Medicare Part D Benzodiazepine Exclusion and Use of Psychotropic Medication by Patients With New Anxiety Disorders
Abstract
Objective:
The Medicare Modernization Act (MMA) specifically excluded benzodiazepines from Medicare Part D coverage starting in 2006; however, benzodiazepines are an effective, low-cost treatment for anxiety. This study evaluated the effect of the Medicare Part D benzodiazepine coverage exclusion among patients with new anxiety disorders.
Methods:
The authors used a quasi-experimental cohort design to study patients with new anxiety diagnoses from a large national health plan during the first six months of 2005, 2006, and 2007. Logistic and zero-truncated negative-binomial regression models using covered claims for behavioral, medical, and pharmaceutical care linked with eligibility files were used to estimate utilization and costs of psychotropic medication and health care utilization among elderly Medicare Advantage enrollees (N=8,397) subject to the MMA benzodiazepine exclusion and a comparison group of near-elderly (ages 60–64) enrollees (N=1,657) of a managed care plan.
Results:
Medicare Advantage enrollees diagnosed in 2005 had significantly (p<.05) higher rates of covered claims for benzodiazepines and all psychotropic drugs, lower rates of covered claims for nonbenzodiazepines, and lower expenditures for psychotropic drugs than enrollees diagnosed in 2006 and 2007. There were no significant differences over time in utilization or expenditures related to psychotropic medication among the comparison group. There also were no significant changes over time in outpatient visits for behavioral care by either cohort.
Conclusions:
Among elderly patients with new anxiety diagnoses, the MMA benzodiazepine exclusion increased use of nonbenzodiazepine psychotropic drugs without substitution of increased behavioral care. Overall, the exclusion was associated with a modest increase in covered claims for psychotropic medication. (Psychiatric Services 63:637–642, 2012; doi: 10.1176/appi.ps.201100331)
The Medicare Modernization Act (MMA) established prescription drug coverage through Medicare Part D starting in 2006 but specifically excluded coverage of benzodiazepines. As a result, only patients with supplemental drug insurance—through Medicaid or private secondary insurance, for example—have access to benzodiazepine coverage. The exclusion of benzodiazepines from Medicare Part D plans was eliminated by the Patient Protection and Affordable Care Act, but this change will not take effect until 2013. However, California's state auditor recently recommended that benzodiazepines be excluded from Medicaid coverage in an effort to reduce budget costs (1).
The MMA's benzodiazepine exclusion was based on studies showing that benzodiazepine use by the elderly increases the risk of falls and hip fractures and worsens conditions such as emphysema, urinary incontinence, and depression (2). However, when used appropriately, benzodiazepines are an effective, low-cost treatment for anxiety. A major concern regarding the MMA's benzodiazepine exclusion was potential adverse effects on patients with anxiety disorders (3).
Few studies have examined the effect of implementation of the MMA benzodiazepine exclusion. One study of benzodiazepine use among nursing home residents in states with varying supplemental Medicaid coverage of benzodiazepines found that in the only state without supplemental Medicaid benzodiazepine coverage, use of benzodiazepines declined but hip fracture rates increased (4). However, the effects of the MMA's exclusion on use of benzodiazepines among nursing home residents, who represent a small proportion of benzodiazepine users, may not be generalizable. Another study found that prescriptions filled for benzodiazepines by a national retail pharmacy chain fell by 5% immediately after the MMA benzodiazepine exclusion, and prescriptions filled for antidepressants and antipsychotics increased by 7% and 18%, respectively (5). However, the study did not evaluate potential effects of substitution of benzodiazepines by mental health services and cannot account for benzodiazepine users who may have switched pharmacies. Finally, no study has examined the effect of the MMA benzodiazepine exclusion on people with anxiety disorders, the population most likely to be adversely affected by the policy's implementation.
