Pharmacotherapy of Alcohol Use Disorders by the Veterans Health Administration: Patterns of Receipt and Persistence
Abstract
Objective:
This study assessed changes since 2007 at Veterans Health Administration (VHA) facilities (N=129) in use of the medications approved by the U.S. Food and Drug Administration for treatment of alcohol use disorders.
Methods:
VHA data from fiscal years (FYs) 2008 and 2009 were used to identify patients with a diagnosis of an alcohol use disorder who received oral or extended-release naltrexone, disulfiram, or acamprosate as well as the proportion of days covered (PDC) in the 180 days after initiation and the time to first ten-day gap in possession (persistence) for each medication. Multilevel, mixed-effects logistic regression models examined the association between patient and facility characteristics and use of medications.
Results:
Nationally, 3.4% of VHA patients with an alcohol use disorder received medications in FY 2009 (11,165 of 331,635 patients), up from 3.0% in FY 2007. Use of medications by patients at the facilities ranged from 0% to 12%. In fully adjusted analyses, facilities offering evening and weekend services had higher rates of medication receipt, but other facility characteristics, such as having prescribers on the addiction program's staff or using medication to treat opioid or tobacco dependence, were unrelated to medication receipt. The mean PDC of acamprosate was significantly lower than mean PDCs of the other medications (p<.05), and persistence in use of naltrexone was significantly greater than use of acamprosate and significantly less than use of disulfiram (p<.05).
Conclusions:
Use of these medications is increasing but remains variable across the VHA system. Interventions are needed to optimize initiation of and persistence in use of these medications. (Psychiatric Services 63:679–685, 2012; doi: 10.1176/appi.ps.201000553)
Consensus standards for evidence-based treatment of alcohol dependence endorse making available and considering use of medications approved by the U.S. Food and Drug Administration (FDA) (oral and extended-release [XR] naltrexone, disulfiram, and acamprosate). This standard has been approved by the National Quality Forum (1) and adopted as a performance measure by the American Psychiatric Association Physician Consortium for Performance Improvement and the National Committee for Quality Assurance (2). All Veterans Health Administration (VHA) facilities are mandated to make available and consider the use of medications for alcohol dependence (3). Nevertheless, receipt of the medications by patients is rare overall and varies highly among and within health care systems (4,5).
We previously reported that in fiscal years (FY) 2006 and 2007, FDA-approved pharmacotherapy was received by 2.8% and 3.0%, respectively, of VHA patients with clinically diagnosed alcohol use disorders (4). Medication receipt was more likely among patients receiving specialty addiction care, patients with alcohol dependence (versus abuse), patients younger than 55 years old, and females. However, in no subgroup did receipt of medications exceed 11.6%. Further, across all VHA facilities, the proportion of patients with alcohol use disorders who received pharmacotherapy ranged from 0% to 7% among patients who received no specialty addiction treatment and up to 20.5% among patients who received specialty addiction treatment. The prior study did not explore facility-level predictors of receipt, an important omission because a substantial proportion of the variation in receipt was between facilities rather than between patients (intraclass correlation=.27). Nor did our previous study describe patterns of use after initiation, such as the 180-day proportion of days covered and time to ten-day gap in possession (persistence) (6).
Very few studies have examined how these medications are utilized in day-to-day clinical practice. A study of patterns of dispensing naltrexone and disulfiram by one VHA network from 1998 to 2001 found that the median supply per treatment episode was two months for both medications, with 35% receiving only one month's supply (7). A large commercial claims data set indicated that a vast majority of patients initiating naltrexone received less than three months' supply, with roughly 50% getting only one month's supply (8). Thus outside academically managed clinical trials, initiation of these medications appears to be rare, and even patients who start taking them usually stop within one or two months.
No consensus exists regarding what constitutes an adequate course of pharmacotherapy for alcohol use disorders (9). Some evidence from experimental protocols linked better outcomes of naltrexone with greater persistence (10,11). In these studies, patients who took 80% of the intended 12-week course of naltrexone had better outcomes. Examining commercial claims data, Kranzler and others (12) found that 14.2% of patients filled prescriptions for 80% or more of a six-month period; these patients had less intensive subsequent treatments—for example, emergency and inpatient admissions—than patients who filled prescriptions covering less than 80% of the six-month period. Analogous data linking persistence and outcomes for disulfiram and acamprosate have not been reported.
