Multimodal Treatment for ADHD Among Youths in Three Medicaid Subgroups: Disabled, Foster Care, and Low Income
Abstract
OBJECTIVE: This study compared the use of treatments for attention-deficit hyperactivity disorder (ADHD) among three distinct subpopulations of Medicaid-insured youths who have very different mental health needs and patterns of service use: those with federally documented disability, those in foster care, and those in families with low income. METHODS: This one-year, cross-sectional study of community mental health services used administrative data. Individuals who were younger than 20 years, who were continuously enrolled in one Mid-Atlantic state Medicaid program, and who had two or more medical encounters associated with an ADHD diagnosis in 1998 were identified (N=1,296). Measures of the use of mental health services were the number of different classes of psychopharmacologic medications, the psychopharmacologic regimen, and the combined use of pharmacotherapy and psychotherapy treatments (multimodal treatment). RESULTS: Use of multiple psychopharmacologic agents was greater in the disabled and foster care groups compared with the low-income group. Significantly fewer mental health provider visits, but greater use of stimulant treatment only, were observed in the low-income group compared with the other groups. Youths in the disabled group were significantly more likely than youths in the low-income group, but not more likely than youths in the foster care group, to receive multimodal treatments. Children in foster care were significantly more likely than those in the other groups to use a substance abuse service. CONCLUSIONS: Among a cohort of Medicaid-enrolled youths with ADHD, co-existing psychiatric disorders and complex psychopharmacologic treatments were more common in the disabled and foster care groups than in the low-income group. Youths with disabilities were significantly more likely than youths in the low-income group to receive multimodal treatment.
Epidemiologic findings show a 3 to 5 percent prevalence of attention-deficit hyperactivity disorder (ADHD) among school-aged children (1,2). Other studies have noted a prevalence of 7 to 16 percent (3,4). Identification of ADHD in pediatric primary care visits for four- to 15-year-old youths increased from 1.4 percent in 1979 to 9.2 percent in 1996 (5). Data from studies of youths in a health maintenance organization and in Medicaid programs indicate that use of stimulants rose from .7 to 1.6 percent in 1991 to 2.5 to 3.8 percent in 1996 (6,7), and national estimates of stimulant use increased from .9 percent in 1987 to 3.4 percent in 1997 (8). Primary care physicians often prescribe only stimulants for ADHD (9,10), and mental health providers use more nonstimulant psychopharmacologic treatments (10,11,12). Among youths enrolled in Medicaid, ADHD prevalence and mental health service use is higher among youths in foster care than among those with a disability or in families with low income (13,14,15,16,17).
Stimulants have a well-established short-term efficacy, are a first-line treatment (18,19), and have a relatively safe profile (20)—with the exception of a black-box warning for hepatotoxicity with pemoline (21)—yet symptoms persist for about 20 to 30 percent of stimulant-treated youths (20,22,23). This persistence of symptoms can be higher in the presence of comorbid psychopathology or developmental delays (24). Stimulants do not necessarily improve self-esteem (25), and the two studies that reported an association between stimulants and improved self-esteem (26,27) involved small samples and newly diagnosed, never-medicated children in mainstream classrooms. Also, medications for behavior problems often do not improve peer relations (28,29).
Limited success with pharmacotherapy or psychotherapy alone warrants combined use of both treatments, that is, multimodal treatment (23,30). Children with comorbid internalizing disorders (anxiety and depression) (22,31)—which commonly occur with ADHD (10,32,33,34,35) and often are related to age of onset and symptom severity (36)—may benefit from multimodal treatment. Although results of the Multimodal Treatment Study of Children With ADHD (MTA) favored medication management, multimodal treatments led to better outcomes than medication management for children with comorbid anxiety disorder (32,37).
Previous research has focused on access (10,12,14,38,39,40,41,42) or the cost of care (43,44) rather than on the use of multimodal treatment. Consequently, relatively little is known about the variety of treatments that youths with ADHD receive in community outpatient practice settings. Medicaid-enrolled youths who have disabilities, are in foster care, or are in a low-income group represent youths with very different mental health needs. Despite the known differences in mental health service use patterns, to our knowledge no other study has explored the use of multimodal treatments among these three groups. Therefore, our study examined the use of psychopharmacologic and multimodal treatments in community practice and examined the variation in treatment among Medicaid-enrolled youths with ADHD who were disabled, in foster care, or met income qualifications for Medicaid.
Methods
Study design and sample
Medicaid administrative data from one Mid-Atlantic state were used for a one-year cross-sectional study of mental health services for child and adolescent enrollees. This article focuses on a continuously enrolled cohort who received ADHD services in 1998. The institutional review board at the Johns Hopkins Bloomberg School of Public Health approved the study.
