between more severe symptoms and longer lithium use (7,8).
Few previous studies assessed the prevalence of lithium toxicity among ambulatory lithium users. We found that over the course of five years, 1.6% of ambulatory lithium users utilized ACS for lithium toxicity. The overall low prevalence of lithium toxicity in this population suggests that current lithium monitoring practices are effective in preventing toxicity for most patients. A previous population-based study found that 3.9% of individuals older than age 65 were hospitalized for lithium toxicity (12). One reason for the small difference may be a lower average age in our study. Additionally, KPCO’s integrated care delivery system along with its utilization of critical drug interaction software and other decision support tools may have facilitated closer monitoring and prevention of toxicity. The rate of lithium toxicity observed in this study, 2.2%, was much lower than in a recent population-based study, which reported that 7.2% of ambulatory lithium users developed lithium toxicity over a 17-year period (4). The higher prevalence could be partially attributable to inclusion of patients with intentional overdose of lithium.
This study had limitations. Lithium toxicity was confirmed by chart review, which is limited to interpretation of signs and symptoms reported in the medical record. However, this is also a strength relative to previous population-based studies that defined lithium toxicity based on ICD-9 codes or on lithium levels alone without chart review. Data on use of over-the-counter NSAIDs could be missing, given that identification was limited to patients’ reported use. Our results are limited to details of lithium toxicity reported and recorded in the KPCO system, which could miss a small proportion of patients who received ACS from a non-KPCO provider. We identified our population of lithium users under the assumption that anyone who filled a prescription for lithium at least once was adherent to the regimen during the time period, which is consistent with a previous similar study (12). Furthermore, the majority of lithium users in the KPCO system are white, insured patients, and therefore the results may have limited generalizability to populations treated in other systems. Finally, some patients had missing measurements of SCr and BUN, which limited the analysis of renal function as a risk factor for lithium toxicity.
Conclusions
This study identified risk factors for utilization of ACS for lithium toxicity, including potentially interacting medications, higher CDS, older age, and higher total daily lithium dose. The risks identified were similar to those found in a previous case series of inpatients with lithium toxicity. The overall prevalence of lithium toxicity in this study was less than reported in previous studies, suggesting that current lithium monitoring practices are effective in preventing toxicity for many patients. However, new prescriptions for potentially interacting medications were a major factor driving use of additional services for lithium toxicity. Future targeting of this preventable risk factor by providers and health systems could lower the prevalence of lithium toxicity and any related consequences. Elderly patients with a new prescription for a medication with the potential to interact dangerously with lithium are especially vulnerable to lithium toxicity. Future research on methods to target these risk factors and further minimize risk of lithium toxicity is needed.
1 Recommendations; in Bipolar Disorder: Assessment and Management. London, National Institute for Health and Care Excellence, 2014. https://www.nice.org.uk/guidance/cg185/chapter/1-RecommendationsGoogle Scholar
2 American Psychiatric Association: Practice guideline for the treatment of patients with bipolar disorder (revision). American Journal of Psychiatry 159(Apr suppl):1–50, 2002Google Scholar
3 : Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology 29: 459–525, 2015Google Scholar
4 : Lithium intoxication: incidence, clinical course and renal function—a population-based retrospective cohort study. Journal of Psychopharmacology 30:1008–1019, 2016Crossref, Medline, Google Scholar
5 : Effects of 10 to 30 years of lithium treatment on kidney function. Journal of Psychopharmacology 29:608–614, 2015Crossref, Medline, Google Scholar
6 : Long-term effect of lithium maintenance therapy on estimated glomerular filtration rate in patients with affective disorders: a population-based cohort study. Lancet Psychiatry 2:1075–1083, 2015Crossref, Medline, Google Scholar
7 : Lithium intoxication. Journal of the American Society of Nephrology 10:666–674, 1999Medline, Google Scholar
8 : Lithium toxicity: an iatrogenic problem in susceptible individuals. Australian and New Zealand Journal of Psychiatry 35:833–840, 2001Crossref, Medline, Google Scholar
9 : Lithium levels and toxicity among hospitalized patients. Psychiatric Services 52:229–231, 2001Link, Google Scholar
10 : Implication of serum concentration monitoring in patients with lithium intoxication. Psychiatry and Clinical Neurosciences 58:25–29, 2004Crossref, Medline, Google Scholar
11 : Drug-drug interactions as a determinant of elevated lithium serum levels in daily clinical practice. Bipolar Disorders 7:274–280, 2005Crossref, Medline, Google Scholar
12 : Drug-induced lithium toxicity in the elderly: a population-based study. Journal of the American Geriatrics Society 52:794–798, 2004Crossref, Medline, Google Scholar
13 : Randomized trial to improve laboratory safety monitoring of ongoing drug therapy in ambulatory patients. Pharmacotherapy 26:619–626, 2006Crossref, Medline, Google Scholar
14 : The effects of patient education in lithium therapy on quality of life and compliance. Archives of Psychiatric Nursing 17:270–275, 2003Crossref, Medline, Google Scholar
15 : Estimation of resource utilisation difference between lithium and valproate treatment groups from the VALID study. Journal of Medical Economics 14:350–356, 2011Crossref, Medline, Google Scholar
16 : A chronic disease score from automated pharmacy data. Journal of Clinical Epidemiology 45:197–203, 1992Crossref, Medline, Google Scholar
17 : Drug interactions with lithium: a guide for clinicians. CNS Drugs 11:41–48, 1999Crossref, Google Scholar
18 : A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Annals of Internal Medicine 130:461–470, 1999Crossref, Medline, Google Scholar
19 KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD). Kidney International 7:3–56, 2017Google Scholar
20 : Risk factors for the development of lithium-induced polyuria. British Journal of Psychiatry 182:319–323, 2003Crossref, Medline, Google Scholar
21 : Overriding of drug safety alerts in computerized physician order entry. Journal of the American Medical Informatics Association 13:138–147, 2006Crossref, Medline, Google Scholar
22 : Recommendations to improve the usability of drug-drug interaction clinical decision support alerts. Journal of the American Medical Informatics Association 22:1243–1250, 2015Crossref, Medline, Google Scholar
23 : Drug interaction alerts in software—what do general practitioners and pharmacists want? Medical Journal of Australia 195:676–680, 2011Crossref, Medline, Google Scholar
24 : What do patients in a lithium outpatient clinic know about lithium therapy? Journal of Psychiatry and Neuroscience 26:319–324, 2001Medline, Google Scholar
25 : Long-term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory data. Lancet 386:461–468, 2015Google Scholar



