Cost-Effectiveness of Telemedicine-Based Collaborative Care for Posttraumatic Stress Disorder
Abstract
Objective:
The study examined the cost-effectiveness of a telemedicine-based collaborative care model designed to increase rural veterans’ engagement in evidence-based treatments for posttraumatic stress disorder (PTSD).
Methods:
The Telemedicine Outreach for PTSD (TOP) study used a pragmatic randomized effectiveness trial to examine effects of PTSD care teams located at Veterans Affairs medical centers and supporting primary care providers in satellite clinics. Teams included a nurse care manager, pharmacist, psychologist, and psychiatrist. Effectiveness was estimated with quality-adjusted life years (QALYs) derived from the Short Form Health Survey for Veterans and Quality of Well-Being (QWB) scale. Intervention and health care costs were collected to evaluate the cost-effectiveness of the intervention.
Results:
The sample (N=265) included mostly rural, unemployed, middle-aged men with a military service–connected disability for PTSD randomly assigned to TOP or usual care. Only minor improvements in QWB QALYs were found. The TOP intervention was relatively expensive, with costs totaling $2,029 per patient per year. Intervention costs were not offset by reductions in health care utilization costs, resulting in an incremental cost-effectiveness ratio of $185,565 per QALY (interquartile range $57,675 to $395,743).
Conclusions:
Because of the upfront training costs and the resource-intensive nature of this intervention, associated expenses were high. Although PTSD-specific effectiveness measures were significantly improved, these changes did not translate to QALYs in the main analysis. However, analyses focusing on patient subgroups with comorbid mental disorders indicated greater QALY improvement for TOP at lower cost.
Posttraumatic stress disorder (PTSD) is prevalent, persistent, and frequently disabling (1). The 12-month prevalence of PTSD in the U.S. population is higher than the prevalence of panic disorder, generalized anxiety disorder, bipolar disorder, or alcohol abuse (2). PTSD is even more prevalent in the Department of Veterans Affairs (VA) health care system. Magruder and colleagues (3) have conducted the largest epidemiological study of PTSD in VA primary care clinics and found the prevalence of PTSD (based on the Clinician-Administered PTSD Scale [CAPS]) was 12%. PTSD is increasingly diagnosed in the VA health care system, with an estimated increase in prevalence of 5% per year (4). Compared with depression, PTSD has a significantly greater impact on quality of life (5).
Costs associated with PTSD present a substantial economic burden to society and to veterans and the VA. Between 2004 and 2009, the VA spent $1.4 billion on health care costs of patients with PTSD (6). RAND estimated that the average two-year postdeployment societal cost attributable to PTSD ranges from $5,904 to $10,298 per veteran (7). The Congressional Budget Office estimated that the average cost for a patient with PTSD in the first year after diagnosis is $8,300, and approximately half of this total is directly attributable to formal care for PTSD (6). Veterans of Iraq and Afghanistan who have PTSD are more likely to use VA services, with two-thirds using VA services at least once within four years after diagnosis (6). Increased utilization by these veterans has been seen in inpatient days, outpatient visits, and prescriptions filled (6).
Although psychotherapy and pharmacotherapy treatments for PTSD have been found to be efficacious in controlled trials, geographic barriers often prevent rural veterans from accessing these evidence-based treatments. Although most parent VA medical centers (VAMCs) offer specialized PTSD programs, the small community-based outpatient clinics (CBOCs) that serve rural veterans often do not find it feasible to hire on-site psychiatrists or other mental health specialists with PTSD expertise. Whereas medication management for PTSD is often provided via interactive video, the delivery of evidence-based psychotherapy via interactive video is far less common. The Telemedicine Outreach for PTSD (TOP) study was designed to address this gap. The TOP intervention (described below) resulted in a significant reduction in PTSD symptoms as measured by the Posttraumatic Diagnostic Scale (PDS) at six months (β=−3.81, p<.01) and 12 months (β=–2.49, p=.04) (8). Although clinical effectiveness is a necessary condition for further implementation of an intervention, it is not sufficient to justify allocation of scarce resources within the VA system. For this reason, a cost-effectiveness analysis of the TOP intervention was needed to justify implementation. The literature on cost-effectiveness of PTSD treatment is very limited. No previous analyses have examined the incremental cost per quality-adjusted life year (QALY) for any PTSD intervention in the VA. Furthermore, only one non-VA study has been published comparing the cost-effectiveness of PTSD treatments (comparing prolonged exposure therapy and sertraline) (9).
