Integration of Care: Integrated Treatment of Schizophrenia
Abstract
The importance of combining psychosocial and pharmacologic interventions for optimum outcome in the treatment of schizophrenia is now well recognized. However, less research is available on ways in which the two types of treatment may affect each other. This article reviews research and principles applying to the integrated use of current psychosocial and pharmacologic treatments for schizophrenia. Emphasis is placed on areas of interaction, including patient adherence and the effect of antipsychotic medications on cognitive functioning. Recommendations are presented for specific clinical situations.
Persons with schizophrenia may experience a variety of symptoms across multiple functional domains. These symptoms can include problems with reality testing, such as delusions and hallucinations; disorganized speech or behavior; deficits in cognitive and social functioning; and abnormalities of affect (1). The direct and indirect costs of schizophrenia are immense, estimated at $32.5 billion in 1990 in the United States alone (2). These figures do not include the considerable additional burden associated with the high rate of comorbidity of schizophrenia with general medical conditions and substance use disorders (3).
Before the introduction of chlorpromazine in the 1950s, the care of persons with schizophrenia was limited to various forms of psychotherapeutic, rehabilitative, and custodial interventions. The advent of relatively safe and effective pharmacologic treatments allowed marked improvement in symptoms and functioning to the extent that it became feasible for the vast majority of persons with schizophrenia to live in community settings rather than in residential facilities (4).
However, it soon became clear that medications alone were not sufficient for the treatment of most people with schizophrenia. Studies showed that 14 to 40 percent of patients who were being treated with adequate dosages of medication experienced relapses within a one-year period (5), and many patients continued to have significant impairment in social and cognitive functioning even when florid psychotic symptoms were no longer present (6). The impact of environmental factors on the course of the illness was supported by identification of family dynamics that correlated with increased rates of relapse (7) and by the finding in the World Health Organization's studies of schizophrenia of better outcomes among persons living in less industrially developed societies (8). In addition, concerns arose about the side effects associated with antipsychotic medications.
Such factors have helped contribute to a resurgence in psychosocial interventions for schizophrenia over the past three decades (9). It is no longer questioned whether medication is a necessary part of the management of schizophrenia. Instead, research has focused on how psychosocial interventions can improve outcomes in the context of ongoing pharmacologic treatment.
In this review, we do not attempt to cover the entire field of psychosocial and pharmacologic treatment, which has already been reviewed elsewhere (3,10,11). Instead, we focus on the integration of approaches for the treatment of schizophrenia.
The importance of integrated treatment
The need for integrated treatment is supported by current theories of the pathophysiology of schizophrenia, which are summarized in Figure 1. The widely held stress-diathesis model (11) proposes that symptoms arise from a combination of internal and external factors. Persons who are at risk of developing schizophrenia carry an underlying biologic vulnerability, possibly related to genetic factors or early insult, such as prenatal infections or obstetric complications. Symptoms arise from the interaction of this vulnerability with environmental influences, such as situational stressors and substance abuse. The fact that the onset of more severe psychopathology usually occurs during adolescence implies that maturational changes may also facilitate the development of symptoms.
The course is most often chronic, characterized by intermittent relapses of acute psychotic symptoms in a context of ongoing functional impairment of widely varying severity (12). Research associating long-term outcomes with duration of untreated psychosis or number of relapses has raised interest in the possibility that early intervention—perhaps even in the prodromal phase—may be a way of improving long-term outcomes. It has also discouraged the use of targeted or intermittent treatment, because concern has grown about potential long-term adverse effects associated with relapses.
The impact of multiple factors on symptoms also opens the door to multiple ways of positively affecting the course of the illness. Underlying biologic vulnerabilities related to abnormalities in the function of neurotransmitters such as dopamine can be addressed with antipsychotic medications. Situational stressors that exacerbate symptoms can be lessened through environmental supports such as case management and family interventions. Individual and group therapy can also help people with schizophrenia to better understand and manage their own patterns of stress and response. Substance abuse can be treated through dual diagnosis programs. Different types of rehabilitative interventions, such as social skills training, vocational rehabilitation, and cognitive remediation, can be targeted at each patient's individual needs.
The deinstitutionalization of persons with schizophrenia has made it necessary to develop new ways of providing mental health care that can accommodate the increased complexity of community-based treatment. Institutions inherently provided a form of integrated treatment, albeit of varying quality and at a high cost to individual freedom. Community-based treatment requires a more explicit determination of the key components of integrated treatment and of how to make these components accessible to patients and families in a way that is flexible enough to accommodate their varying needs. Evidence-based practice has arisen as a way of incorporating research findings into clinical practice. However, a significant difficulty in using evidence-based practice for guiding integrated treatment of schizophrenia is the limited number of studies that used appropriate methodology, including measurement of dosages of both pharmacologic and psychosocial aspects of treatment.