This study examined psychotropic drug use, psychotropic drug costs, and health care utilization among two cohorts of older patients with anxiety disorders—elderly patients enrolled in Medicare Advantage and near-elderly patients with managed care insurance—before and after the MMA benzodiazepine exclusion. Before implementation of Medicare Part D, Medicare Advantage plans were one of the few options available to Medicare-eligible patients that provided prescription drug coverage; all Medicare Advantage plans were subject to the MMA' s categorical exclusion of benzodiazepines. We limited the sample to patients with new anxiety diagnoses because we hypothesized that the benzodiazepine exclusion would affect their prescription patterns more than those of patients with established diagnoses of anxiety, who may be less willing to switch medications.
Methods
The study used behavioral care, general medical, and pharmaceutical claims from 2004 to 2007 that were linked with eligibility files from a large national health plan. We developed an intervention cohort of elderly (age 65 or older) individuals drawn from one of the health plan's national Medicare Advantage plans who were not eligible for Medicaid. We developed a comparison cohort of near-elderly (age 60 to 64) individuals from one of the health plan's national managed care plans. Both cohorts had prescription drug coverage before and after the MMA's implementation, but benzodiazepine coverage was excluded for the intervention cohort after MMA implementation.
Within each cohort, we identified patients with new anxiety diagnoses during the first six months of 2005, 2006, and 2007. These patients were identified by examining the behavioral and medical claims for anxiety diagnoses that included ICD-9-CM codes 300.0, 300.00, 300.01, 300.02, 300.09, 300.21, 300.22, 300.23, 300.29, 300.3, 308.0, 309.24, 309.28, and 309.81 (6). A new diagnosis was defined as an index encounter claim by an individual with no other encounter claims with anxiety diagnoses in the prior six months (“washout” period). Patients who did not have continuous general medical, behavioral care, and pharmaceutical insurance coverage for at least six months before and after the index encounter were excluded. The final intervention cohort had 8,397 individuals, and the final comparison cohort had 1,657 individuals.
The main outcomes were any use and days' supply of and total expenditures for all psychotropic medications and for classes of psychotropic medication. Total expenditures included the patients' deductibles and copayments plus plan reimbursement. Psychotropic medications were subclassified as benzodiazepines, antidepressants, other anxiolytics, and other medications listed by the American Hospital Formulary System under 28.00: central nervous system (7). We included all medications within the following subclasses: barbiturates (28.12.04 and 28.24.04), benzodiazepines (28.12.08 and 28.24.08), antidepressants (28.16.04), antipsychotic agents (28.16.08), amphetamines (28.20.04), and antimanic agents (28.28.00). We also included buspirone, droperidol, meprobamate, chloral hydrate, eszopiclone, ramelteon, zaleplon, and zolpidem from the subclass miscellaneous anxiolytics, sedatives, and hypnotics (28.24.92) and atomoxetine and selegiline from the subclass miscellaneous central nervous system agents (28.92.00).
Additional outcomes included outpatient visits for behavioral care, any hospitalizations related to benzodiazepine withdrawal, any hospitalizations related to inappropriate benzodiazepine use, and any emergency department visits. Behavioral care outpatient visits were defined by using the Current Procedural Terminology procedure codes on the claim and included psychiatric assessment or evaluation, psychotherapy, psychotropic medication management, electroconvulsive therapy, and other psychiatric services. Hospitalizations were identified as potentially related to benzodiazepine withdrawal if the principal diagnosis was the ICD-9-CM code for either drug withdrawal (292.0), insomnia (307.4x, 327.0x, 780.52), delirium (293.x), nausea (787.0x),malaise and fatigue (780.79), seizures (345.0x, 345.1x, 345.9x, and 780.39), tachycardia (785.0), or hypertension (401.xx, 405.xx) (8). Hospitalizations were identified as potentially related to inappropriate benzodiazepine use if the principal diagnosis was the ICD-9-CM code for syncope (780.2), depression (296.xx, 300.4,or 311), chronic obstructive pulmonary disease (491.xx, 492.xx, or 496), urinary incontinence (625.6 or 788.3x), or fractures, including fracture of the hip (820.xx or 81.40), wrist (814.xx), ankle (824.x), and shoulder (812.00–812.02 and 812.10–812.12) (2).