Methods
Study Setting and Enrollment Procedures
The intervention, methods, and clinical outcomes of the TOP clinical trial have been described in detail elsewhere (8). To summarize, the TOP study was a pragmatic randomized effectiveness trial comparing telemedicine-based collaborative care for PTSD with enhanced usual care in 11 VA CBOCs without on-site psychiatrists. Veterans receiving care at these clinics were eligible for enrollment if they met diagnostic criteria for current PTSD according to the CAPS and were not currently receiving care for PTSD at a VAMC and did not have a current diagnosis of schizophrenia, bipolar disorder, or substance dependence. Enrollment occurred between November 23, 2009, and September 28, 2011, with 265 veterans randomly assigned to either usual care or the TOP intervention. Veterans were followed for 12 months postenrollment.
Usual Care
Patients randomly assigned to usual care received the already high standard of care for PTSD at CBOCs, including pharmacotherapy from a primary care physician, psychiatric nurse practitioner, or telepsychiatrist and psychotherapy (individual or group sessions) from an on-site midlevel mental health specialist. Patients receiving usual care were eligible to receive any available services at either their CBOC or the distant VAMC.
TOP Intervention
A more detailed description of the intervention has been published elsewhere (8). The TOP intervention involved off-site PTSD care teams located at parent VAMCs to support on-site CBOC providers. The intervention involved five types of providers: primary care providers located at CBOCs, off-site PTSD telephone nurse care managers, off-site telephone clinical pharmacists, off-site interactive video psychologists, and off-site interactive video psychiatrists. Nurse care managers conducted care management activities, pharmacists reviewed medication histories, psychologists delivered cognitive processing therapy (an evidence-based, trauma-focused psychotherapy), and psychiatrists supervised the team and conducted psychiatric consultations. The off-site PTSD care teams used the electronic health record system (Computerized Patient Record System), telephones, and interactive video to communicate with providers and patients at CBOCs.
Data Collection
Consistent with current recommendations for reference case cost-effectiveness analyses (10,11), we used generic QALYs to measure the clinical effectiveness of the TOP intervention. The primary effectiveness outcome of interest was QALYs based on the Quality of Well-Being (QWB) scale. The QWB scale was designed for cost-per-QALY analyses and comprises four subscales: symptom and problem complex, physical activity, social activity, and mobility (12,13). Each subscale score is determined by preference weights derived from a representative community sample by using a categorical rating scale method and a multiattribute utility model. Subscale scores are subtracted from 1.0 (perfect health) to determine the QWB scale index score, ranging from .0, death, to 1.0, perfect health. For a secondary analysis of the cost-effectiveness denominator, QALYs derived from the 12-item Short Form Health Survey for Veterans (SF-12V) as developed by Brazier and Roberts (14) were examined.
Health care costs were collected to assess the cost-effectiveness of the intervention from the payer's perspective (VA). VA costs in fiscal years 2008 to 2012 were assessed by using Decision Support Systems (DSS) National Data Extracts, which use an activity-based cost allocation method and include fixed direct, variable direct, and fixed indirect costs (15). Outpatient costs for the main analysis were organized in the following groups by primary stop code (that is, encounter type) field: primary care, specialty mental health care, ancillary, specialty physical health care, and other (that is, costs that do not fall into one of the other categories). Telepsychiatry consults and telepsychology encounters by the off-site intervention team were captured in the activity-based cost allocation method. Other intervention costs (for example, care manager activities and weekly intervention meetings) were collected by activity logs. Outpatient VA medication costs were assessed by using VA DSS data. The cost of each prescription was determined on the basis of the drug product costs and the supplies needed to dispense the prescription according to dispensing location (centralized mail order pharmacy versus pharmacy window). In a secondary analysis, inpatient cost was added to the main analysis cost.