Studies of integrated pharmacologic and psychosocial treatments
Most research on the treatment of schizophrenia has focused on either pharmacologic intervention or psychosocial intervention. Other potential concurrent treatments, such as use of medication among patients participating in a trial of a psychosocial intervention, have been typically designated as "standard treatment" and not systematically controlled. Comparatively little research has been done on integrated treatment as such. Studies that simultaneously control for multiple aspects of treatment can be difficult to implement but have the potential to provide information that is not otherwise available. Such information can include the clinical relevance of possible interactions of different types of treatment on different symptoms or functional domains.
The first efforts to examine both pharmacologic and psychosocial treatment were early studies that compared the two types of treatment and established the preeminent role of antipsychotic medications in the treatment of positive symptoms. May (13) placed inpatients with moderately severe schizophrenia into five treatment groups: milieu therapy, insight-oriented individual psychotherapy, antipsychotic medication, electroconvulsive therapy, and a combination of antipsychotic medication and individual psychotherapy. Both of the groups that received medication had significantly better outcomes, followed by the group that received electroconvulsive therapy. The group that received combination treatment did not do better than the group that received medication alone.
A collaborative National Institute of Mental Health (NIMH) study that compared individual supportive psychotherapy with a control condition among patients who were receiving chlorpromazine or placebo had a different result. Although medication was still necessary for a positive outcome, the incorporation of the psychotherapeutic intervention had a positive effect, indicating that the approaches could be additive (14). This finding was supported by a later study in which patients who received the depot form of fluphenazine had a better response to psychotherapy than those who received the less reliably delivered oral form of the same medication (15). These studies supported the combination of supportive rather than insight-oriented therapy with pharmacologic treatment.
Brown and Rutter (16) developed the concept of expressed emotion in an attempt to understand why some patients who were stable in the hospital rapidly relapsed after returning home. They described a family's level of expressed emotion according to an empirically derived index of three factors, including the frequency of critical comments, hostility, and "emotional overinvolvement." The recognition from studies of expressed emotion that the family environment could have an impact on relapse rates led investigators to question whether patients could be successfully treated with lower dosages of medication if family stressors were also addressed. This question had added urgency because of the significant dose-related neurologic side effects of the typical antipsychotics available at the time.
Goldstein and colleagues (17) examined the effect of a six-week crisis-oriented family intervention on relapse rates among recently discharged patients receiving either standard-dosage (25 mg) or low-dosage (6.25 mg) fluphenazine decanoate every two weeks. They found that relapse rates at the end of the six-week family intervention period ranged from zero among patients who received both standard-dosage fluphenazine and the family intervention to 48 percent among patients who received only the low-dosage fluphenazine. At six weeks both groups showed a beneficial effect from the addition of psychotherapy. However, at six months this effect had persisted only among the patients who received the higher dosage of fluphenazine, indicating that the effect of the family intervention was not sustained in the absence of adequate medication.
Hogarty and colleagues (18) examined the relationship of rates of relapse to household levels of expressed emotion among patients who were treated with a standard dosage (25 mg every two weeks) or a minimal dosage (20 percent of the standard dosage) of depot fluphenazine. They found that patients who were receiving the lower dosage were more likely to experience minor relapses in environments with high levels of expressed emotions but that at the end of the two-year study period the lower-dosage group showed more improvement in measures of social adjustment. This finding supported the hypothesis that lower medication dosages could be sufficient for patients living in households with lower levels of expressed emotions and that minimizing dosages might be advantageous for other areas of functioning.
A study by Marder and colleagues (19) assessed the relative impacts of social skills training (problem-solving model) and supportive therapy over a two-year period among patients who received a low dosage of fluphenazine decanoate (5 to 10 mg every two weeks). Patients were also treated with supplemental active oral fluphenazine or placebo if they started to manifest prodromal symptoms suggestive of relapse. At the end of the study, the group that received the social skills training showed a small but significant advantage over the group that received supportive therapy in two of six measures of social adjustment. Social skills training did not appear to affect relapse rates. On the other hand, the supplemental medication decreased rates of relapse during the second year but did not affect measures of social adjustment. The authors concluded that this finding could be evidence that the different interventions affected different areas of outcome—that is, medications for relapse and social skills training for social functioning, with the best overall results among patients who received both (20).
The large multicenter NIMH Treatment Strategies in Schizophrenia Study assigned patients who were in the maintenance phase of treatment to three different dosage strategies of fluphenazine decanoate: standard dosage, low dosage, and targeted treatment—that is, placebo plus supplemental medication if the patient developed prodromal symptoms. All patients could receive a higher dosage of antipsychotic as rescue medication if their symptoms worsened. The study compared the interaction of each medication strategy with two different types of family intervention. The family intervention took the form of monthly multifamily support groups or a combination of these support groups with more intensive and individualized behavioral family treatment.