All continuously (ten or more months) Medicaid-enrolled youths who were younger than 20 years in 1998 and had two or more medical encounters related to ADHD were identified (N=1,296). The criterion of two or more ADHD visits was used to identify individuals who were actively in treatment. The sample included 494 out of 4,828 Medicaid-enrolled youths who were eligible because of disability (10.2 percent), 87 of 1,211 who were eligible because they were in foster care (7.2 percent), and 715 of 48,576 who were eligible because of low family income (1.5 percent). Youths were assigned to the category with the longest period of enrollment in 1998. Multiprogram enrollment affected only 19 youths (1.5 percent): ten who were in both the low-income and disabled groups (.8 percent) and nine who were in both the low-income and foster care groups (.7 percent).
Data sources
Data on Medicaid eligibility, medical encounters, and medication use were used for our study. Eligibility files included information on the individual's age, gender, race and ethnicity, enrollment dates, and state-assigned eligibility category, which related to disability, foster care, and low-income status. Data on disabling conditions were not available from Medicaid claims, but disability eligibility was determined by federal standards.
Medical encounter files produced service use variables, including service date, provider type, medical diagnosis, medical procedures, and service setting. Provider categories were mental health (psychiatrists, psychologists, mental health and substance abuse clinics, and psychiatric clinics), primary care (physicians, pediatricians, managed care, nurse practitioners, Early and Periodic Screening, Detection, and Treatment providers, and general health clinics), or other specialty (home or personal care providers, outpatient hospital clinics, neurology, and rehabilitation).
International Classification of Disease, 9th Edition (ICD-9) codes represented clinician-reported diagnoses. Claims-based diagnostic information has been found to be fairly complete, with 54 to 100 percent corresponding with the medical record data (45,46,47,48). The three-digit major category code has been found to be more reliable than specific five-digit codes (49). Organized by major group, diagnoses found in our study included ADHD, adjustment disorder, anxiety, autism, bipolar disorder, conduct disorder, depression, developmental disability, learning disability, mental retardation, oppositional defiant disorder, personality disorders, psychoses, substance use disorders, and tic disorders. All other codes were categorized as "other psychiatric disorders."
Procedures, recorded as Current Procedural Terminology 1998, Standard Edition (CPT), were classified as mental health, developmental, and general medical. Mental health-related procedures included CPT codes 90801 to 90899, methadone drug level testing, psychological testing, and several state-specific codes, including individual and group mental health treatments, substance abuse counseling and services, psychiatric rehabilitation, residential treatment-based behavioral therapy, and day treatment services. These codes were classified further as psychopharmacologic management, psychotherapy, school-based services, substance abuse and mental health treatment, and "all other" (for example, psychiatric evaluations). Developmental procedures included speech and language as well as physical and occupational therapy. General procedures were all other evaluation and management visits.
Psychopharmacologic medications were identified from the pharmacy claims. The major therapeutic classifications were stimulants, antidepressants, antipsychotics, antiparkinsonian medications, sedatives and hypnotics, anxiolytics, anticonvulsants, and lithium. Antidepressants were also identified as selective serotonin reuptake inhibitors (SSRI) and tricyclic antidepressants, given the more frequent use of SSRIs for ADHD (50). Because carbamazepine and valproic acid are used in treating patients with psychiatric disorders, these medications were subgrouped as mood-stabilizing anticonvulsants.
Analytic plan
Descriptive statistics were used to characterize the population, and bivariate chi square analyses were used to test associations between demographic characteristics, mental health services, and psychopharmacologic treatments by Medicaid subgroup. Three polytomous logistic regression models were used to test the associations between Medicaid subgroup and each dependent variable: number of different classes of psychopharmacologic medications (model 1), psychopharmacologic regimen (model 2), and single versus combined pharmacotherapy and psychotherapy treatment (model 3). Use of medications from different classes was the basis for identifying receipt of none, one, two, or three or more psychopharmacologic medications. Psychopharmacologic regimen was stimulant only, stimulant with at least one other medication, or nonstimulant only. Psychopharmacologic medication only, psychotherapy only, or both was used to define single versus multimodal treatment. No psychopharmacologic treatments (model 1 and 2) and multimodal treatments (model 3) were the reference groups. The independent variable was Medicaid subgroup, as defined by disability (referent), foster care, and low-income. Models adjusted for age, gender, race and ethnicity, psychiatric diagnoses, and provider specialty. To avoid small cell sizes, diagnostic categories were collapsed as externalizing disorder (conduct and oppositional defiant disorders), internalizing disorder (depression and anxiety), severe mental illness (psychoses and bipolar disorder), developmental disorder (mental retardation, autism, developmental delays, and tics), adjustment disorder, and all other disorders.