Age and gender were obtained from the eligibility files and used as covariates. We also used ICD-9-CM diagnoses from encounter claims during the six-month washout period to create indicators for comorbid psychiatric conditions (substance use disorder, depression, and psychotic disorder) and general medical conditions (arthritis, anemia, asthma, heart failure, chronic obstructive pulmonary disease, diabetes, ulcer or liver problems, hypertension, malignant cancer, paralysis or other neurological disorders, obesity, peripheral vascular disease, pulmonary circulation disease, renal failure, valvular disease, and weight loss) (9,10).
For statistical analyses, logistic regression models were estimated for any use of psychotropic medication and health care utilization outcomes. Two-part models were estimated for the psychotropic medication expenditure and days' supply outcomes, with logistic regression used to predict any use and zero-truncated negative binomial regression used to predict expenditures (or days' supply) given use of the psychotropic medications. All of the regression models included an indicator for the year (2005, 2006, or 2007) and were adjusted for gender, age, age squared, and comorbid psychiatric and general medical conditions. Significance was determined as p<.05.
To facilitate interpretation of the estimates, we present the predicted probabilities or expected values associated with each covariate along with normal-based standard errors, bootstrapped with 2,000 replicate samples. SAS 9.1.3 was used for data management and Stata 11 was used for all statistical analyses. This study was declared exempt from review by the University of California, Los Angeles, Institutional Review Board.
Results
Table 1 shows characteristics of both cohorts. Because of the different age restrictions placed on each cohort, the intervention cohort was significantly older than the comparison cohort (75.5 versus 61.9 years, respectively). Hence, it had higher percentages of women (79.0% versus 68.9%, respectively) and individuals with a medical comorbidity excluding hypertension (65.7% versus 52.5%, respectively) but a significantly lower percentage of individuals with a psychiatric comorbidity (23.4% versus 25.9%) than the comparison cohort. The differences between the two groups in rates of comorbid psychiatric illnesses were due primarily to differences in rates of psychoses, whereas the differences in comorbid medical conditions were found for nearly all conditions except oncologic and rheumatologic conditions and obesity.
The characteristics of the cohorts over time were also compared (results not shown). In 2006 and 2007, the intervention and comparison cohorts were significantly older than in 2005, but the groups did not differ significantly by gender or comorbid psychiatric conditions. In 2005 and 2006, similar percentages of individuals in the intervention cohort had hypertension (71.6% [N=1,922] and 72.4% [N=2,079], respectively) and other comorbid general medical conditions (67.3% [N=1,806] and 67.0% [N=1,393], respectively). In the 2007 cohort, the rates of hypertension and other comorbid general medical conditions, though only slightly lower, were significantly different (68.2% [N=1,264] and 63.0% [N=1,167], respectively). For the comparison cohort, rates of hypertension were higher in 2007 than in 2005 and 2006 (57.0% [N=276], 50.1% [N=324], and 51.2% [N=269], respectively).
Significant differences were found in predicted use over time of psychotropic medication by the intervention cohort (Table 2). Individuals diagnosed in 2005 had significantly higher rates of covered claims for psychotropic medication than those diagnosed in 2006 or 2007 (75.4% versus 45.9% and 50.0%, respectively); this difference was largely due to significant reductions in covered claims for benzodiazepines (63.0% in 2005 versus .9% in 2006 and 1.3% in 2007). Claims for nonbenzodiazepine psychotropic medication by the intervention cohort increased significantly, from 40.3% in 2005 to 45.5% in 2006 and 49.5% in 2007. More specifically, significant increases occurred in rates of covered claims for antidepressants, from 35.0% in 2005 to 39.6% in 2006 and 44.2% in 2007, and for anxiolytics, from 7.7% in 2005 to 9.7% in 2006 and 10.9% in 2007.