The study found higher rates of relapse in the targeted and low-dosage medication groups. No statistically significant difference in relapse rates was found between the two family treatment groups (21). Measures of patients' social functioning, family attitudes, and family burden were also examined. Families who received the intensive behavioral intervention showed significantly less rejecting attitudes toward patients over the two-year period. However, patients' social functioning and family burden were not related to family treatment or medication (22). The investigators speculated that the lack of a substantial differential effect between the two family treatments may have been due to a positive effect of the less intensive family treatment, because the rate of rehospitalization for both family treatment groups was lower than what had been reported in other studies for usual community care. They also questioned whether patients who met the criteria for participation in the maintenance phase were already so stable that the differences in relapse rates that may have been seen in a less stable population were lost. Such issues illustrate the complexity of conducting research on integrated psychosocial and pharmacologic treatment.
The recent finding of the importance of cognitive functioning to overall outcome among patients with schizophrenia has stimulated interest in whether the effects of antipsychotic medications on cognitive measures correlate with response to psychosocial treatments. Rosenheck and colleagues (23) compared participation in psychosocial treatment between patients with treatment-resistant illness who were receiving clozapine or haloperidol. They found that patients receiving clozapine were more likely to actively participate in rehabilitation.
In a different study of patients with treatment-resistant schizophrenia, Liberman and colleagues (24) assessed the relative improvement in activities of daily living among patients who received risperidone or haloperidol while residing on an inpatient unit with a well-developed behavioral rehabilitation program. They found that although all patients experienced significant improvement, there was no difference between patients receiving the different medications.
These studies suggest that the increased efficacy seen with clozapine in this population can improve patients' ability to make use of other elements of treatment but that for patients receiving other currently available antipsychotics, a sufficiently robust psychosocial intervention may have much more of an effect on clinically significant outcome measures.
Several key points can be drawn from these studies of integrated approaches. The chief message is that medication and psychosocial interventions are efficacious for different outcomes. Although medication is essential for preventing relapse, psychosocial treatments affect other domains that are important for the quality of life. In addition, a synergistic aspect is evident. Medication can facilitate participation in psychosocial treatment, and psychosocial interventions such as family therapy can decrease relapse rates beyond what is seen for a given dosage of medication alone.
Recommendations for integrated treatment
An essential aspect of guiding community-based treatment is determining which outcomes should be prioritized. This decision is particularly complicated for an illness such as schizophrenia, which can affect so many areas of functioning and involves multiple stakeholders with potentially different priorities—for example, patients, families, clinicians, administrators, and law enforcement personnel. Lehman (25) and Attkisson and colleagues (26) have suggested a framework for evaluating outcomes in schizophrenia, based on recommendations of an NIMH expert panel. This framework divides outcomes into four general domains: clinical, rehabilitative, humanitarian, and public welfare. The clinical domain includes issues such as psychopathology, symptoms, and treatments. The rehabilitative domain emphasizes the individual's strengths and ability to function adaptively, both socially and vocationally. The humanitarian domain includes concerns such as quality of life and subjective sense of well-being, and the public safety domain focuses on issues relating to the balance between the rights of the patient and the community to both liberty and to personal safety and well-being.
Lehman also proposed categorizing outcomes as either proximal or distal outcomes, according to the likely temporal relationship between an intervention and its outcome. For example, an intervention with an antipsychotic medication is likely to have an effect on easily observable clinical symptoms—and within a short period after the medication is administered—in addition to improving long-term quality of life. Results of a social skills program may not be as quickly detectable but may have a significant impact on longer-term outcomes such as ability to maintain employment. Such a framework can help to clarify the different effects of an intervention and their possible interactions. It can also help to keep a focus on more distal outcomes or those not directly within the clinical domain, which may be more difficult to study but are nonetheless important.
Effective community-based treatment also takes into account the importance of maintaining a collaborative relationship between patients, families, and treatment providers. Patients and families are more likely to remain engaged with treatment if they perceive it as being relevant to their personal concerns. The consumer movement and associated recovery model of mental illness have helped empower patients and their families to articulate their goals and the means they find most helpful to reach them (27).
A strong therapeutic alliance based on collaborative engagement and attention to multiple outcome domains is the foundation of integrated treatment and should be maintained regardless of the stage of illness (28). Different specific interventions can then be called upon within this framework for particular clinical situations.