Variables were added sequentially to the model on the basis of original hypotheses and significant bivariate associations. Model fit was ascertained by using log-likelihood tests. Medicaid subgroup was entered first, followed by demographic variables (age, gender, and race and ethnicity), mental health service use indicators, provider specialty, and co-occurring diagnoses, retaining only variables that improved the model fit. A two-tailed, 5 percent significance level with a Bonferroni correction (p<.002) for multiple comparisons was used. All analyses were performed by using SAS version 8.2.
Results
Demographic and clinical description
As Table 1 shows, the ADHD cohort (N=1,296) differed significantly across Medicaid subgroups in terms of age (p<.001) and race and ethnicity (p=.001). Most youths were five to 14 years old, but the proportion of youths who were younger than ten years was higher in the low-income group (60 percent) than in the foster care (35 percent) or disabled (44 percent) groups. African Americans accounted for a larger proportion of the disabled and foster care groups than of the low-income group. The disabled group contained more Hispanics than the other groups.
As shown in Table 1, youths in the disabled and foster care groups were more likely than youths in the low-income group to have psychiatric diagnoses other than ADHD (p<.001). Fifty-nine percent of youths from the low-income group had a diagnosis of ADHD only, compared with 33 percent and 32 percent of those in the disabled group and the foster care group, respectively. One quarter of youths with disabilities and an equal proportion of youths in foster care also had an externalizing disorder. A larger proportion of youths in foster care had an internalizing disorder compared with the disabled and low-income groups (p<.001).
Mental health service use
As Table 2 shows, youths in the low-income group were significantly less likely than youths in the other groups to see a mental health provider (p<.001). Also, youths in the foster care group were significantly less likely than those in the other groups to have had a visit with a primary care provider (p<.001). Psychopharmacologic management and psychotherapy visits did not differ across groups (Table 2), but school-based services did. Youths in the disabled group were significantly more likely than youths in the other groups to use school-based services (p<.001). Use of substance abuse services also differed between groups (p<.001): youths in foster care (59 percent) were 1.5 to three times as likely as those in the disabled group (36 percent) or the low-income group (19 percent) to receive a substance abuse service (p<.001). Moreover, 37 percent of youths in the low-income group did not have a visit associated with a specific mental health service, compared with 23 percent of youths in the disabled group and 16 percent of youths in the foster care group.
Psychopharmacologic treatment
Approximately 90 percent of youths in each of the three Medicaid subgroups received at least one psychopharmacologic medication in 1998 (Table 2). Stimulants were the most common psychopharmacologic medication prescribed across all groups. Antidepressant use was greater in the disabled (27 percent) and foster care (38 percent) groups than in the low-income group (18 percent). SSRI use in the foster care group (28 percent) was considerably higher than in the disabled (18 percent) and low-income (11 percent) groups. Although antipsychotic use was more common in the disabled (28 percent) and foster care (29 percent) groups than in the low-income group (14 percent), more than 85 percent of antipsychotic use across all groups involved an atypical agent. Mood-stabilizing anticonvulsant use was similar in the disabled (12 percent) and foster care (13 percent) groups, but both groups had higher use than the low-income group (4 percent).
Use of multiple classes of psychopharmacologic medication differed significantly across Medicaid subgroups (p<.001) (Table 2). A larger proportion of the foster care group (46 percent) and disabled group (45 percent) received psychopharmacologic medications from multiple classes compared with the low-income group (24 percent). The low-income group (61 percent) was more likely than the disabled (48 percent) and foster care (38 percent) groups to receive stimulant monotherapy. The disabled (41 percent) and foster care (43 percent) groups were more likely than the low-income group (22 percent) to receive stimulants along with other psychopharmacologic agents. Nonstimulant psychopharmacologic therapy alone was more common in the foster care group than in the other groups.
Odds ratios (ORs) and 95 percent confidence intervals (CIs) were adjusted for age, gender, race and ethnicity, co-occurring psychiatric diagnoses, and provider specialty. Table 3 shows that the odds of receiving more than one psychopharmacologic medication were 60 to 75 percent lower among youths in the low-income group than among youths in the disabled group. The low-income group was one-third as likely as the disabled group to receive stimulants along with other psychopharmacologic agents. Notably, psychopharmacologic treatment did not differ significantly among the disabled and foster care groups.
Use of multimodal treatment
Multimodal treatment differed significantly (p<.001) across groups (Table 2); the adjusted ORs and CIs are presented in Table 3. The only significant difference was that the odds of receiving psychotherapy alone, instead of receiving multimodal treatment, was three times as great in the low-income group as in the disabled group. In general, multimodal treatment was more frequent in the disabled group than in the other groups.
