Best Practices: The Electronic Medical Record Is an Invaluable Clinical Tool: Let’s Start Using It
Abstract
This column describes the potential of an enhanced electronic medical record (EMR) to advance best practices by displaying patient history, measuring progress, and facilitating clinical research. To create a graphical, single-page display of patient history, the authors examined data in the Minneapolis Department of Veterans Affairs EMR system, including 1.8 million encounters for 50,000 mental health patients. The prototype dashboard presents information on a patient’s current and past providers, diagnoses, therapeutic interventions, prescriptions, dosages, and outcomes. To provide needed outcome data to monitor patient progress, the authors tested two questions with 212 patients. Patient and clinician responses to the questions provide reliable and clinically useful data that can be used in the EMR to track patient change over time. Use of EMRs can bridge gaps between science and practice to inform diagnosis and treatment decisions and permit more accurate prognoses.
Editor's Note: William M. Glazer, M.D., died in June at age 66 in his home on Martha's Vineyard. Bill was the editor of the Best Practices column for nearly 20 years—he introduced the column in the November 1994 issue. Best Practices is an important feature of the journal. As psychiatric services changed, so did Bill's column. He responded to new ideas and editorial suggestions with flexibility and creativity. Authors benefited from his guidance and readers from his dedication to improving current practice and patient care. He will be missed by the editor and the staff of the journal.—Howard H. Goldman, M.D., Ph.D.
Every practicing clinician has experienced a mental health intake appointment with a patient whose file contained thousands of notes. Not long ago, the patient’s file would have been made of paper and weighed upwards of 25 pounds. Sometimes patient files were so large that multiple file folders were necessary. The paper is now largely gone, but the general form and function of the medical record remains: clinicians type and read narrative notes.
To prepare for an intake, the clinician must scan the many notes, integrate them into a preliminary case conceptualization, and use the interview to gather data to test clinical hypotheses. This method works when there is sufficient time. However, manual chart review is easy to curtail or even omit when resources are scarce, productivity demands increase, or no-show rates are high. By the same token, these demands on clinical time may make it difficult for clinicians to write detailed notes. The result can be a medical record containing greatly abbreviated information that is rarely reviewed in detail. In addition, the electronic medical record (EMR) can make even the most earnest manual chart review difficult, because the EMR often contains useless information that obscures clinically relevant material. This occurs is because the information in most EMRs is presented in an inefficient and cumbersome manner—almost exclusively in template or narrative notes that the clinician must read or, more accurately, speed read.
In this column, we envisage an enhanced EMR that makes daily clinical tasks more efficient and precise and that advances best-practice standards in the digital age. This EMR can instantaneously summarize thousands of notes from previous clinical encounters to inform diagnostic and treatment decisions and prognoses. Such an EMR provides a rich data set for clinical research and can improve patient privacy by preventing unauthorized individuals from viewing information. Realizing the EMR’s promise, however, requires a shift in perspective in regard to documentation—from a series of unrelated cross-sectional snapshots to an integrated longitudinal data compilation that provides the user with insight and knowledge about a patient’s clinical experience up to the present moment.
Graphical maps of patient care
On the computer screen, EMRs look like a series of notes, but on the inside they are massive databases. Instead of the clinician’s rifling through notes and visually scanning text, the computer can extract and compile relevant clinical data and present the collated data in digestible and actionable forms. Use of existing clinical information in the EMR to generate charts and graphs of patient history, clinical characteristics, and treatment progress requires a modicum of technical support. We envision a graphical map of the patient’s mental health history, with current and past providers, diagnoses, therapeutic interventions, prescriptions, dosages, and outcomes displayed on a single page. It is an at-a-glance summary of the patient at the click of the mouse, orienting the clinician at the macro level, as well as providing quick links to important micro-level documents, such as assessment reports and admission and discharge summaries. It is a map of the patient’s clinical life that allows the provider to scroll through the dashboard, focus on particular aspects of the patient’s history, and click on relevant landmarks for more information or access to a clinical note.
