Psychopharmacology: Atypical Antipsychotic Dosing: The Effect of Co-medication With Anticonvulsants
The dosing recommendations for atypical antipsychotics that are provided in the package inserts are derived from double-blind studies—in which clinician researchers are generally forbidden from prescribing additional medications—and are based on the dose-response of the "average subject." These recommendations may be appropriate for the "average subject" who is taking only the atypical antipsychotic but not for patients in monotherapy who are not "average" because of genetic reasons—they may lack or have too much of the main metabolic enzyme—or for those who are taking several other psychiatric or medical medications.
Very little information has been published describing the relationship between atypical antipsychotic plasma concentrations and dosages. However, it is very likely that most or all atypical antipsychotics have a linear relationship between typical dosages and concentrations (first-order kinetics), particularly for the same patient. Pharmacologists use an easy formula, the concentration-dose ratio (C/D), to represent this relationship. According to the limited data that has been published from one of the risperidone multicenter studies (1), the average total risperidone plasma concentration, which was calculated by adding risperidone and 9-hydroxyrisperidone concentrations, for patients who were taking 2 mg a day was 14 ng per ml (C/D=7.0). For patients taking 6 mg a day, it was 43 ng per ml (C/D=7.2); for those taking 10 mg a day, it was 73 ng per ml (C/D=7.3); and for those taking 16 mg a day, it was 111 ng per ml (C/D=6.9). Therefore, the average C/D for risperidone is approximately 7.
A change in the C/D by a factor of 2 is probably meaningful from the clinician's point of view. For example, for a patient taking 6 mg of risperidone, one would expect a total risperidone concentration of approximately 42 ng per ml. If this patient's blood level of risperidone is higher than 84 ng per ml, one should suspect that a genetic or environmental factor—or both—is causing the patient to metabolize risperidone two times more slowly than the average patient. The level of 84 ng per ml is equivalent to the blood level of an average patient taking 12 mg of risperidone daily. Similarly, if the patient takes 6 mg a day and a blood level of 21 ng per ml is found, one should suspect that either the patient is not taking the medication as prescribed or that some genetic or environmental factor—or both—is causing the patient to metabolize risperidone two times faster than the average patient. The level of 21 ng per ml is equivalent to the blood level of an average patient taking 3 mg of risperidone daily.
A similar argument can be made for clozapine. It is believed that plasma clozapine concentrations exceeding 350 ng per ml are therapeutic and that most patients require 300 to 600 mg a day to reach these levels. If it is assumed that a patient needs a dosage of 300 mg a day to reach 350 ng per ml, then the C/D would be 1.2. Conversely, if a patient needs a dosage of 600 mg a day to reach 350 ng per ml, the C/D would be .6. Therefore, the average patient taking clozapine has a C/D of .6 to 1.2. Patients who need high clozapine dosages to reach therapeutic concentrations have a low C/D. For example, in one published case, a patient who was thought to have a high metabolic capacity to destroy clozapine had a C/D of less than .17.
One situation that may change a C/D for a patient is the presence of inducers. Four "old" anticonvulsants —phenobarbital, primidone, phenytoin, and carbamazepine—are powerful inducers of the cytochrome P450 (CYP) enzymes (2) and of the less well-understood UDP-glucuronosyltransferases (UGTs) (3). Phenytoin and carbamazepine may be frequently co-prescribed with antipsychotics and are powerful inducers of CYP3A and of several UGTs, which may have clinical implications for patients taking atypical antipsychotics. CYP2D6 cannot be induced. Phenytoin and carbamazepine induce CYP2C isoenzymes (2); however, these enzymes have limited roles in the metabolism of atypical antipsychotics, as shown in Table 1. It is unclear if CYP1A2 can be induced. Moreover, CYP1A2 and its relationship to olanzapine is an example of our limited understanding in this area. Initially, the pharmaceutical company marketing olanzapine suggested that the effects of carbamazepine on olanzapine metabolism could be explained by the induction of CYP1A2 by carbamazepine (3). More recent studies suggest that carbamazepine may increase olanzapine metabolism by inducing UGTs (3).