Discussion
This study offers new information about psychopharmacologic and multimodal treatment for ADHD in community-based practice. Compared with youths in the low-income Medicaid eligibility group, youths who were disabled or in foster care had similar rates of mental health services, were more likely to have a co-existing psychiatric disorder, and were more likely to receive more complex psychopharmacologic treatment. The disabled group was significantly more likely than the low-income group to receive multimodal treatment instead of psychotherapy alone, even after the analysis controlled for comorbid psychopathology. Although 33 to 41 percent of youths in all three groups received multimodal treatment, nearly 90 percent received at least one psychopharmacologic medication.
The findings reported here corroborate previous reports of mental health service use by Medicaid-enrolled youths in foster care and disabled eligibility groups (14,15,16,17,51,52). Several researchers have reported higher use and costs for youths in foster care compared with youths in other Medicaid-insured groups (15,16). Studies have also found that services increased for youths who are disabled after policies in child disability qualifications were changed (51). On the basis of southwestern Pennsylvania Medicaid claims data, higher rates of psychiatric diagnoses and mental health service use were reported for youths in foster care compared with youths in the Aid to Families with Dependent Children (AFDC) category, but rates among youths in the foster care group and in the disabled group were similar (17). In a countywide population of Medicaid-enrolled youths in a Mid-Atlantic state, the prevalence of psychiatric diagnoses and psychopharmacologic treatment was higher among those in foster care than among those who were disabled or in another Medicaid category (largely AFDC) (14).
Our study extends our earlier work (14) by including mental health treatments provided during medical visits; however, the findings across Medicaid groups in the current study are different from those reported earlier. This may be due to differences in the definition of mental health services, the criterion of more than one ADHD-related visit, the inclusion of continuously enrolled youths, and variation in state Medicaid programs and provider practices. Even so, the general trends across the low-income, foster care, and disabled groups coincide with those found in earlier studies (14,17).
Variation in multimodal treatment is likely to be related to different mental health needs across the three groups. Youths in foster care use more mental health services given the multiple placements (53) and the abuse and neglect that are associated with psychological and behavioral problems in this population (54,55,56). Furthermore, youths with an identified disability have frequent contact with a health care provider for ongoing management of chronic illnesses (52,57). By comparison, multimodal treatment was less common in the low-income group, which also has less psychological impairment.
Less than half of the ADHD cohort received multimodal treatment; yet recent evidence supports the use of multimodal treatment for ADHD and co-existing psychiatric disorders. About one-third of youths with ADHD have a co-existing psychiatric disorder (32,34). Some of the initial analyses from the MTA study reported the beneficial effects of combined treatments for ADHD and comorbid anxiety disorder, particularly when oppositional or conduct disorder also were present (32,37). Post-hoc analysis of MTA findings that used a single composite outcome instead of the 19 individual measures revealed a statistically significant improvement for the combined treatment group compared with the group that received medication management alone (58). Because evidence of the effectiveness of multimodal treatment in community practice is limited and because certain factors—such as family support and financial resources—may enhance combined treatment, further research is warranted (23).
Because our study is a descriptive analysis of community standards of care for youths with ADHD, the data cannot address the appropriateness or effectiveness of treatment. However, the data do highlight important differences in the complexity of psychopathology across the Medicaid subgroups. The association between psychopharmacologic complexity and co-existing psychiatric disorders has been reported elsewhere. In a managed care population of three- to 17-year-olds in the Pacific Northwest region of the United States, Guevara and colleagues (34) reported that among those with ADHD and an internalizing disorder, 18 percent received only an SSRI and 26 percent received a stimulant plus an SSRI. Using automated medical record data for five- to 12-year-olds in the Kaiser Permanente Northwest Region health plan, Boles and colleagues (59) reported that youths with ADHD and a comorbid mental illness were more likely to receive nonstimulant psychopharmacologic agents, with or without stimulants, and to be given a prescription for two or more psychopharmacologic agents.
Notably, 28 to 29 percent of youths in the disabled and foster care groups received antipsychotics; yet the prevalence of severe mental illness (psychoses or bipolar disorder) for which these medications are used clinically was relatively low. Furthermore, more than 85 percent of antipsychotic use involved atypical agents, which suggests that their use is primarily for the management of aggressive behavior (60,61). Given the associated risk of weight gain and of diabetes with the atypical antipsychotics (62,63), our findings emphasize the need for systematic side effect monitoring. This issue is worthy of further study in a larger sample and with more detailed information on the indication, dosing, and duration of treatment.