Patients experiencing an acute psychotic episode
Priorities during an acute psychotic episode should include attention to safety, appropriate assessment for factors that may have contributed to the onset of the episode, and establishment of a positive treatment alliance. Although many patients will require inpatient treatment, avoiding an inpatient admission—if the appropriate resources are available—can help diminish stigmatization and maintain as much participation in normal role functions as is feasible.
Antipsychotic medication is currently the single most important intervention for patient stabilization during any acute episode. The medications and their effects are listed in Table 1 (29,30,31,32,33,34,35,36). Given that there is as yet no clear evidence of significantly increased efficacy with any particular antipsychotic—with the exception of clozapine for treatment-resistant illness—the choice of antipsychotic medication should be made on the basis of factors such as previous treatment response, individual side-effect profile, patient acceptability, and long-term treatment planning (37).
An atypical antipsychotic should usually be chosen as a first-line agent, because these agents are generally better tolerated and are associated with a lower risk of neurologic side effects. An individual's first psychotic break represents an especially crucial period. It is a time in which the foundations can be laid for a positive relationship between the treatment team and the patient and his or her family. Psychoeducation has the potential to be particularly effective during this time, when a period of crisis can facilitate openness to change. In addition, recent research has been showing that schizophrenia may respond differently to treatment early in the course of illness. Patients appear to have a better response to medications early on, and there is some evidence to suggest that identification and control of symptoms soon after the onset of symptoms may result in a better long-term outcome. Unfortunately, patients are also more likely to experience adverse side effects during this stage of their illness and to have a higher risk of nonadherence to treatment (38,39).
Patients beginning to recover from an acute psychotic episode
The weeks following an acute psychotic episode are generally referred to as the stabilization phase, during which the likelihood of a rapid relapse is high if medications are stopped or if the patient is exposed to excessive levels of stress. However, as the acute symptoms of psychosis begin to recede, treatment can start to focus on other areas, such as environmental stressors, adherence issues, and the impact of the episode on the patient and his or her family. Pharmacologic treatment should be adjusted to treat associated symptoms, such as depression, or to manage side effects, including switching antipsychotics if necessary.
Stabilization is a period during which it is essential to give adequate attention to ensuring a smooth transition between acute care and postacute follow-up, particularly for patients who have required hospitalization. Lack of adherence to medication regimens after discharge from an acute hospitalization is common and represents the single most significant risk factor for relapse. Between 30 and 60 percent of patients do not show up for their first outpatient appointment after hospitalization, and 50 percent are noncompliant within the first year after discharge (40).
Velligan and colleagues (41) recently published preliminary results from a longitudinal prospective study of adherence in recently discharged patients with schizophrenia. They found that 25 percent of patients had already started missing doses in the first two weeks after discharge from the hospital. One major contributory element identified was that patients often did not understand what their treatment regimen was, which resulted in significant departures from what had been prescribed even when the patients were motivated to stay in treatment. These researchers expressed concern about current constraints on mental health services that have meant that many patients are discharged while still actively psychotic and without the cognitive resources necessary to adhere to a treatment plan.
A course of treatment in a partial hospitalization program or day treatment program may be indicated during this phase, particularly for patients who are discharged from inpatient units while still experiencing significant psychotic or mood symptoms. For patients who have significant contact with their families, initiating family treatment can significantly reduce rates of relapse. Studies have shown that monthly family meetings have had as positive an effect as more intensive forms of family therapy (21). However, the duration of family treatment is important. This form of therapy should optimally be continued for at least nine months, because this duration has been shown to result in more lasting positive effects (37,42,43). Patients who are likely to require additional support should be referred for case management services. In addition, attention should be paid to whether economic or housing support is needed.
Patients whose symptoms have resolved or reached a plateau
A patient is considered to have entered the stable phase when his or her symptoms cease to improve but the patient is no longer in a crisis situation. This situation can occur when patients are continuing to experience varying degrees of positive or negative symptoms. Most will have persisting impairments in cognitive or social functioning. During this period rehabilitative interventions that have a greater likelihood of improving distal outcomes can start to be incorporated into treatment. A variety of psychosocial interventions are listed in Table 2 (44,45,46,47,48,49,50,51,52,53,54,55,56,57,58). For example, as described above, social skills training in conjunction with pharmacologic treatment can improve measures of social adjustment. Vocational rehabilitation, such as supported employment, should be made available for any patient who has a good employment history or who expresses an interest in working.
The more distal effects of medications should also be taken into account, particularly if this has not been done previously. The relative impact of different antipsychotics on cognitive functioning is currently the subject of intense investigation. Although the positive effect of any of the antipsychotics on cognitive functioning is not yet clear, both anticholinergic side effects and parkinsonism are associated with impairments in cognition.