Statistical Analysis
Case-mix adjusters included demographic variables, social support, Operation Enduring Freedom/Operation Iraqi Freedom status, prognostic indicators, and disability claim status. Incremental costs and QALYs were calculated by using intent-to-treat analysis to measure the effect of treatment allocation (16). Because of the large number of available covariates, only those found to significantly predict dependent variables in bivariate analyses were included in multivariate analyses. After model specification was finalized, costs from the one-year preintervention period were added as a covariate to cost models to control for baseline cost differences. Because total costs were nonnormally distributed, we used generalized linear models (GLMs) to estimate the effect of the intervention on total costs. To calculate the incremental treatment effect on costs, we computed two predicted costs for each participant on the basis of the coefficients from the GLM regressions and the covariate values for each participant. The first cost prediction was for costs as if the participant had been randomly assigned to the TOP intervention, and the second cost prediction was for costs as if the participant had been randomly assigned to usual care. The difference between these two cost predictions represented the incremental effect of the intervention on costs for a participant, because all covariate effects were identical for the two estimates for each patient.
Incremental cost-effectiveness ratios (ICERs) are reported from the VA perspective by using intervention costs and the VA health care service utilization costs (main analysis). The numerator was the incremental difference in total costs between the intervention and usual care. The denominator was the incremental difference in QALYs between the intervention and usual care. For the primary analysis, we examined QWB QALYs and only outpatient and pharmacy costs. Typical standard error estimation methods do not apply to ICERs because the possibility of having a zero or near-zero denominator is not negligible and cost and effectiveness estimates are rarely independent (17). Therefore, we used a nonparametric bootstrap method with replacement and 1,000 replications to generate an empirical joint distribution of incremental costs and QALYs (17,18). Acceptability curves representing the probability of falling below ICER thresholds ranged from $0 to $500,000 per QALY (19). ICERs were calculated for the full sample for the primary as well as secondary effectiveness and cost measures. In a post hoc exploratory analysis to better understand for whom the intervention was most beneficial, subgroups with a comorbid mental disorder diagnosis were examined. The parent study included screening information for anxiety, depression, and panic disorder—mental disorders that commonly co-occur with PTSD.
Results
Baseline sociodemographic, clinical, and depression-related variables are presented in Table 1. In general, patients lived in rural areas and were unemployed middle-aged men with military service–connected disability for PTSD. The sample had severe PTSD symptoms, with a mean CAPS score of 75. None of the outcome measures or case-mix factors differed significantly between the TOP and usual care groups.