It is important to consider several limitations in light of the study findings. First, these data were derived from cross-sectional data and do not speak to the continuity of care or individual trajectories. Nonetheless, this work identified areas for future longitudinal studies on the patterns and use of multimodal treatment in community practice settings. Second, although this study is specific to one state Medicaid program and may not be representative of other states, corroboration of previously reported findings is encouraging. Third, these data are from reimbursement claims for conditions that prompted a medical encounter and may underestimate the prevalence of chronic mental disorders in the community, particularly if professional help for the chronic condition was not sought (64). However, our study examined mental health service use for a one-year period by continuously enrolled individuals, which should be adequate time to detect the extent of mental illness in this population. Finally, these data do not include services rendered in the specialty mental health carve-out plan when the maximum 30 unit-hours of mental health care allowed in the fee-for-service and managed care capitated system were exceeded. Although the intensity of mental health service use may be underestimated, the data accurately represent youths who received psychopharmacologic and psychotherapy interventions for ADHD in the community.
Conclusions
The findings of this study can be usefully applied to future community-based child mental health services research, particularly in examinations of possible differences in use among a privately insured population. Previous studies reported more stimulant monotherapy treatment (12,59,65) and fewer mental health, psychotherapy (65,66), and multimodal treatments (10) in primary care compared with community mental health practices. According to MTA findings, community-based physicians prescribed lower stimulant dosages and their patients had fewer follow-up visits than physicians participating in the other active treatment arms (67). A patient-oriented and need-driven model has been proposed to improve ADHD management (68). Future studies should explore effective dissemination of evidence-based treatments in community settings.
Acknowledgments
This work was possible with the support of David Michalik, Paula Hibbert, and Nancy Widdoes. Paul Lipkin, M.D., and Daniel Safer, M.D., provided a thoughtful review.
Dr. dosReis is affiliated with the division of child and adolescent psychiatry at Johns Hopkins Medicine, Johns Hopkins Hospital, CMSC 346, 600 North Wolfe Street, Baltimore, Maryland 21287 (e-mail, [email protected]). Ms. Puccia was a research assistant in the division of child and adolescent psychiatry at Johns Hopkins Medicine in Baltimore at the time of the study. Dr. dosReis, Dr. Owens, and Dr. Leaf are with the Bloomberg School of Public Health at Johns Hopkins University in Baltimore. Dr. Owens is currently with the Agency for Healthcare Research and Quality in Rockville, Maryland. At the initiation of this study she was with Johns Hopkins Bloomberg School of Public Health in Baltimore. These data were presented at the annual meeting of the American Academy of Child and Adolescent Psychiatry, held October 14 to 19, 2003, in Miami.
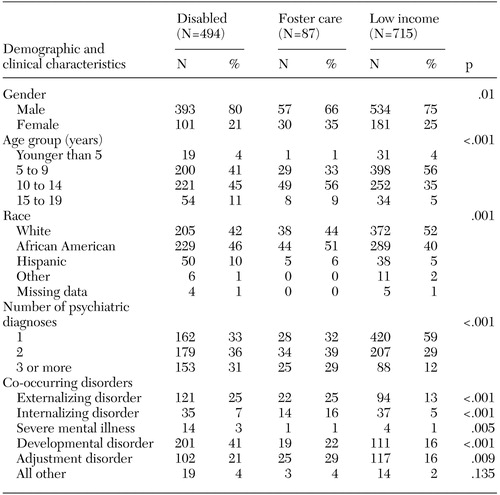 |
Table 1. Demographic and clinical characteristics of youths with attention-deficit hyperactivity disorder by Medicaid subgroup (N=1,296)
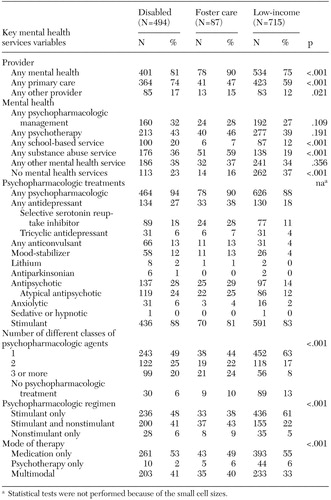 |
Table 2. Medicaid subgroup comparisons of mental health services for youths with attention-deficit hyperactivity disorder (N=1,296)
 |
Table 3. Odds ratio comparisons of psychopharmacologic treatment regimens of youths with attention-deficit hyperactivity disorder, by Medicaid subgroup (N=1,296)
1. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC, American Psychiatric Association, 1994Google Scholar
2. Mental Health: A Report of the Surgeon General. Rockville, Md, Center for Mental Health Services, National Institute of Mental Health, 1999Google Scholar
3. Rowland AS, Umbach DM, Catoe KE, et al: Studying the epidemiology of attention-deficit hyperactivity disorder: screening method and pilot results. Canadian Journal of Psychiatry 46:931–940, 2001Crossref, Medline, Google Scholar