Patients who experience frequent relapses
For patients who experience frequent relapses or the need for multiple rehospitalizations, the first step is to investigate whether the patient is having problems with adherence. Additional psychosocial interventions that have been found to be effective in this population include assertive community treatment or, for individuals who have close family involvement, family therapy. Substance abuse is a strong risk factor for relapse, and if it is present the patient should be referred to a dual diagnosis program.
Patients who are not adherent to treatment
Difficulties with adherence are not unique to patients with schizophrenia but are common with any chronic illness in which treatment is primarily prophylactic. Some types of psychopathology increase the likelihood of poor adherence among patients with schizophrenia—for example, paranoia with grandiose delusions, poor insight, comorbid substance abuse, and neurocognitive deficits. Contextual elements such as chronic homelessness or residing with a family or caregivers who are not supportive of medications can also decrease adherence. Akathisia and subjective dysphoria are the side effects most commonly associated with lack of adherence to antipsychotic medications (59).
The first step is to determine the cause of the problems with adherence. If poor adherence is related to adverse effects of medication, these should be treated through changing the dosage or adding an adjunctive medication, such as benztropine in the case of parkinsonism. If this is not adequate, the patient should have a different antipsychotic prescribed. Depot antipsychotics should be considered when the inconvenience of maintaining oral treatment is a significant factor. Additional supportive measures—for example, the use of pill boxes for behavioral cueing, intensive case management, or compliance therapy—may be helpful for some patients (60).
Patients with treatment-resistant symptoms
Patients with persistent psychotic symptoms may benefit from initiating therapy with clozapine early. Cognitive-behavioral therapy may also be used for patients who have ongoing delusions and hallucinations (61,62). If a patient has functional impairment related to ongoing significant negative symptoms, it should be determined whether these symptoms are being confounded by factors such as psychotic withdrawal, extrapyramidal symptoms, or untreated depression. Psychotic withdrawal should be addressed by increasing or changing the antipsychotic treatment. If extrapyramidal symptoms are prominent, and if the patient is being treated with a conventional antipsychotic, the patient should switch to an atypical agent. If depression is present, a full course of antidepressant therapy should be instituted. Social skills training is currently recommended for patients who have persistent primary negative symptoms that affect psychosocial functioning. It is also possible that pharmacologic agents such as NMDA-receptor agonists will be available in the future for clinical use in this population (32).
Patients with comorbid substance abuse
The Epidemiologic Catchment Area study showed that the rate of lifetime substance use disorders was 48 percent among persons with schizophrenia, compared with 17 percent in the general population (63). Patients with comorbid schizophrenia and substance use problems have typically been unable to remain in substance abuse treatment programs because of their mental illness, and they respond poorly in treatment programs for schizophrenia, because substance abuse issues are not addressed (64). Patients with comorbid schizophrenia and substance abuse should be treated in an integrated dual diagnosis program that allows ongoing assessment of substance abuse and specialized treatment approaches.
Efficacy, effectiveness, efficiency, and implementation
The preceding discussion focuses on what is known about the efficacy of integrated treatment approaches. However, it has become increasingly recognized that there are several necessary steps between identifying an efficacious intervention and changing practice in community clinics to reflect those findings (65). These steps include giving attention to the difference between efficacy, or the potential of an intervention to result in a particular outcome in a research setting, and effectiveness, defined as what the outcomes are likely to be when an intervention is transplanted into a real-world setting with a more heterogeneous patient population and more obstacles in maintaining fidelity to a given treatment protocol.
The next step is determining whether the potential intervention is an efficient use of available resources. Finally, questions of how easy or difficult it may be to implement the intervention must be considered. A growing body of literature about dissemination of changes shows that education alone is seldom effective and that a multipronged approach—including strong administrative support, hands-on exposure, redesign of professional incentives to support new methods, and ongoing support and feedback—are necessary to make a sustained difference in the actual practice of mental health care delivery (37,66,67,68). Such changes may represent a significant burden to already beleaguered mental health care systems and must be planned well ahead of time if proposed changes are to succeed.
Conclusions
Schizophrenia arises from a complex interaction between genetic and environmental factors that affect multiple functional domains in a heterogeneous fashion. The ways in which these different domains contribute to the psychopathology of schizophrenia are gradually being delineated. It has become recognized that pharmacologic and psychosocial interventions both have a necessary place in the treatment of schizophrenia, and the past decades of research on these different forms of intervention are beginning to clarify how each affects specific types of symptoms and areas of functioning.
The importance of cognitive deficits in psychosocial outcomes emphasizes the need to systematically examine other variables besides relapse, hospitalization, and positive symptoms. As our understanding of schizophrenia continues to become more sophisticated, it will be important to conduct research studies that are designed to clarify the ways in which different treatment methods and patterns of psychopathology interact (20). Such studies will provide the evidence needed to guide the integration of these interventions in the service of patients, their families, and communities.