To create a graphical map, the authors examined available data in the Minneapolis Department of Veterans Affairs (VA) EMR, including 1.8 million encounters for 50,000 mental health patients. Figure 1 displays a prototype. The graphic is dense with information, allowing the clinician to learn a great deal about the patient before reviewing other records. In current practice, it takes at least ten minutes to gather the information presented in the figure, and manual record reviews are hurried and incomplete. In contrast, a one-minute scan of the dashboard in Figure 1 provides a consistent and accurate review of key information. The information already exists in the Minneapolis VA EMR, but it is not accessible in a single place. Currently, patient age is contained in one note and hospitalization history in another. To obtain diagnostic and treatment history, the clinician must read dozens of notes. The dashboard is one way to organize information automatically and to free up clinical time for direct patient contact.
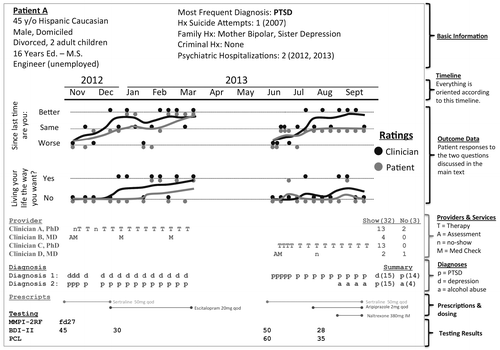
aThe dashboard is organized by month. In November 2012 this fictional patient was assessed (A) by Clinician B, given a medication evaluation (M), prescribed sertraline, diagnosed as having depression (d) and PTSD (p), and administered the Beck Depression Inventory (BDI-II) and Minnesota Multiphasic Personality Inventory (MMPI-2RF). The patient was then seen for depression by Clinician A. He did not attend his first appointment (n, no show) and engaged in therapy (T) around a week later. From November to March, he had diagnoses of depression and PTSD and experienced improvement (the running averages in the Outcome Data section increase over time for the two outcome measures). His BDI-II score decreased from 45 to 30. During that time, the medication was switched from sertraline to escitalopram. Despite ending treatment successfully in March, he was readmitted in June with prominent PTSD. His care was transferred to Clinician C, who provided psychotherapy, and Clinician D, who represcribed sertraline, eventually augmented with aripiprazole. The BDI-II was readministered, along with the PTSD Checklist (PCL). In August he was given a diagnosis of alcohol dependence (a) and given a naltrexone injection.
Critical information presented in Figure 1—the outcome measures and corresponding trajectories of change—was unavailable at our VA facility. In fact, no outcomes were consistently tracked, which meant that it was difficult to make clinical or administrative decisions that were informed by patient outcome (for example, by considering the outcome of a prior course of treatment). A major obstacle to tracking outcomes is that clinical time must be devoted to regularly gathering outcome data to measure patient change. Our approach was to use two items, which, with many repeated measurements averaged over time, could show smooth trajectories of change.
Collecting outcome measures
Outcome questionnaires are typically long (more than ten items), infrequently administered, and specific to a disorder or provider type, such as the Beck Depression Inventory or the Patient Health Questionnaire. In our experience, outcome measure results are often not entered into the EMR in a way that can easily be extracted by the computer (for example, scores are typed directly into the progress note narrative). In addition, outcome data are often collected only after scheduled treatment termination, with no baseline measure of functioning and no information about patients who terminate early. Information that might predict early termination or noncompliance may be lost.
Overcoming these challenges demands systematized outcome assessment at every clinical encounter. One solution is the administration of a brief instrument with demonstrated reliability and validity that does not burden the clinician or patient. Even ten items would be far too many. It should be cheap or, ideally, free. To be comparable across patient groups, it must be general. To get buy-in from frontline clinicians, it must prove clinically useful. Our solution was to use two brief and direct questions: “Are you living the life you want to live?” and “Since the last time you were seen by Mental Health, are you doing the same, worse, or better?” Both questions are rated at every encounter by the patient and by his or her provider. These questions were derived by a committee composed of the mental health leadership at our facility. Questions were selected for simplicity, generalizability, and consistency with overarching clinical orientations in our facility, which helped ensure that they are clinically useful.