This study had the following objectives: to determine if receipt by VHA patients of medications for treatment of alcohol use disorders has changed since 2007, to examine the patient- and facility-level predictors of pharmacotherapy receipt, and to characterize and compare patterns of use and persistence in use of these medications.
Methods
Medication receipt
We identified patients from the VHA National Patient Care Database (NPCD) who received an alcohol use disorder diagnosis (ICD-9-CM codes 303.9x or 305.0x) in FYs 2008 and 2009 and used VHA Decision Support System (DSS) inpatient and outpatient pharmacy benefits files to determine the proportion of the patients who received acamprosate, disulfiram, oral naltrexone, or XR naltrexone. Even though these medications are approved only for alcohol dependence, we also included patients with diagnoses of alcohol abuse for several reasons. First, the fidelity of these diagnostic distinctions in clinical practice is low, and many patients have both diagnoses concurrently. Second, an appreciable percentage of VHA patients who take these medications have only an alcohol abuse diagnosis. Third, the substance disorders-related work group of the upcoming revision of the DSM (www.dsm5.org) has proposed elimination of the abuse-dependence distinction. However, we also included subgroup and sensitivity analyses restricted to patients with a diagnosis of alcohol dependence because it may act as a proxy for severity.
Patient- and facility-level predictors
NPCD data about patients' characteristics, for example, age and gender, and their clinical diagnoses (alcohol abuse or dependence, comorbid drug use disorder, or other psychiatric diagnoses) were examined as predictors of medication receipt. DSS specialty care stop codes and bed section codes were used to identify patients with an alcohol use disorder at each facility who received treatment during the year at one of VHA's specialty substance use disorder clinics or inpatient or residential programs. Other potentially relevant demographic and clinical predictors, such as income and addiction severity, were not included because VHA administrative data were not available. Because information in the VHA administrative data about patients' race is unreliable, race was not included as a predictor (13–15).
Facility-level variables with plausible links to medication receipt were derived from the 2008 Drug and Alcohol Program Survey (DAPS) of programs' practices and available services. The 2008 DAPS was administered to 260 addiction treatment programs by the VHA Program Evaluation and Resource Center and aggregated to the 129 VHA facilities defined by the NPCD three-digit station codes. The DAPS is usually completed by the program manager and had a 100% response rate in 2008. The DAPS variables in this study included those with obvious structural links to medication provision (number of staff with prescribing privileges and ratio of prescribers to total specialty care patients), those that may be indicators of openness to the use of medications in treatment (having medication available to aid detoxification from alcohol or opioids, having inpatient beds available for withdrawal management, and providing medication for smoking cessation), and those that signify flexible and patient-centered care (providing services on nights or weekends, using treatment improvement protocols in decision making regarding patient treatment, and treating patients who are actively using substances). Formulary policy was not included because it is determined nationally for all facilities. Disulfiram and oral naltrexone are on the formulary and are available to VHA patients at all facilities. National criteria for nonformulary use of acamprosate and XR naltrexone ensure consistency across facilities in case-by-case approval of prescriptions. Therefore, there is no variation between facilities in formulary policy.
All patient- and facility-level analyses were performed by using multilevel, mixed-effects logistic regression models, with a random effect for facility to account for the clustering of patients within VHA facilities.
Medication utilization patterns
Two metrics were used to characterize longitudinal medication utilization patterns—180-day proportion of days covered (PDC) and time to first clinically significant gap in possession (TTG). The PDC is the total days' supply, including overlapping prescriptions, dispensed during the observation period divided by the length of the period (180 days) and truncated to 100%, if necessary (16). Each XR naltrexone injection was counted as 30 days of supply. To calculate the 180-day PDC, we included patients who did not have a prescription filled in FY 2007 and who initiated use of a medication during the 18 months from the start of FY 2008 to 180 days before the end of FY 2009.