| All (N=265) | TOP (N=133) | Usual care (N=132) | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | p |
| Age (M±SD) | 52.2±13.8 | 51.9±14.0 | 52.5±13.6 | .73 | |||
| Male | 238 | 90 | 118 | 90 | 120 | 91 | .56 |
| Race-ethnicity | .15 | ||||||
| White | 169 | 64 | 77 | 58 | 92 | 70 | |
| African American | 52 | 20 | 31 | 23 | 21 | 16 | |
| Hispanic | 20 | 8 | 12 | 9 | 8 | 6 | |
| Other | 24 | 9 | 13 | 10 | 11 | 8 | |
| Annual household income <$20,000 | 67 | 25 | 35 | 26 | 32 | 24 | .70 |
| Married | 185 | 70 | 91 | 68 | 94 | 71 | .62 |
| High school graduate | 249 | 94 | 124 | 93 | 125 | 95 | .62 |
| Employed | 68 | 26 | 38 | 29 | 30 | 23 | .28 |
| Distance to closest VA medical center (M±SD km) | 92.8±40.8 | 91±40.8 | 94.6±41.0 | .49 | |||
| Living in urbanized area | 55 | 21 | 30 | 23 | 25 | 19 | .47 |
| Social support score (M±SD)a | 3.5±1.0 | 3.5±1.1 | 3.5±.9 | .72 | |||
| Period of wartime service | .52 | ||||||
| OEF/OIF/ONDb | 77 | 29 | 41 | 31 | 36 | 27 | |
| Other | 188 | 71 | 92 | 69 | 96 | 73 | |
| Combat experience | 131 | 49 | 62 | 47 | 69 | 52 | .36 |
| Service-connected disability for PTSD | .39 | ||||||
| Never applied | 42 | 16 | 19 | 14 | 23 | 17 | |
| Applied, denied | 25 | 10 | 13 | 10 | 12 | 9 | |
| Applied, pending | 61 | 23 | 35 | 27 | 26 | 20 | |
| Approved | 135 | 51 | 64 | 50 | 71 | 54 | |
| Psychotropic medications acceptable | .85 | ||||||
| Definitely | 119 | 45 | 58 | 44 | 61 | 46 | |
| Probably | 91 | 34 | 49 | 37 | 42 | 32 | |
| Probably not | 24 | 9 | 11 | 8 | 13 | 10 | |
| Definitely not | 31 | 12 | 15 | 11 | 16 | 12 | |
| Individual psychotherapy acceptable | .82 | ||||||
| Definitely | 169 | 64 | 85 | 64 | 84 | 64 | |
| Probably | 74 | 28 | 35 | 26 | 39 | 30 | |
| Probably not | 20 | 8 | 12 | 9 | 8 | 6 | |
| Definitely not | 2 | 1 | 1 | 1 | 1 | 1 | |
| Prior PTSD-specific treatment | 207 | 78 | 99 | 74 | 108 | 82 | .15 |
| Prior use of any psychotropic medication | 238 | 90 | 121 | 91 | 117 | 89 | .53 |
| Prior use of any psychotherapy | 241 | 91 | 117 | 88 | 124 | 94 | .09 |
| PTSD treatment provider | .38 | ||||||
| Primary care physician | 44 | 17 | 17 | 13 | 27 | 21 | |
| Psychiatric advanced practice nurse | 87 | 33 | 49 | 37 | 38 | 29 | |
| Social worker | 88 | 33 | 41 | 31 | 47 | 36 | |
| Telepsychiatrist | 17 | 6 | 10 | 8 | 7 | 5 | |
| Other | 29 | 11 | 16 | 12 | 13 | 10 | |
| CAPS score (M±SD)c | 75±12.7 | 75.9±13.3 | 74.0±12.0 | .22 | |||
| PDS score (M±SD)d | 34.2±8.1 | 35.0±8.0 | 33.5±8.2 | .12 | |||
| HSCL-20 depression severity score (M±SD)e | 2.1±6.0 | 2.2±.6 | 2.1±.7 | .47 | |||
| SF-12V physical component summary (M±SD)f | 35±12.8 | 34.9±12.0 | 35.2±13.6 | .86 | |||
| SF-12V mental component summary (M±SD)f | 32.8±10.3 | 31.9±10.2 | 31.8±10.4 | .14 | |||
| N of chronic general medical illnesses (M±SD) | 4.3±2.3 | 4.2±2.4 | 4.4±2.3 | .38 | |||
| Current major depressive disorder | 209 | 79 | 107 | 81 | 102 | 77 | .53 |
| Current panic disorder | 117 | 44 | 63 | 47 | 54 | 41 | .29 |
| Current generalized anxiety disorder | 178 | 67 | 88 | 66 | 90 | 68 | .73 |
| AUDIT treatment recommendationg | .82 | ||||||
| Alcohol education | 206 | 78 | 106 | 80 | 100 | 76 | |
| Simple advice | 32 | 12 | 14 | 10 | 18 | 14 | |
| Brief counseling and continued monitoring | 10 | 4 | 5 | 4 | 5 | 4 | |
| Referral to specialist | 17 | 7 | 8 | 6 | 9 | 7 | |
TABLE 1. Baseline characteristics of veterans with PTSD randomly assigned to Telemedicine Outreach for PTSD (TOP) or to usual care
Table 2 summarizes intervention and health care costs incurred by patients in the intervention and usual care groups. Health care costs were separated into outpatient (primary care, mental health specialty, PTSD-specific care, physical health specialty, ancillary, and other) and pharmacy (mental health related and non–mental health related). The only statistically significant unadjusted differences in one-year preintervention health care costs were higher physical health specialty outpatient costs in the intervention group ($2,706 versus $1,481) and lower mental health pharmacy costs in the intervention group ($306 versus $340).