4. Wolraich ML, Hannah JN, Pinnock TY, et al: Comparison of diagnostic criteria for attention-deficit hyperactivity disorder in a county-wide sample. Journal of the American Academy of Child and Adolescent Psychiatry 35:319–324, 1996Crossref, Medline, Google Scholar
5. Kelleher KJ, McInerny TK, Gardner WP, et al: Increasing identification of psychosocial problems:1979–1996. Pediatrics 105:1313–1321, 2000Google Scholar
6. Zito JM, Safer DJ, dosReis S, et al: Psychotropic practice patterns for youth: a 10-year perspective. Archives of Pediatrics and Adolescent Medicine 157:17–25, 2003Crossref, Medline, Google Scholar
7. Zito JM, Safer DJ, dosReis S, et al: Trends in the prescribing of psychotropic medications to preschoolers. JAMA 283:1025–1030, 2000Crossref, Medline, Google Scholar
8. Olfson M, Gameroff MJ, Marcus SC, et al: National trends in the treatment of attention deficit hyperactivity disorder. American Journal of Psychiatry 160:1071–1077, 2003Link, Google Scholar
9. Zarin DA, Tanielian TL, Suarez AP, et al: Treatment of attention-deficit hyperactivity disorder by different physician specialties. Psychiatric Services 49:171, 1998Link, Google Scholar
10. Bussing R, Zima BT, Belin TR: Variations in ADHD treatment among special education students. Journal of the American Academy of Child and Adolescent Psychiatry 37:968–976, 1998Crossref, Medline, Google Scholar
11. Zarin DA, Suarez AP, Pincus HA, et al: Clinical and treatment characteristics of children with attention-deficit/hyperactivity disorder in psychiatric practice. Journal of the American Academy of Child and Adolescent Psychiatry 37:1262–1270, 1998Crossref, Medline, Google Scholar
12. Zito JM, Safer DJ, dosReis S, et al: Psychotherapeutic medication patterns for youths with attention deficit/hyperactivity disorder. Archives of Pediatrics and Adolescent Medicine 153:1257–1263, 1999Crossref, Medline, Google Scholar
13. Halfon N, Berkowitz G, Klee L: Children in foster care in California: an examination of Medicaid reimbursed health services utilization. Pediatrics 89:1230–1237, 1992Medline, Google Scholar
14. DosReis S, Zito JM, Safer DJ, et al: Mental health services for youths in foster care and disabled youths. American Journal of Public Health 91:1094–1099, 2001Crossref, Medline, Google Scholar
15. Halfon N, Berkowitz G, Klee L: Mental health service utilization by children in foster care in California. Pediatrics 89:1238–1244, 1992Medline, Google Scholar
16. Takayama JI, Bergman AB, Connell FA: Children in foster care in the state of Washington. JAMA 271:1850–1855, 1994Crossref, Medline, Google Scholar
17. Harman JS, Childs GE, Kelleher KJ: Mental health care utilization and expenditures by children in foster care. Archives of Pediatrics and Adolescent Medicine 154:1114–1117, 2000Crossref, Medline, Google Scholar
18. Dulcan M: Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder: American Academy of Child and Adolescent Psychiatry. Journal of the American Academy of Child and Adolescent Psychiatry 36(suppl 10):85S-121S, 1997Google Scholar
19. Weisz JR, Jensen PS: Efficacy and effectiveness of child and adolescent psychotherapy and pharmacotherapy. Mental Health Services Research 1:125–157, 1999Crossref, Medline, Google Scholar
20. Greenhill LL, Pliszka S, Dulcan MK, et al: Practice parameters for the use of stimulant medications in the treatment of children, adolescents, and adults. Journal of the American Academy of Child and Adolescent Psychiatry 41(suppl 2):26S-49S, 2002Google Scholar
21. Safer DJ, Zito JM, Gardner JF: Pemoline hepatotoxicity and post-marketing surveillance. Journal of the American Academy of Child and Adolescent Psychiatry 40:622–629, 2001Crossref, Medline, Google Scholar
22. Burns BJ, Hoagwood K, Mrazek PJ: Effective treatment for mental disorders in children and adolescents. Clinical Child and Family Psychology Review 2:199–254, 1999Crossref, Medline, Google Scholar
23. Pelham WE Jr, Gnagy EM, Greiner AR, et al: Behavioral versus behavioral and pharmacological treatment in ADHD children attending a summer treatment program. Journal of Abnormal Child Psychology 28:507–525, 2000Crossref, Medline, Google Scholar
24. Barkley RA: Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York, Guilford, 1998Google Scholar
25. Bussing R, Zima BT, Perwien AR: Self-esteem in special education children with ADHD: relationship to disorder characteristics and medication use. Journal of the American Academy of Child and Adolescent Psychiatry 39:1260–1269, 2000Crossref, Medline, Google Scholar