Significant differences also occurred over time in predicted days' supply of psychotropic drugs for the intervention cohort (Table 2). Those diagnosed in 2005 had significantly higher days' supply (N=124.9) of psychotropic drugs than those diagnosed in 2006 (N=66.0) or 2007 (N=76.1); this difference was largely due to a significant reduction in claims covered for benzodiazepine use. The days' supply of benzodiazepines were much greater for those diagnosed in 2005 (N=71.1) than for those diagnosed in 2006 (N=1.1) and 2007 (N=1.5). Among those in the intervention group with any use of psychotropic drugs, the days' supply of all psychotropic drugs also significantly declined between 2005 (N=163.5) and 2006 (N=139.4) and 2007 (N=148.2) (data not shown). Among those in the intervention group with any use of benzodiazepines, the days' supply of benzodiazepines did not significantly differ in 2005 (N=112.9), 2006 (N=118.0), and 2007 (N=120.8) (data not shown). Days' supply of nonbenzodiazepines among the intervention cohort significantly increased from 53.3 in 2005 to 64.9 and 74.6, respectively, in 2006 and 2007. Significant increases in days' supply occurred specifically for antidepressants, from 43.4 in 2005 to 51.7 in 2006 and 59.3 in 2007, and for anxiolytics, from 4.8 in 2005 to 7.3 in 2006 and 9.8 in 2007.
Significant differences also occurred over time in predicted expenditures for psychotropic drugs by the intervention cohort (Table 2). Annual covered expenditures for all psychotropic drugs increased from $125 in 2005 to $129 in 2006, a nonsignificant increase, and to $154 in 2007, a significant increase. Covered expenditures for benzodiazepines were greater among the 2005 cohort than among the 2006 and 2007 cohorts ($35 versus $.50 and $.80). Expenditures for nonbenzodiazepines by the intervention cohort increased significantly, from $91 in 2005 to $128 in 2006 and $153 in 2007. Expenditures for antidepressants ($58 in 2005 versus $79 in 2006 and $89 in 2007) and anxiolytics ($11 in 2005 versus $20 in 2006 and $25 in 2007) also increased significantly.
There were no significant changes in use over time by the intervention cohort in any of the measures of predicted health care utilization, including hospital stays potentially related to inappropriate benzodiazepine use or benzodiazepine withdrawal, emergency department visits, and outpatient visits for behavioral care (Table 2).
For the comparison cohort, there were marginally significant declines in benzodiazepine expenditures, from $67 in 2005 to $39 in 2006 (p=.044) and $38 in 2007 (p=.051). Expenditures for psychotropic drugs overall and for non-benzodiazepine psychotropic drugs, antidepressants, and anxiolytics were not significantly different from 2005 to 2007. Rates of claims covered or days' supply for all psychotropic drugs as well as for benzodiazepines, nonbenzodiazepine psychotropic drugs, antidepressants, and anxiolytics did not significantly change over time. There were no significant changes over time in any measure of health care utilization, including hospital stays potentially related to inappropriate benzodiazepine use or benzodiazepine withdrawal, emergency department visits, and outpatient visits for behavioral care.
Discussion
Prior studies of drugs and out-of-pocket payments (copayments) show that individuals faced with higher levels of copayment reduce their general utilization of drugs (11), although individuals are less likely to reduce use of a drug for a chronic condition than other drugs (12). Population-level analyses also have not always observed reductions in psychotropic drug utilization as a result of higher copayments, possibly because of other competing factors (13). Nonetheless, prior efforts to regulate use of benzodiazepines suggest that many individuals who appropriately use benzodiazepines go without treatment when restrictions are imposed. When New York's Medicaid system implemented triplicates for benzodiazepines in 1989, benzodiazepine use was reduced by 50% and was only partially (10%) offset by substitution by anxiolyticdrugs (14–16). Eighteen months after a temporary ban on triazolam was implemented in the United Kingdom, 45% of chronic and 66% of intermittent users stopped using benzodiazepinesaltogether, even though other benzodiazepines were available by prescription (17).