| Preintervention | Postintervention | |||||
|---|---|---|---|---|---|---|
| Service type | Usual care | TOP | p | Usual care | TOP | p |
| Outpatient | ||||||
| Primary care | 906.59 | 1,032.32 | .45 | 836.43 | 960.10 | .42 |
| Mental health specialty | 1,688.07 | 2,630.77 | .92 | 2,159.26 | 2,964.63 | .01 |
| PTSD-specific care, nontelephone | 227.12 | 226.39 | .28 | 174.96 | 238.00 | .03 |
| PTSD-specific care, telephone | 262.72 | 265.32 | .95 | 6.94 | 799.61 | .01 |
| Physical health specialty | 2,706.39 | 1,481.34 | .02 | 2,284.71 | 1,555.88 | .93 |
| Ancillary | 1585.65 | 1,749.52 | .93 | 1,457.42 | 1,586.24 | .70 |
| Other | 42.48 | 66.62 | .15 | 24.41 | 45.74 | .25 |
| Total | 7,419.01 | 7,452.26 | .28 | 6,944.13 | 8,150.20 | .02 |
| Pharmacy | ||||||
| Mental health | 340.05 | 305.64 | .01 | 348.49 | 365.61 | .65 |
| Non-mental health | 1,310.25 | 894.02 | .36 | 2,251.76 | 967.06 | .15 |
| Total | 1,650.30 | 1,199.66 | .34 | 2,600.25 | 1,332.68 | .13 |
| Total | 9,069.31 | 8,651.92 | 9,544.38 | 9,482.88 | ||
| Intervention activity | Hours | Average salary rate ($)a | Average fringe ($) | Total ($) | Per patient ($) (N=133) | |
| Weekly caseload review | 561 | 63.11 | 21.04 | 47,240.23 | 355.19 | |
| Nurse care manager activities | 2,126 | 41.35 | 13.78 | 11,7231.96 | 881.44 | |
| PTSD telephone care | 267,867.50 | 792.67 | ||||
| Total intervention | 2,688 | 432,339.69 | 2,029.30 | |||
TABLE 2. Unadjusted mean costs for veterans with PTSD assigned to usual care or to Telemedicine Outreach for PTSD (TOP), in the year before and the year after the intervention
In the year postbaseline, for the intervention group the unadjusted mental health specialty costs ($2,965 versus $2,159) and PTSD-specific care, both nontelephone provided ($238 versus $175) and telephone provided ($800 versus $7), were higher than for the usual care group, as were total outpatient costs ($8,150 versus $6,944). Preintervention total costs were $9,069 and $8,652 for the usual care and intervention groups, respectively. Postintervention total costs were $9,544 for usual care and $11,512 (including the intervention costs of $2,029) for the intervention group. Total unadjusted health care expenditures increased by $475 for usual care patients and by $830 for intervention patients. The inclusion of intervention costs resulted in a difference in total unadjusted incremental costs of $2,384, favoring usual care.