The authors are affiliated with the department of psychiatry at the University of New Mexico School of Medicine, 2400 Tucker N.E., Albuquerque, New Mexico 87131 (e-mail, [email protected]). This paper is part of a special section on integrated care for persons with mental illness.
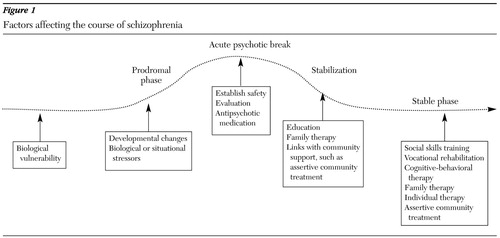
Figure 1. Factors affecting the course of schizophrenia
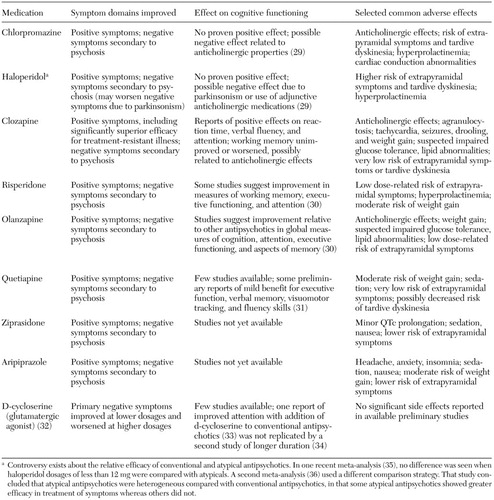 |
Table 1. Medications for treatment of patients with schizophrenia
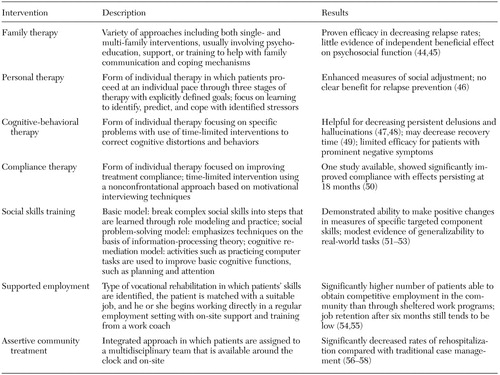 |
Table 2. Psychosocial interventions for patients with schizophrenia
1. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, rev. Washington, DC, American Psychiatric Association, 2000Google Scholar
2. Rice DP: The economic impact of schizophrenia. Journal of Clinical Psychiatry 60:4–6, 28–30, 1999Google Scholar
3. Mental Health: A Report of the Surgeon General. Rockville, Md, US Department of Health and Human Services, 1999Google Scholar
4. Buchanan RW, Carpenter WT: Schizophrenia: introduction and overview, in Comprehensive Textbook of Psychiatry, vol 1. Edited by Sadock BJ, Sadock VA. Philadelphia, Lippincott Williams & Wilkins, 2000Google Scholar
5. Hogarty GE, Ulrich RF: The limitations of antipsychotic medication on schizophrenia relapse and adjustment and the contributions of psychosocial treatment. Journal of Psychiatric Research 32:243–250, 1998Crossref, Medline, Google Scholar
6. Liberman RP: Psychosocial treatments for schizophrenia. Psychiatry 57:104–114, 1994Crossref, Medline, Google Scholar
7. Brown GW, Birley JL, Wing JK: Influence of family life on the course of schizophrenic disorders: a replication. British Journal of Psychiatry 121:241–258, 1972Crossref, Medline, Google Scholar
8. Jablensky A, Sartorius N, Ernberg G, et al: Schizophrenia: manifestations, incidence, and course in different cultures: a World Health Organization ten-country study. Psychological Medicine Monograph Supplement 20:1–97, 1992Crossref, Medline, Google Scholar
9. Bustillo JR, Lauriello J, Keith SJ: Schizophrenia: improving outcome. Harvard Review of Psychiatry 6:229–240, 1999Crossref, Medline, Google Scholar
10. American Psychiatric Association: Practice guideline for the treatment of patients with schizophrenia. American Journal of Psychiatry 154:1–63, 1997Google Scholar
11. Herz MI, Marder SR: Schizophrenia: Comprehensive Treatment and Management. Philadelphia, Lippincott Williams & Wilkins, 2002Google Scholar
12. Hafner H, an der Heiden W: Course and outcome of schizophrenia, in Schizophrenia. Edited by Hirsch SR, Weinberger D. Malden, Mass, Blackwell, 2003Google Scholar
13. May PRA: Treatment of Schizophrenia: A Comparative Study of Five Treatment Models. New York, Science House, 1968Google Scholar
14. Hogarty GE, Goldberg SC, Schooler NR: Drug and sociotherapy in the aftercare of schizophrenic patients: III. adjustment of nonrelapsed patients. Archives of General Psychiatry 31:609–618, 1974Crossref, Medline, Google Scholar
15. Hogarty GE, Schooler NR, Ulrich R, et al: Fluphenazine and social therapy in the aftercare of schizophrenic patients: relapse analyses of a two-year controlled study of fluphenazine decanoate and fluphenazine hydrochloride. Archives of General Psychiatry 36:1283–1294, 1979Crossref, Medline, Google Scholar
16. Brown GW, Rutter M: The measurement of family activities and relationships: a methodological study. Human Relations Supplement 2:10–15, 1966Google Scholar
17. Goldstein MJ, Rodnick EH, Evans JR, et al: Drug and family therapy in the aftercare of acute schizophrenics. Archives of General Psychiatry 35:1169–1177, 1978Crossref, Medline, Google Scholar
18. Hogarty GE, McEvoy JP, Munetz M, et al: Dose of fluphenazine, familial expressed emotion, and outcome in schizophrenia: results of a two-year controlled study. Archives of General Psychiatry 45:797–805, 1988Crossref, Medline, Google Scholar