Recording patient and clinician answers at every encounter enables sequential tracking of clinical change during treatment. Systematic divergence between clinician and patient answers allows for the evaluation of clinician or patient bias. Simultaneous ratings also enable clinicians to compare their perception of a patient’s change with the patient’s perception. When ratings are congruent, all is well. When responses diverge, the clinician can have a productive conversation with the patient: “I noticed that you don’t feel you’re improving, but I see a lot of improvement. Why do you think that is?”
These simple questions appear to be psychometrically sound. In a pilot study conducted in June and July 2012 at the Minneapolis VA, nine providers collected these measures for 212 clients over 254 visits. Provider and patient agreement at any particular encounter was good (κ=.74). For the 35 patients who were seen more than once, 28 reported improvement, and the longitudinal clinician-patient agreement was good (κ=.85). Clinicians appreciated the questions and reported that the wording facilitated productive conversations about recovery. Administration was efficiently conducted by clerical staff and took less than a minute per client. In short, these questions appear effective and would allow most facilities to collect clinically relevant outcome information for every patient. [More information on the development and testing of the questions is provided in an online data supplement to this column.]
Collecting outcome measures is a step in the right direction, but fully realizing the advantages of the EMR also requires reconsidering existing documentation practices, for which we provide concrete suggestions in the next section.
Visions for best practices from the EMR
Traditional documents can be changed to leverage computational resources inherent in EMRs while retaining the ability to convey clinical nuance. Consider the diagnostic report, which records patient history, usually in a free-flowing text narrative that is valuable for communicating clinical nuance but very difficult for a computer to search and analyze. To improve analyzability, template fields could be designated for important information, such as key dates (for example, birth, marriage, divorce, illness onset and remission, hospitalization, discharge, abuse, and criminal convictions). Other standardized information, such as years of education, GPA, occupation, homelessness, and test results (IQ, personality, and symptom checklists), could be recorded in a way that allows the EMR to export, analyze, and summarize it (for example, in the dashboard in Figure 1).
The template fields would be updated as needed. Clerical staff would not have to take a full psychosocial history more than once, and the information could be automatically extracted, analyzed, summarized, and used to tailor services. For example, the computer could analyze the income and occupation fields and inform the clinician that an indigent client qualifies for homeless services or job retraining. It could also provide links to information, such as brochures, for a specific institution in a specific community.
The creation of an optimized EMR is central to the advancement of best-practice standards and to the realization of personalized medicine (1)—the notion that a patient’s medical information (diagnostic, demographic, environmental, and genetic data) can be used to create a treatment plan tailored for that individual. Although personalized medicine sounds individualistic, it is not. Only by comparing a patient with similar patients can we understand how to appropriately modify standard therapy to create an optimal treatment. Such analyses will require computer-assisted integration of science and practice at a level unseen in health care today. Any computer algorithm, no matter how sophisticated, will require information about other patients (ideally the entire patient population) to understand how one patient is unique.
Pushing the envelope in best practices and beginning to realize the promise of personalized medicine will benefit greatly if patients consent to the use of their clinical data for health care research. Routine consent would build a database of patient characteristics (signs, symptoms, demographic and environmental factors, and genes) that can be used in real time, perhaps through EMR applications such as the dashboard in Figure 1, to assist practitioners in diagnosis and treatment. Major clinical research initiatives, such as the VA’s Million Veteran Program and Kaiser Permanente’s Program on Genes, Environment, and Health, are excellent starts, but extremely large, clinically focused research programs should be the norm rather than the exception. The generated data would be routinely used in daily clinical tasks, case conferences, and administration to provide critical decision-making information, such as disorder prevalence, number needed to treat, treatment effectiveness, and so on. We routinely “Google” factual information in our personal lives. With a living research database, the Google analog would also exist in our clinical lives. However, realizing these changes will require a cultural transformation in how we document and how we use that documentation.
Conclusions
One way to evolve our discipline is to reengineer how the EMR interfaces with our day-to-day clinical practice. Achieving this goal involves data visualization, measurement of outcomes, and routinely conducting clinical research with the data that we gather. The EMR has great potential to inform decisions about diagnosis and treatment and to permit more accurate prognoses. The EMR can help bridge gaps between science and practice and reserve valuable human resources for clinical tasks that cannot be performed by a computer.
1 : The path to personalized medicine. New England Journal of Medicine 363:301–304, 2010Crossref, Medline, Google Scholar