After adjustment for case-mix variables and preintervention costs, the overall incremental cost of the intervention (considering the combined differences in health care costs and the intervention costs) was $2,495 (p<.01) per patient. Adding inpatient costs to the model decreased the incremental cost to $2,294 (p=.03). Inpatient costs were included in the secondary analysis because of the generally highly skewed distribution of these costs; this approach is consistent with the literature (20).
As previously reported, compared with veterans randomly assigned to usual care, those randomly assigned to TOP experienced significantly greater improvements in PTSD and depression severity, although the effect sizes ranged from small to medium. We calculated incremental generic QALYs by using QWB (primary) and SF-12V standard gamble (secondary). The intervention resulted in positive but insignificant differences in QALYs with these measures. Although the intervention had a significant effect on the PTSD-specific measure (PDS), no significant difference was seen in these generic measures.
Mean ICERs from the original sample varied greatly depending on the effectiveness measure examined (Table 3). Because of the high sensitivity of the ICER to changes in the very small effectiveness differences, median ICER values were calculated by using an adjusted bootstrapped sample (1,000 replications with replacement) (21). The primary analysis examining outpatient and pharmacy costs and QWB QALYs resulted in a median ICER of $185,565 per QALY (interquartile range $57,675 to $395,743) (Table 3). Figure 1 shows the ICER distribution for this bootstrapped sample, and Figure 2 shows the cost-effectiveness acceptability curve. When the $50,000 per QALY threshold was used, the primary analysis was cost-effective in 5% of the samples. When a less conservative cost per QALY threshold of $150,000 was used, the primary analysis was cost-effective in 23% of the samples.
| ICERb | ||||
|---|---|---|---|---|
| Analysis and measure | Effectiveness differencea | Cost difference | Median | IQRc |
| Primary analysis QWB (full sample N=265)d | .008 | $2,495e | 185,565 | 57,675 to 395,743 |
| Secondary analyses | ||||
| SF-12Vf | .001 | $2,495e | 138,108 | – to 579,501 |
| QWBd | .008 | $2,294g | 167,247 | 14,228 to 364,334 |
| SF-12Vf | .001 | $2,294g | 101,355 | – to 534,181 |
| Subgroup analyses QWBd | ||||
| Anxiety disorder (N=140) | .011 | –$5,525e | —h | – to 98,583 |
| Depression (N=160) | .001 | –$5,521e | —h | – to 524,377 |
| Panic disorder (N=102) | .012 | –$4,718e | —h | – to 121,654 |
TABLE 3. Incremental cost per quality-adjusted life year ratios and net health benefit (bootstrap sample) for veterans with PTSD assigned to Telemedicine Outreach for PTSD
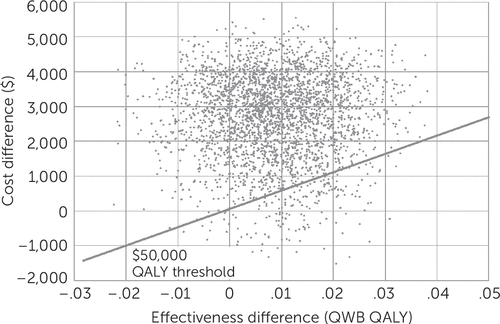
FIGURE 1. Incremental cost-effectiveness plane: Telemedicine Outreach for PTSD versus usual carea
aQWB, Quality of Well-Being scale; QALY, quality-adjusted life year
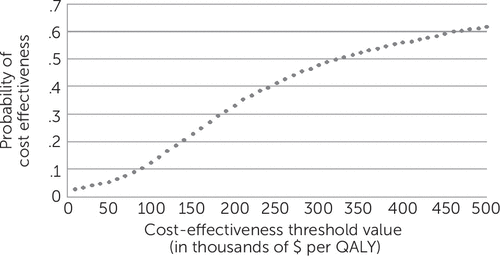
FIGURE 2. Cost-effectiveness acceptability curve for Telemedicine Outreach for PTSDa
aThe acceptability curve is for the entire sample (main analysis).