Other, newer anticonvulsants are less powerful inducers (2). Lamotrigine is probably a weak UGT inducer (it induces its own metabolism). Topiramate may also have mildly inductive CYP3A properties—and it inhibits CYP2C19. Oxcarbazepine probably mildly induces CYP3A and UGTs. Therefore, it is unlikely that topiramate, lamotrigine, or oxcarbazepine drug interactions have major important clinical consequences, except in rare cases. Valproic acid definitely is not an inducer; moreover, it inhibits UGTs and some CYPs, particularly CYP2C9, which is not important for antipsychotic metabolism. Gabapentin, levetiracetam, and tiagabine are not metabolic inducers (2).
When interpreting drug interactions associated with metabolic induction, clinicians must remember that the chronology of inhibition and induction is different. Inhibition reaches maximum effect when the inhibitor has reached steady state. However, inducers usually take several weeks—up to three weeks for carbamazepine and up to two weeks for phenytoin—to get maximum effects because they require new enzyme synthesis for their effects (2). Similarly, inducer effects may take a few weeks to disappear.
A literature review of the interactions between atypical antipsychotics and anticonvulsant inducers provides limited information. Unfortunately, the presence or lack of information in the literature is not a random event but is significantly influenced by the openness of the drug companies and by requirements of the Food and Drug Administration (FDA). In the past five years, after several drugs were withdrawn from the market because of toxic drug interactions, the FDA has been asking companies to conduct pharmacokinetic studies before marketing new drugs. More recently marketed drugs, such as olanzapine, quetiapine, ziprasidone, and aripiprazole, have been subjected to well-controlled pharmacokinetic studies using inducers. Obviously, these studies may be more controlled than case reports published by clinicians that describe naturalistic studies of plasma levels, but these controlled pharmacokinetic studies may have been too short to reach maximum inductive effects (Table 1). Until clinicians and researchers begin publishing their experiences with drug levels after co-prescription with anticonvulsant inducers, it will be difficult to establish whether these well-controlled but short studies of the newer atypical antipsychotics reflect the real world well.
Clozapine and risperidone were marketed before these studies were required. Therefore, one needs to use the available information published by clinicians and researchers. When trying to extrapolate from the literature to help real-world clinicians calculate dosages of atypical antipsychotics when a patient is taking a medication that is an inducer, well-thought-out published longitudinal case reports may be crucial as long as they apply an on-off design and require repeated measures of blood levels over several months. In an on-off design, the anticonvulsant is not present (off) and is added (on), or the anticonvulsant is present (on) and discontinued (off). This design controls by individual variability as long as other factors, such as co-prescribed medications, are stable. It is important to note that some on-off studies that explored carbamazepine's inductive properties used oxcarbazepine as the off phase. Because oxcarbazepine may have mild inductive properties, the estimations calculated from those studies underestimate the inductive effects of carbamazepine (Table 1).
More formally trained researchers sometimes publish parallel retrospective and cross-sectional designs comparing one or a few samples of blood levels in two patient groups taking atypical antipsychotics, one "with" inducers and another "without" inducers (with-without studies). The problem with this type of more sophisticated design is that patients "with" and "without" need to be appropriately matched for other factors influencing metabolic activity. Such matching may be difficult; for most CYPs, we cannot control for genetic differences.
Table 1 provides an average correction factor that can be used in two ways. If a patient is taking one of the anticonvulsant inducers and an atypical antipsychotic, discontinuing the anticonvulsant would probably cause in the average patient an increase in the blood level by the correction factor. For example, the correction factor for carbamazepine for the average patient taking aripiprazole is 2. Assuming an average patient is stable on 30 mg a day of aripiprazole and carbamazepine, discontinuing carbamazepine would be associated with slowly increasing aripiprazole levels, equivalent to multiplying the dosage by a factor of 2, or prescribing 60 mg a day. The opposite situation may occur. If an average patient is stable on 15 mg a day of aripiprazole, adding carbamazepine would be associated with slowly decreasing aripiprazole levels, equivalent to dividing the dosage by a factor of 2, or prescribing only 7.5 mg a day. For some atypical antipsychotics, drug interaction data are available only for phenytoin or carbamazepine (Table 1). In those cases, one can simply assume that the inductive effects of phenytoin and carbamazepine are roughly similar.