19. Marder SR, Wirshing WC, Mintz J, et al: Two-year outcome of social skills training and group psychotherapy for outpatients with schizophrenia. American Journal of Psychiatry 153:1585–1592, 1996Link, Google Scholar
20. Marder SR: Integrating pharmacological and psychosocial treatments for schizophrenia. Acta Psychiatrica Scandinavica Supplement 102:87–90, 2000Crossref, Google Scholar
21. Schooler NR, Keith SJ, Severe JB, et al: Relapse and rehospitalization during maintenance treatment of schizophrenia: the effects of dose reduction and family treatment. Archives of General Psychiatry 54:453–463, 1997Crossref, Medline, Google Scholar
22. Mueser KT, Sengupta A, Schooler NR, et al: Family treatment and medication dosage reduction in schizophrenia: effects on patient social functioning, family attitudes, and burden. Journal of Consulting and Clinical Psychology 69:3–12, 2001Crossref, Medline, Google Scholar
23. Rosenheck R, Tekell J, Peters J, et al: Does participation in psychosocial treatment augment the benefit of clozapine? Archives of General Psychiatry 55:618–625, 1998Google Scholar
24. 24.Liberman RP, Gutkind D, Mintz J, et al: Impact of risperidone versus haloperidol on activities of daily living in the treatment of refractory schizophrenia. Comprehensive Psychiatry 43:469–473, 2002Crossref, Medline, Google Scholar
25. Lehman AF: Developing an outcomes-oriented approach for the treatment of schizophrenia. Journal of Clinical Psychiatry 60(suppl 19):30–35, 1999Medline, Google Scholar
26. Attkisson C, Cook J, Karno M, et al: Clinical services research. Schizophrenia Bulletin 18:561–626, 1992Crossref, Medline, Google Scholar
27. Mead S, Copeland ME: What recovery means to us: consumers' perspectives. Community Mental Health Journal 36:315–331, 2000Crossref, Medline, Google Scholar
28. Corrigan PW, Liberman RP, Engel JD: From noncompliance to collaboration in the treatment of schizophrenia. Hospital and Community Psychiatry 41:1203–1211, 1990Abstract, Google Scholar
29. Spohn HE, Strauss ME: Relation of neuroleptic and anticholinergic medication to cognitive functions in schizophrenia. Journal of Abnormal Psychology 98:367–380, 1989Crossref, Medline, Google Scholar
30. Purdon SE, Jones BDW, Stip E, et al: Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. Archives of General Psychiatry 57:249–258, 2000Crossref, Medline, Google Scholar
31. Velligan DI, Miller AL: Cognitive dysfunction in schizophrenia and its importance to outcome: the place of atypical antipsychotics in treatment. Journal of Clinical Psychiatry 60:25–28, 1999Medline, Google Scholar
32. Goff DC, Coyle JT: The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. American Journal of Psychiatry 158:1367–1377, 2001Link, Google Scholar
33. Goff DC, Tsai G, Manoach DS, et al: Dose-finding trial of d-cycloserine added to neuroleptics for negative symptoms in schizophrenia. American Journal of Psychiatry 152:1213–1215, 1995Link, Google Scholar
34. Goff DC, Tsai G, Levitt J, et al: A placebo-controlled trial of d-cycloserine added to conventional neuroleptics in patients with schizophrenia. Archives of General Psychiatry 56:21–27, 1999Crossref, Medline, Google Scholar
35. Geddes J, Freemantle N, Harrison P, et al: Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. British Medical Journal 321:1371–1376, 2000Crossref, Medline, Google Scholar
36. Davis JM, Chen N, Glick ID: A meta-analysis of the efficacy of second-generation antipsychotics. Archives of General Psychiatry 60:553–564, 2003Crossref, Medline, Google Scholar
37. Lehman AF, Steinwachs DM: At issue: translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophrenia Bulletin 24:1–10, 1998Crossref, Medline, Google Scholar