The second metric, TTG, is a measure of persistence. Assessment of TTG requires specification of the size of the gap in possession of medication that is considered clinically significant (17). Although no standards exist in this area, ten days was chosen for this study because pharmacologic benefits of these medications would not be expected after ten days of nonuse. For every patient, all gap sizes were calculated from the first prescription filled to the last fill or end of the observation period. The TTG of at least ten days was calculated for each patient, and those without such gaps were considered censored.
Like other time-to-event analyses, TTG calculation and analysis do not require a uniform observation period. Therefore, the sample included patients who did not have a prescription filled in FY 2007 and initiated one of the medications during the 24 months from FY 2008 to the end of FY 2009. For every patient, all gap sizes were calculated from the first prescription filled to the last fill or the end of the observation period. Then the TTG of at least ten days was calculated for each patient. Those with no such gaps in the observation period were considered censored at the end of the observation period.
PDC and TTG describe two somewhat distinct dimensions of medication utilization. The 180-day PDC can accrue over multiple episodes of care—for example, patients' participation in treatment may be episodic or they may take the medication only as needed (PRN). TTG focuses on the length of mostly continuous availability after initiating treatment. Analysis of variance with post hoc, pairwise comparisons were used to compare the PDC of the four medications, and TTG of the four medications was compared with Cox regression.
Institutional review boards of Stanford University and the Department of Veterans Affairs Palo Alto Health Care System approved all aspects of this study.
Results
Medication receipt
Table 1 presents the number of VHA patients with alcohol use disorders, as well as patients with only alcohol dependence, who received any of the FDA-approved pharmacotherapies from FY 2006 to FY 2009. (Data from 2006 and 2007 are from our earlier study [4]). The percentage of patients with an alcohol use disorder who received at least one medication rose from 3.0% in FY 2007 to 3.4% in FY 2009, an increase due mostly to use of oral naltrexone. The percentage of patients with an alcohol use disorder who received at least one medication in FY 2009 varied by facility (range 0%–12%) and increased from FY 2007 to FY 2009 by a mean±SD of 1.5%±1.2% (range −1% to 7%; paired t=13.65, df=128, p<.001). Although rates of receipt were somewhat higher among patients with an alcohol dependence diagnosis, the general trends were the same.
The rate of pharmacotherapy receipt among patients with an alcohol use disorder and contact with addiction specialty treatment during the fiscal year was 8.2% in FY 2009, up from 6.4% in FY 2007, and ranged by facility from 1% to 24% in FY 2009. The rate by facility increased by 1.5%±3.2% (range −8% to 20%; paired t=4.84, df=127, p<.001). The rate of pharmacotherapy receipt among patients with an alcohol use disorder and without contact with addiction specialty treatment was 1.3% in FY 2009, up from 1.2% in FY 2007, and ranged by facility from 0% to 5% in FY 2009. The rate by facility increased by .1%±.5%, but the increase was not statistically significant.
Predictors of medication receipt
Table 2 presents the multivariate, mixed-effects logistic regression model of associations between patient and facility factors and the receipt of any of the medications among the patients with alcohol dependence in FY 2009. Patients who were female, were age 31 to 55, had no comorbid drug use disorder, had contact with specialty addiction care (especially inpatient or residential addiction treatment), and had a comorbid psychiatric diagnosis were more likely than patients without these characteristics to receive at least one of these medications. The intraclass correlation of the intercept-only model was .27, meaning that 27% of the total variance in medication receipt was explainable by facility factors rather than by patient factors. However, the only significant facility-level predictor of medication receipt was providing services on nights or weekends; patients at facilities that provided such services (82%) were 50% more likely to receive medication. Contrary to expectations, the other facility-level characteristics were not significant independent predictors of medication receipt. These results were the same for analyses of patients with specialty addiction program contact and patients with alcohol use disorders.
Proportion of days covered
A total of 10,779 patients initiated the medications during the 18 months beginning in FY 2008. The mean 180-day PDC of disulfiram, XR naltrexone, oral naltrexone, and acamprosate was .464 (84 days), .447 (80 days), .422 (76 days), and .385 (69 days), respectively. Analysis of variance with post hoc, pairwise comparisons revealed that the mean PDC of acamprosate was significantly lower than the mean PDCs of disulfiram, XR naltrexone, and oral naltrexone (p<.05). The mean PDC of disulfiram was significantly higher than the mean PDC of oral naltrexone (p<.05) (Table 3).