To better understand the findings, we conducted post hoc exploratory analyses examining clinically relevant subgroups. We examined the primary analysis in three overlapping subgroups: those with depression (N=160), anxiety (N=140), or panic disorder (N=102). In each of these subgroups, the intervention was dominant (greater QWB QALY improvement at lower cost) (Table 3). In the bootstrap analysis, most samples showed the TOP intervention to be not only cost-effective but cost-saving for patients with comorbid depression (53%), anxiety (82%), and panic disorder (53%). [An online supplement to this article includes a figure with the cost-effectiveness acceptability curves.]
Discussion
Publications describing the cost-effectiveness of PTSD care are extremely scarce. This is the first cost-effectiveness analysis of any intervention for veterans with PTSD and the first examination in the PTSD literature of the costs associated with collaborative care or telemedicine. The evidence of disease-specific effectiveness of the TOP intervention warranted further investigation through a cost-effectiveness analysis; however, the disease-specific evidence did not translate into quality-of-life improvements for the overall sample. The lack of quality-of-life improvements coupled with the relatively high cost of the intervention resulted in cost-effectiveness ratios that were higher than the recommended thresholds for implementation in the full sample. However, because other primary care–based interventions in the VA or patients with a PTSD diagnosis have not been effective (22,23), and there are no other cost-effectiveness analyses for this patient population, it is somewhat difficult to make policy recommendations.
Although the results for the full sample were less than encouraging, the subgroup analyses each showed that the TOP intervention was not only cost-effective but cost-saving among patients with PTSD who had comorbid anxiety, depression, or panic disorder. These findings are in accord with previous literature that examined collaborative care for depression. In this literature, cost-effectiveness, and even cost savings, has been demonstrated in more complicated patient populations in which comorbid conditions are present (20,24). However, the marginal effectiveness and the smaller samples in these subgroups resulted in extremely wide variability in the ICERs. The findings of the subgroup analyses highlight the importance of selecting an appropriate target population for resource-intensive interventions such as TOP.
Moreover, the cost of the intervention ($2,029) compared with the total annual health care costs for patients in the sample ($11,512) puts the magnitude of these costs in perspective. The TOP intervention is resource intensive, resulting in an 18% increase in outpatient care costs for these veterans. Because collaborative care is generally a resource-intensive approach to care, strategies for minimizing intervention costs have been discussed with respect to depression care (25). Solutions proposed for minimizing these costs include decreasing nurse care manager efforts on ancillary activities, such as call preparation and unsuccessful call attempts. Nearly three-quarters of the total intervention costs were related to nurse care manager activities, and thus efforts to replace their time by streamlining processes or introducing technological solutions could result in large savings.
Several limitations of this analysis are worth noting. First, although the VA is the largest provider of PTSD care in the world and the largest health care organization in the United States, the results of this study may not be generalizable to other settings. This is of particular importance when examining PTSD, because veterans are much more likely than persons in the general population to experience PTSD resulting from a combat-related trauma. The setting of this study is also important because, as an integrated general medical and mental health care provider, the VA does not reflect the systems of care experienced by most patients with PTSD in the United States, including veterans who receive care outside the VA. The demographic and clinical characteristics of VA patients are also typically different from patients outside the VA. These findings are also limited by the one-year follow-up time; this may explain the lack of quality-of-life improvement despite symptomatic improvement, because quality-of-life improvements typically take longer to develop. Finally, the subsamples in the exploratory analysis had considerable overlap, thereby limiting our ability to estimate the independent effect of each comorbid disorder.
Conclusions
This cost-effectiveness analysis examined the results of a pragmatic effectiveness trial of a telemedicine-based collaborative care intervention. Although the intervention was marginally effective, the high costs of delivering care for PTSD via this model underline the importance of streamlining the intervention to make it more efficient. The findings also suggest that more favorable cost-effectiveness results could be achieved by targeting veterans with comorbid mental health conditions, such as anxiety, depression, or panic disorder.