26. Frankel F, Cantwell DP, Myatt R, et al: Do stimulants improve self-esteem in children with ADHD and peer problems? Journal of Child and Adolescent Psychopharmacology 9:185–194, 1999Google Scholar
27. Kelly PC, Cohen ML, Walker WO, et al: Self-esteem in children medically managed for attention deficit disorder. Pediatrics 83:211–217, 1989Medline, Google Scholar
28. DosReis S, Zito JM, Safer DJ, et al: Parental perceptions and satisfaction with stimulant medication for ADHD. Journal of Developmental and Behavioral Pediatrics 24:155–161, 2003Crossref, Medline, Google Scholar
29. Bagwell CL, Molina BSG, Pelham WE Jr, et al: Attention-deficit hyperactivity disorder and problems in peer relations: predictions from childhood to adolescence. Journal of the American Academy of Child and Adolescent Psychiatry 40:1285–1292, 2001Crossref, Medline, Google Scholar
30. Klassen A, Miller A, Raina P, et al: Attention-deficit hyperactivity disorder in children and youth: a quantitative systematic review of the efficacy of different management strategies. Canadian Journal of Psychiatry 44:1007–1016, 1999Crossref, Medline, Google Scholar
31. Cunningham CE: In the wake of the MTA: charting a new course for the study and treatment of children with attention-deficit hyperactivity disorder. Canadian Journal of Psychiatry 44:999–1006, 1999Crossref, Medline, Google Scholar
32. Jensen PS, Hinshaw SP, Kraemer HC, et al: ADHD comorbidity findings from the MTA Study: comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry 40:147–158, 2001Crossref, Medline, Google Scholar
33. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder: the MTA Cooperative Group: Multimodal Treatment Study of Children With ADHD. Archives of General Psychiatry 56:1073–1086, 1999Crossref, Medline, Google Scholar
34. Guevara J, Lozano P, Wickizer T, et al: Psychotropic medication use in a population of children who have attention-deficit/hyperactivity disorder. Pediatrics 109:733–739, 2002Crossref, Medline, Google Scholar
35. Costello EJ, Mustillo S, Erkanli A, et al: Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry 60:837–844, 2003Crossref, Medline, Google Scholar
36. Connor DF, Edwards G, Fletcher KE, et al: Correlates of comorbid psychopathology in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 42:193–200, 2003Crossref, Medline, Google Scholar
37. Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder: the Multimodal Treatment Study of children With Attention-deficit/hyperactivity disorder. Archives of General Psychiatry 56:1088–1096, 1999Crossref, Medline, Google Scholar
38. Cox ER, Motheral BR, Henderson RR, et al: Geographic variation in the prevalence of stimulant medication use among children 5 to 14 years old: results from a commercially insured US sample. Pediatrics 111:237–243, 2003Crossref, Medline, Google Scholar
39. LeFever GB, Dawson KV, Morrow AL: The extent of drug therapy for attention-deficit-hyperactivity disorder among children in public schools. American Journal of Public Health 89:1359–1364, 1999Crossref, Medline, Google Scholar
40. LeFever GB, Hannon PH, Dawson KV, et al: Prevalence of medication use for attention deficit hyperactivity disorder (ADHD): a population-based study of Virginia school children. Pediatric Research 41:14a, 1997Crossref, Google Scholar
41. Rappley MD, Eneli IU, Mullan PB, et al: Patterns of psychotropic medication use in very young children with attention-deficit hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics 23:23–30, 2002Crossref, Medline, Google Scholar
42. Zito JM, Safer DJ, dosReis S, et al: Methylphenidate patterns among Medicaid youths. Psychopharmacology Bulletin 33:143–147, 1997Medline, Google Scholar
43. Chan E, Zhan C, Homer CJ: Health care use and costs for children with attention-deficit/hyperactivity disorder: national estimates from the medical expenditure panel survey. Archives of Pediatrics and Adolescent Medicine 156:504–511, 2002Crossref, Medline, Google Scholar
44. Guevara J, Lozano P, Wickizer T, et al: Utilization and cost of health care services for children with attention-deficit/hyperactivity disorder. Pediatrics 108:71–78, 2001Crossref, Medline, Google Scholar
45. Lessler JT, Harris BSH: Medicaid as a Source for Postmarketing Surveillance Information, Final Report. Research Triangle Park, NC, Research Triangle Institute, 1984Google Scholar