In this study of elderly patients with new anxiety diagnoses enrolled in Medicare Advantage, the benzodiazepine exclusion implemented by the MMA resulted in increased use and days' supply of nonbenzodiazepine psychotropic medications but in decreased use and days' supply of psychotropic medications overall. The decline in covered claims for psychotropic medication following the implementation of the MMA may reflect an overall reduction in treatment of anxiety, given that we did not find evidence that outpatient behavioral care use had increased to substitute for psychotropic medications. Despite the reduction in overall use of psychotropic medication by the intervention group, their expenditures for psychotropic medication increased. Even if implementation of the MMA reduced inappropriate benzodiazepine use, any reductions achieved did not result in reduced hospitalizations or emergency department visits and were accompanied by slightly higher medication expenditures.
There are several limitations to this study. Because the data were restricted to paid claims, we could not measure benzodiazepine use paid for by patients completely out of pocket. As a result, the reductions in psychotropic drug use we observed could have been offset by unobserved benzodiazepine use paid for completely out of pocket. However, if patients had paid for substantial benzodiazepine use out of pocket, the overall pharmaceutical costs after Part D implementation would have been even higher.
Our study does not have clinical data to determine whether treatment outcomes changed as a result of Part D implementation. Additional studies could help elucidate this issue. Another limitation of administrative claims without clinical data is that anxiety and related conditions may not have been coded. As a result, it is possible that our sample of individuals with new anxiety disorders may not reflect the entire population of such individuals or that we included individuals with chronic anxiety disorder. Future studies that have clinical information could help determine whether use or costs of medication among individuals with uncoded new anxiety disorders differ substantially from that of individuals with coded new anxiety disorders. Because individuals with chronic anxiety disorder are less likely to switch medications, we may have overestimated the degree to which antidepressants or other anxiolytics have been used as substitutes for benzodiazepines since implementation of the MMA.
It is possible that outpatient medical visits could have increased in response to the change in benzodiazepine coverage. Unfortunately, we did not have access to data to evaluate this possibility, and future studies could help determine if such an effect occurred. We examined patients with Medicare Advantage insurance, and it is possible that our findings may not generalize to the Medicare fee-for-service population. However, Medicare fee-for-service patients had little prescription drug coverage before Medicare Part D, which makes it difficult to study this issue in the larger Medicare fee-for-service population.
Our control cohort was smaller than our intervention cohort, which made it more difficult to detect significant differences in their use of and expenditures for psychotropic medications over time. However, the point estimates for the comparison cohort suggest that because the rates and amounts of use and expenditures were generally stable over time, the lack of significant differences was not due to low statistical power. The comparison cohort also had more use of psychotropic drugs than the intervention cohort, which raises the concern of whether secular time trends would have been the same. However, the comparison cohort and the intervention cohort did not significantly differ in the rates of depression or substance use disorder. The only difference between the cohorts occurred in rates of psychosis diagnoses, which were likely due to the age differences.
Conclusions
Although the MMA's benzodiazepine exclusion may have improved care for older Medicare patients without anxiety disorders who were using benzodiazepines, it was associated with increased overall prescription costs and with reduced treatment of older Medicare patients with anxiety. These findings suggest that states seeking to reduce budget costs through restrictions of benzodiazepines may actually increase overall costs. It will be important to evaluate whether these changes persist after benzodiazepines are made available in 2013 through Medicare Part D.