Table 3 also presents the percentage of patients initiating each medication who received fewer than 31, 61, 91, and 144 days' supply within the 180-day observation period. The percentage of patients initiating medication who received fewer than 31 days' supply was highest for acamprosate (40%) and lowest for disulfiram (31%). Lower percentages signify more patients remaining on the medication through the time period; for example, 69% of patients who initiated disulfiram received more than 31 days' supply. A four-sample test for equality of proportions and pairwise comparisons of proportions with a Bonferroni correction suggested that the proportion of patients who received fewer than 31 days' supply was significantly higher among patients who received acamprosate than among those who received oral naltrexone (p<.001). In turn, the proportion of patients who received fewer than 31 days' supply was significantly higher among patients who received oral naltrexone than among those who received disulfiram (p<.001). Comparisons of the percentages of patients who received fewer than 31 days' supply of XR naltrexone and of other medications were not significant.
This general pattern was maintained across the 61-, 91-, and 144-day cutoffs. The proportion of patients who received 61, 91, and 144 days' supply of medication varied for each cutoff (χ2=66.7, df=3, p<.001; χ2=44.3, df=3, p<.001; and χ2=79.6, df=3, p<.001, respectively). At all time cutoffs, the proportion of patients receiving less than a given days' supply was significantly lower for disulfiram than for oral naltrexone and acamprosate (p<.05). At all time cutoffs, the proportion of patients receiving less than a given days' supply was significantly higher for acamprosate than for oral naltrexone and disulfiram (p<.05).
The average PDC of medications varied substantially, from 26.4% to 76.4%, by facility. The average PDC for the medications by facility was not related to rates of medication receipt by the patients at each facility. Mixed-effects regression results estimating the associations between characteristics of patients and facilities and PDC are presented in Table 4.
Time to first gap or discontinuation
The sample and observation time for these analyses are different from the PDC analyses because initial prescription could occur any time during FY 2008 and FY 2009, and the observation time could be as long as 24 months. A gap was defined as a lapse in supply that was ended by another prescription refill. Discontinuation involved no refill through the end of the observation period. The percentages of patients without a ten-day gap in supply or discontinuation after 30 and 90 days are presented in Table 5.
The proportion of individuals who reached 30 days without a gap was lowest (38.4%) among those receiving acamprosate and highest (51.9%) among those who received disulfiram. The proportion of individuals who reached 90 days without a gap was lowest (9.4%) among those receiving acamprosate and highest (20.2%) among patients receiving XR naltrexone.
At both the 30- and 90-day time points, the confidence intervals (CIs) for oral naltrexone, disulfiram, and XR naltrexone overlap with each other but not with the CI for acamprosate, revealing that the proportion of patients clearing these time points without a gap was significantly lower for acamprosate than for the other drugs. Cox regression analysis of time to first ten-day gap with oral naltrexone as the reference revealed that the risk of experiencing a gap in supply or discontinuation was significantly higher among patients receiving acamprosate (odds ratio [OR]=1.32, p<.001) and significantly lower among patients receiving disulfiram (OR=.92, p<.001).
Discussion
The use of pharmacotherapy by the VHA to treat alcohol use disorders is increasing, but it is still relatively rare and varies substantially by facility. Increased use of oral naltrexone and the more frequent receipt of pharmacotherapy by patients who had contact with addiction treatment programs accounted for most of the modest but statistically significant increase in use of pharmacotherapy from FY 2007 to FY 2009. The diffusion in use of XR naltrexone remained modest and concentrated in a few facilities. The overall trend was toward increased use of pharmacotherapy for alcohol use disorders, and use at some facilities increased dramatically. Yet at other facilities use of pharmacotherapy to treat alcohol use disorders decreased. These data suggest the need for more targeted evaluation to explain these changes.