46. Walkup JT, Boyer CA, Kellermann SL: Reliability of Medicaid claims files for use in psychiatric diagnoses and service delivery. Administration and Policy in Mental Health 27:129–139, 2000Crossref, Medline, Google Scholar
47. Lurie N, Popkin M, Dysken M, et al: Accuracy of diagnoses of schizophrenia in Medicaid claims. Hospital and Community Psychiatry 43:69–71, 1992Abstract, Google Scholar
48. Steinwachs DM, Stuart ME, Scholle S, et al: A comparison of ambulatory Medicaid claims to medical records: a reliability assessment. American Journal of Medical Quality 13:63–69, 1998Crossref, Medline, Google Scholar
49. Strom BL: Pharmacoepidemiology. New York, John Wiley and Sons, Inc, 1994Google Scholar
50. Rushton JL, Clark SJ, Freed GL: Pediatrician and family physician prescription of selective serotonin reuptake inhibitors. Pediatrics 105:E82, 2000Google Scholar
51. Perrin JM, Kuhlthau K, McLaughlin TJ, et al: Changing patterns of conditions among children receiving supplemental security income disability benefits. Archives of Pediatrics and Adolescent Medicine 153:80–84, 1999Crossref, Medline, Google Scholar
52. Kuhlthau K, Ferris TG, Beal AC, et al: Who cares for Medicaid-enrolled children with chronic conditions? Pediatrics 108:906–912, 2001Google Scholar
53. Rubin DM, Allessandrini EA, Feudtner C, et al: Placement stability and mental health costs for children in foster care. Pediatrics 113:1336–1341, 2004Crossref, Medline, Google Scholar
54. Hochstadt NJ, Jaudes PK, Zimo DA, et al: The medical and psychosocial needs of children entering foster care. Child Abuse and Neglect 11:53–62, 1987Crossref, Medline, Google Scholar
55. Frank G: Treatment needs of children in foster care. American Journal of Orthopsychiatry 50:256–263, 1980Crossref, Medline, Google Scholar
56. Takayama JI, Wolfe E, Coulter KP: Relationship between reason for placement and medical findings among children in foster care. Pediatrics 101:201–207, 1998Crossref, Medline, Google Scholar
57. Kuhlthau K, Perrin JM, Ettner SL, et al: High-expenditure children with supplemental security income. Pediatrics 102:610–615, 1998Crossref, Medline, Google Scholar
58. Conners CK, Epstein JN, March JS, et al: Multimodal treatment of ADHD in the MTA: an alternative outcome analysis. Journal of the American Academy of Child and Adolescent Psychiatry 40:159–167, 2001Crossref, Medline, Google Scholar
59. Boles M, Lynch FL, DeBar LL: Variations in pharmacotherapy for attention deficit hyperactivity disorder in managed care. Journal of Child and Adolescent Psychopharmacology 11:43–52, 2001Crossref, Medline, Google Scholar
60. Buckley PF: The role of typical and atypical antipsychotic medications in the management of agitation and aggression. Journal of Clinical Psychiatry 60:52–60, 1999Medline, Google Scholar
61. Fava M: Psychopharmacologic treatment of pathologic aggression. Psychiatric Clinics of North America 20:427–451, 1997Crossref, Medline, Google Scholar
62. Allison DB, Mentore JL, Heo M, et al: Antipsychotic-induced weight gain: a comprehensive research synthesis. American Journal of Psychiatry 156:1686–1696, 1999Abstract, Google Scholar
63. Lund BC, Perry PJ, Brooks JM, et al: Clozapine use in patients with schizophrenia and the risk of diabetes, hyperlipidemia, and hypertension. Archives of General Psychiatry 58:1172–1176, 2001Crossref, Medline, Google Scholar
64. Bright RA, Avorn J, Everitt DE: Medicaid data as a resource for epidemiologic studies: strengths and limitations. Journal of Clinical Epidemiology 42:937–945, 1989Crossref, Medline, Google Scholar
65. Hoagwood K, Kelleher KJ, Feil M, et al: Treatment services for children with ADHD: a national perspective. Journal of the American Academy of Child and Adolescent Psychiatry 39:198–206, 2000Crossref, Medline, Google Scholar
66. Parr JR, Ward A, Inman S: Current practice in the management of attention deficit disorder with hyperactivity (ADHD). Child: Care, Health, and Development 29:215–218, 2003Crossref, Medline, Google Scholar
67. Jensen PS, Hinshaw SP, Swanson JM, et al: Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): implications and applications for primary care providers. Journal of Developmental and Behavioral Pediatrics 22:60–73, 2001Crossref, Medline, Google Scholar
68. Hazelwood E, Bovingdon T, Tiemens K: The meaning of a multimodal approach for children with ADHD: experiences of service professionals. Child: Care, Health, and Development 28:301–307, 2002Crossref, Medline, Google Scholar