1 : Posttraumatic stress disorder among military returnees from Afghanistan and Iraq. American Journal of Psychiatry 163:586–593, 2006Link, Google Scholar
2 : Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 62:617–627, 2005Crossref, Medline, Google Scholar
3 : Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. General Hospital Psychiatry 27:169–179, 2005Crossref, Medline, Google Scholar
4 : Mental health and substance use disorder spending in the Department of Veterans Affairs, fiscal years 2000–2007. Psychiatric Services 62:389–395, 2011Link, Google Scholar
5 : Posttraumatic stress disorder and health status: the Veterans Health Study. Journal of Ambulatory Care Management 29:71–86, 2006Crossref, Medline, Google Scholar
6 The Veterans Health Administration’s Treatment of PTSD and Traumatic Brain Injury Among Recent Combat Veterans. Washington, DC, Congressional Budget Office, 2012Google Scholar
7 : Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, Calif, RAND, 2008Google Scholar
8 : Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 72:58–67, 2015Crossref, Medline, Google Scholar
9 : Cost-effectiveness of prolonged exposure therapy versus pharmacotherapy and treatment choice in posttraumatic stress disorder (the Optimizing PTSD Treatment Trial): a doubly randomized preference trial. Journal of Clinical Psychiatry 75:222–230, 2014Crossref, Medline, Google Scholar
10 Gold MR, Siegel JE, Russell LB, (eds): Cost-Effectiveness in Health and Medicine. New York, Oxford University Press, 1996Google Scholar
11 : On being NICE in the UK: guidelines for technology appraisal for the NHS in England and Wales. Health Economics 11:185–191, 2002Crossref, Medline, Google Scholar
12 : Assessment of the quality of life of patients with major depression. Psychiatric Services 48:224–230, 1997Link, Google Scholar
13 : A general health policy model: update and applications. Health Services Research 23:203–235, 1988Medline, Google Scholar
14 : The estimation of a preference-based measure of health from the SF-12. Medical Care 42:851–859, 2004Crossref, Medline, Google Scholar
15 VHA Decision Support System (DSS): Introduction. Washington, DC, VA Information Resource Center, 2011. http://www.virec.research.va.gov/DataSourcesName/DSS/DSSintro.htm. Accessed March 3, 2011Google Scholar
16 : Intention-to-treat analysis in clinical trials: principles and practical importance. Revista Portuguesa de Cardiologia 21:1191–1198, 2002Medline, Google Scholar
17 : Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Economics 6:327–340, 1997Crossref, Medline, Google Scholar
18 : Policy space areas and properties of benefit-cost/utility analysis. JAMA 255:794–795, 1986Crossref, Medline, Google Scholar
19 : Uncertainty in decision models analyzing cost-effectiveness: the joint distribution of incremental costs and effectiveness evaluated with a nonparametric bootstrap method. Medical Decision Making 18:337–346, 1998Crossref, Medline, Google Scholar
20 : Cost-effectiveness of collaborative care for depression in HIV clinics. Journal of Acquired Immune Deficiency Syndromes 70:377–385, 2015Crossref, Medline, Google Scholar
21 : Median-based incremental cost-effectiveness ratio (ICER). Journal of Statistical Theory and Practice 6:428–442, 2012Crossref, Medline, Google Scholar
22 : RESPECT-PTSD: re-engineering systems for the primary care treatment of PTSD: a randomized controlled trial. Journal of General Internal Medicine 28:32–40, 2013Crossref, Medline, Google Scholar
23 : Telephone monitoring and support after discharge from residential PTSD treatment: a randomized controlled trial. Psychiatric Services 64:13–20, 2013Link, Google Scholar
24 : Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Archives of General Psychiatry 69:506–514, 2012Crossref, Medline, Google Scholar
25 : Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Archives of General Psychiatry 67:812–821, 2010Crossref, Medline, Google Scholar