Patients who were female, who were age 31 to 55, who had no comorbid drug use disorder, and who had an alcohol dependence (versus abuse) disorder diagnosis, contact with specialty addiction care (especially inpatient or residential addiction treatment), and a comorbid psychiatric diagnosis were more likely than patients without such characteristics to receive at least one of these medications. These patient-level predictors are consistent with and extend the results of our previous analysis of FY 2007 data. In this analysis, only one facility-level characteristic—the availability of specialty substance use disorder treatment programming on nights, weekends, or both—independently predicted pharmacotherapy receipt. Any direct link between extended hours of treatment and the receipt of pharmacotherapy is unknown. It is perhaps more likely that this factor, found by other studies to be linked to outcomes (18), is a general marker for quality and, perhaps, a patient-centered orientation.
The lack of association between the number of full-time-equivalent (FTE)addiction program staff who could prescribe medications or the rate of prescriber FTEs per total patients at a facility and receipt of medications was surprising. Perhaps in an addiction program, availability of prescribers is less important than access to prescribers who are supportive of pharmacotherapy for alcohol use disorders, even if they are not directly linked to the addiction program. In other words, access to providers may be necessary but not sufficient to ensure prescription of these medications. A limitation of this study is that much of the variability found in medication receipt was between facilities, but facility-level variables failed to explain much of this variation.
The results related to use patterns and persistence were roughly consistent with the study at one VHA network that found the median supply of naltrexone and disulfiram per treatment episode was two months and that 35% of patients received only one month's supply. This study updated and extended these findings to the VHA nationally and provided data on acamprosate and XR naltrexone. The relatively low persistence in use of acamprosate might be explained by the need to take this medication three times a day but requires further exploration.
The data on initiation and persistence—especially the lack of data on whether patients took the dispensed oral medications, patients' preferences, medical contraindications, or providers' decision-making processes—are purely descriptive and have limitations. Thus the data used in this study represent the upper bound on alcohol pharmacotherapy utilization. Given that limitation, the low rate of utilization of FDA-approved medications found here is even more discouraging.
Conclusions
The use of FDA-approved medications for the treatment of alcohol use disorders by the VHA is slowly increasing but remains highly variable across facilities. Perhaps the negative results of a well-known, large randomized trial of naltrexone at the VHA (19) have affected the use of pharmacotherapy for alcohol dependence, even though other studies have demonstrated its efficacy. These data provide an updated baseline for targeted efforts to increase consideration of and persistence in use of these medications. Interventions are needed to reduce undesirable systemwide variability in medication initiation and once initiated, to improve medication persistence.
1 National Voluntary Consensus Standards for the Treatment of Substance Use Conditions: Evidence-Based Treatment Practices. Washington, DC, National Quality Forum, 2007 Google Scholar
2