The risk of adding and discontinuing anticonvulsant inducers is related to the therapeutic window of the atypical antipsychotics. Obviously, clozapine has the narrowest difference between appropriate dosages and therapeutic blood levels on the one hand and toxic dosages and toxic blood levels on the other. Several of clozapine's side effects are dose related; therefore, it may be safer to use clozapine levels to orient medication changes for clozapine patients who are taking multiple interacting medications. It is important to remember that levels higher than 1,000 ng per ml have been associated with toxicity and risk of seizures. Risperidone-induced extrapyramidal side effects are also dose related, particularly in dosages of 6 mg a day and higher. For other atypical antipsychotics, the addition of anticonvulsants and the need to use high dosages may be more of a cost issue than a toxicity issue. It appears that extraordinarily high dosages of quetiapine may be required when adding phenytoin (Table 1), with unexpected high costs.
In reviewing Table 1, the reader should remember the difference between large samples and individuals. When our research group reviewed the use of high dosages of typical antipsychotics in two state hospitals, anticonvulsant inducers were not significant in a statistical model that attempted to predict high dosages. Our inability to prove the effects of inducers in large samples of patients does not rule out that drug interactions may have major clinical importance in a specific case; the literature is full of cases in which the addition or discontinuation of anticonvulsants may have clinical implications. Moreover, several cases of drug interaction with atypical antipsychotics that have clinical repercussions have been reported in clinical practice. A patient who is taking an atypical antipsychotic may be declared a nonresponder if he or she is taking an anticonvulsant inducer and the dosage is not adjusted. A patient who is responding to an atypical antipsychotic may become a nonresponder when an anticonvulsant inducer is added. A patient who is taking an atypical antipsychotic and an anticonvulsant inducer with no side effects may develop side effects after the anticonvulsant is discontinued.
Valproic acid is probably free of major interactions with atypical antipsychotics, although some minor effects in clozapine levels may be possible. Thus no correction factor is recommended when valproic acid is co-prescribed with atypical antipsychotics. (A table presenting details of the relevant studies of co-prescription of valproic acid and atypical antipsychotics is available from the author.)
In summary, if prescribing psychiatrists select carbamazepine or phenytoin instead of alternative anticonvulsants, they need to be aware that they may be decreasing blood levels of atypical antipsychotics. Treatment with gabapentin, levetiracetam, and tiagabine probably does not require correction of atypical antipsychotic dosages. Topiramate and lamotrigine probably have no major effects on the blood levels of atypical antipsychotics, but better lamotrigine studies may be needed to rule out clinically significant drug interactions, particularly with olanzapine and clozapine that are metabolized by UGT. Oxcarbazepine probably has less clinically significant effects than carbamazepine because it is a milder inducer. In some cases oxcarbazepine may cause modest increases in the metabolism of atypical antipsychotics, which would require modest increases in dosages. Finally, the limited published information on valproic acid, a metabolic inhibitor, suggests that no changes in atypical antipsychotic dosing should be recommended after addition or discontinuation of valproic acid. Larger studies are needed.
Dr. de Leon is affiliated with the University of Kentucky Mental Health Research Center at Eastern State Hospital, 627 West Fourth Street, Lexington, Kentucky 40508 (e-mail, [email protected]). George M. Simpson, M.D., is editor of this column.
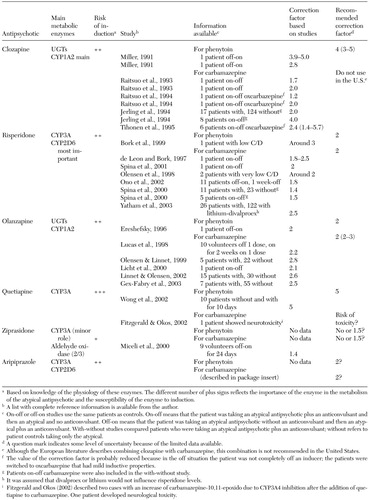 |
Table 1. Recommended correction factors for the co-prescription of anticonvulsant inducers with atypical antipsychotics and supporting data
1. Anderson CB, True JE, Ereshefsky L, et al: Risperidone dose, plasma levels, and response. American Psychiatric Association annual meeting, New Research Program and Abstracts NR 217, San Francisco, May 22–27, 1993Google Scholar
2. Anderson GD: A mechanistic approach to antiepileptic drug interactions. Annals of Pharmacotherapy 32:554–563, 1998Crossref, Medline, Google Scholar
3. De Leon J: Glucuronidation enzyme genes and psychiatry. International Journal of Neuropsychopharmacology 6:57–72, 2003Crossref, Medline, Google Scholar