1 Cutting Government Waste and Increasing Efficiency: Recommendations to the Governor. Sacramento, Calif, Bureau of State Audits, 2011 Google Scholar
2 : Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Archives of Internal Medicine 163:2716–2724, 2003 Crossref, Medline, Google Scholar
3 : The exclusion of benzodiazepine coverage in Medicare: simple steps for avoiding a public health crisis. Psychiatric Services 56:1143–1146, 2005 Link, Google Scholar
4 : Medicare Part D's exclusion of benzodiazepines and fracture risk in nursing homes. Archives of Internal Medicine 170:693–698, 2010 Crossref, Medline, Google Scholar
5 : The impact of Medicare Part D on psychotropic utilization and financial burden for community-based seniors. Psychiatric Services 59:1191–1197, 2008 Link, Google Scholar
6 : Physician office visits of adults for anxiety disorders in the United States, 1985–1998. Journal of General Internal Medicine 17:165–172, 2002 Crossref, Medline, Google Scholar
7 AHFS Drug Information 2005. Bethesda, Md, American Society of Health-System Pharmacists, 2005 Google Scholar
8 Benzodiazepine Dependence, Toxicity, and Abuse: a Task Force Report. Washington, DC, American Psychiatric Association, 1990 Google Scholar
9 HCUP Comorbidity Software, Version 3.2. Rockville, Md, Agency for Healthcare Research and Quality, 2007. Available at www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp Google Scholar
10 : Association of general medical and psychiatric comorbidities with receipt of guideline-concordant care for depression. Psychiatric Services 61:1255–1259, 2010 Link, Google Scholar
11 : The demand for prescription drugs as a function of cost-sharing. Social Science and Medicine 21:1063–1069, 1985 Crossref, Medline, Google Scholar
12 : Pharmacy benefits and the use of drugs by the chronically ill. JAMA 291:2344–2350, 2004 Crossref, Medline, Google Scholar
13 : A time-series analysis of the effect of increased copayments on the prescription of antidepressants, anxiolytics, and sedatives in Sweden from 1990 to 1999. Clinical Therapeutics 25:1262–1275, 2003 Crossref, Medline, Google Scholar
14 : A controlled study of the effects of state surveillance on indicators of problematic and nonproblematic benzodiazepine use in a Medicaid population. International Journal of Psychiatry in Medicine 34:103–123, 2004 Crossref, Medline, Google Scholar
15 : A retrospective data analysis of the impact of the New York triplicate prescription program on benzodiazepine use in Medicaid patients with chronic psychiatric and neurologic disorders. Clinical Therapeutics 26:322–336, 2004 Crossref, Medline, Google Scholar
16 : Effects of state surveillance on new posthospitalization benzodiazepine use. International Journal for Quality in Health Care 15:423–431, 2003 Crossref, Medline, Google Scholar
17 : Withdrawal of triazolam's product license: effect on patients 18 months later. Addiction 90:927–934, 1995 Crossref, Medline, Google Scholar
Figures and Tables
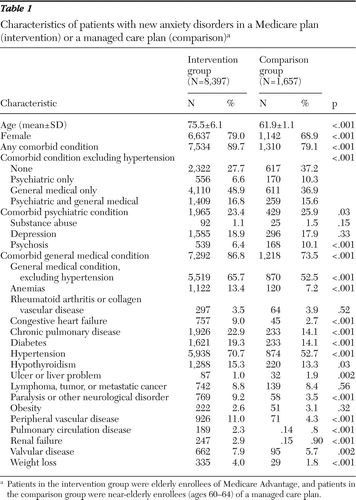
Table 1 Characteristics of patients with new anxiety disorders in a Medicare plan (intervention) or a managed care plan (comparison)
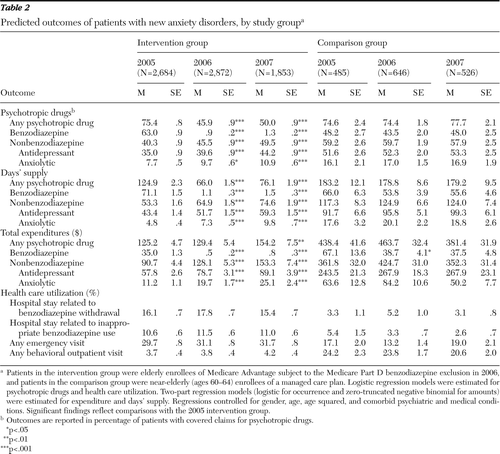
Table 2 Predicted outcomes of patients with new anxiety disorders, by study group