3 Uniform mental health services in VA medical centers and clinics; in VHA Handbook 1160.01. Washington, DC, US Department of Veterans Affairs, Veterans Health Administration, 2008 Google Scholar
4 : Pharmacotherapy of alcohol use disorders in the Veterans Health Administration. Psychiatric Services 61:392–398, 2010 Link, Google Scholar
5 : Alcohol and opioid dependence medications: prescription trends, overall and by physician specialty. Drug and Alcohol Dependence 99:345–349, 2009 Crossref, Medline, Google Scholar
6 : Medication compliance and persistence: terminology and definitions. Value Health 11:44–47, 2008 Crossref, Medline, Google Scholar
7 : Patterns of dispensed disulfiram and naltrexone for alcoholism treatment in a veteran patient population. Alcoholism, Clinical and Experimental Research 28:1229–1235, 2004 Crossref, Medline, Google Scholar
8 : Trends in naltrexone use among members of a large private health plan. Psychiatric Services 55:221, 2004 Link, Google Scholar
9 Incorporating Alcohol Pharmacotherapies Into Medical Practice. DHHS pub no SMA 09-4380. Rockville, Md, Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment, 2009 Google Scholar
10 : Improving naltrexone response: an intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. Journal of Addictive Disorders 19:71–83, 2000 Crossref, Medline, Google Scholar
11 : What role does measuring medication compliance play in evaluating the efficacy of naltrexone? Alcohol Clinical and Experimental Research 31:596–603, 2007 Medline, Google Scholar
12 : Persistence with oral naltrexone for alcohol treatment: implications for health-care utilization. Addiction 103:1801–1808, 2008 Crossref, Medline, Google Scholar
13 : Transition to the new race-ethnicity data collection standards in the Department of Veterans Affairs. Population Health Metrics 4:7, 2006; doi:
14 : Agreement between administrative data and patients' self-reports of race-ethnicity. American Journal of Public Health 93:1734–1739, 2003 Crossref, Medline, Google Scholar
15
16 : Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Annals of Pharmacotherapy 40:1280–1288, 2006 Crossref, Medline, Google Scholar
17 : Estimating medication persistency using administrative claims data. American Journal of Managed Care 11:449–457, 2005 Medline, Google Scholar
18 : Treatment staff's continuity of care practices, patients' engagement in continuing care, and abstinence following outpatient substance-use disorder treatment. Journal of Studies on Alcohol and Drugs 69:747–756, 2008 Crossref, Medline, Google Scholar
19 : Naltrexone in the treatment of alcohol dependence. New England Journal of Medicine 345:1734–1739, 2001 Crossref, Medline, Google Scholar
Figures and Tables
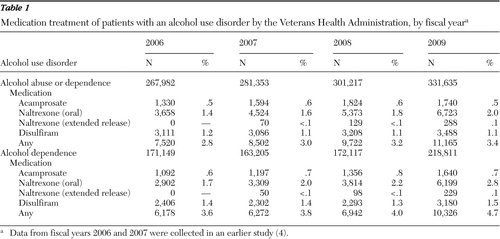
Table 1 Medication treatment of patients with an alcohol use disorder by the Veterans Health Administration, by fiscal year
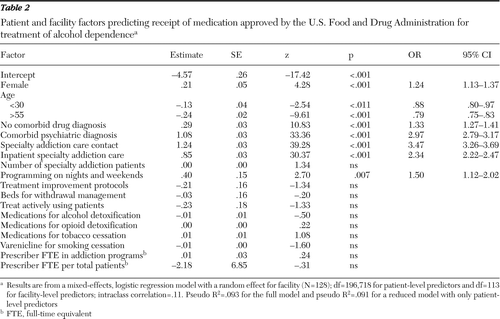
Table 2 Patient and facility factors predicting receipt of medication approved by the U.S. Food and Drug Administration for treatment of alcohol dependence
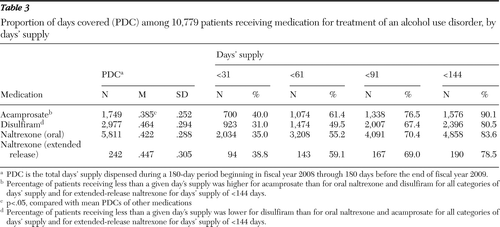
Table 3 Proportion of days covered (PDC) among 10,779 patients receiving medication for treatment of an alcohol use disorder, by days' supply
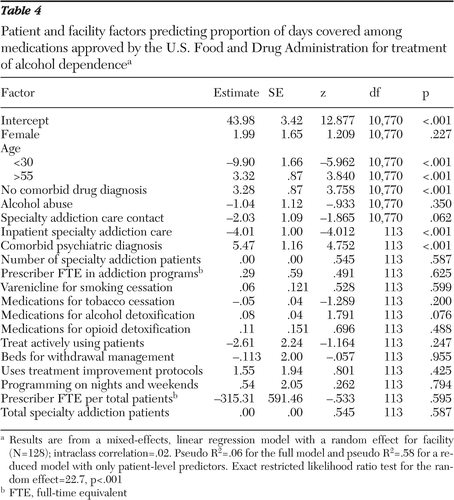
Table 4 Patient and facility factors predicting proportion of days covered among medications approved by the U.S. Food and Drug Administration for treatment of alcohol dependence
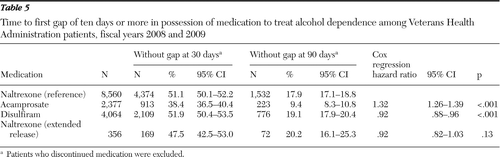
Table 5 Time to first gap of ten days or more in possession of medication to treat alcohol dependence among Veterans Health Administration patients, fiscal years 2008 and 2009