38. Miyamoto S, Stroup TS, Duncan GE, et al: Acute pharmacological treatment of schizophrenia, in Schizophrenia. Edited by Hirsch SR, Weinberger D. Malden, Mass, Blackwell, 2003Google Scholar
39. Lindstrom E, Bingefors K: Patient compliance with drug therapy in schizophrenia: economic and clinical issues. Pharmacoeconomics 18:106–124, 2000Medline, Google Scholar
40. Weiden PJ, Olfson M: Cost of relapse in schizophrenia. Schizophrenia Bulletin 21:419–429, 1995Crossref, Medline, Google Scholar
41. Velligan DI, Lam F, Ereshefsky L, et al: Psychopharmacology: perspectives on medication adherence and atypical antipsychotic medications. Psychiatric Services 54:665–667, 2003Link, Google Scholar
42. Mueser KT, Glynn SM: Family intervention for schizophrenia, in Best Practice: Developing and Promoting Empirically Supported Interventions. Edited by Dobson KS, Craig KD. Newbury Park, Calif, Sage, 1998Google Scholar
43. Tarrier N, Barrowclough C, Porceddu K, et al: The Salford Family Intervention Project: relapse rates of schizophrenia at five and eight years. British Journal of Psychiatry 165:829–832, 1994Crossref, Medline, Google Scholar
44. Pharoah FM, Mari JJ, Streiner D: Family intervention for schizophrenia. Cochrane Review, update software. New York, Oxford University Press, 2003Google Scholar
45. Pilling S, Bebbington P, Kuipers E, et al: Psychological treatments in schizophrenia: I. meta-analysis of family intervention and cognitive behavioral therapy. Psychological Medicine 32:763–782, 2002Medline, Google Scholar
46. Hogarty GE, Kornblith SJ, Greenwald D, et al: Personal therapy: a disorder-relevant psychotherapy for schizophrenia. Schizophrenia Bulletin 21:379–393, 1995Crossref, Medline, Google Scholar
47. Tarrier N, Beckett R, Harwood S, et al: A trial of two cognitive-behavioural methods of treating drug-resistant residual psychotic symptoms in schizophrenic patients: I. outcome. British Journal of Psychiatry 162:524–532, 1993Crossref, Medline, Google Scholar
48. Kuipers E, Garety P, Fowler D, et al: London-East Anglia randomised controlled trial of cognitive-behavioural therapy for psychosis: I. effects of the treatment phase. British Journal of Psychiatry 171:319–327, 1997Crossref, Medline, Google Scholar
49. Drury V, Birchwood M, Cochrane R, et al: Cognitive therapy and recovery from acute psychosis: a controlled trial: I. impact on psychotic symptoms. British Journal of Psychiatry 169:593–601, 1996Crossref, Medline, Google Scholar
50. Kemp R, Kirov G, Everitt B, et al: Randomised controlled trial of compliance therapy:18–month follow-up. British Journal of Psychiatry 172:413–419, 1998Google Scholar
51. Bellack AS, Mueser KT: Psychosocial treatment for schizophrenia. Schizophrenia Bulletin 19:317–336, 1993Crossref, Medline, Google Scholar
52. Braff DL: Information processing and attention dysfunctions in schizophrenia. Schizophrenia Bulletin 19:233–259, 1993Crossref, Medline, Google Scholar
53. Brenner HD, Hodel B, Roder V, et al: Treatment of cognitive dysfunctions and behavioral deficits in schizophrenia. Schizophrenia Bulletin 18:21–26, 1992Crossref, Medline, Google Scholar
54. Drake RE, Becker DR, Biesanz JC, et al: Rehabilitative day treatment vs supported employment: I. vocational outcomes. Community Mental Health Journal 30:519–532, 1994Crossref, Medline, Google Scholar
55. Bond GR, Drake RE, Mueser KT, et al: An update on supported employment for people with severe mental illness. Psychiatric Services 48:335–346, 1997Link, Google Scholar
56. Mueser KT, Bond GR, Drake RE, et al: Models of community care for severe mental illness: a review of research on case management. Schizophrenia Bulletin 24:37–74, 1998Crossref, Medline, Google Scholar
57. Lehman AF, Dixon LB, Kernan E, et al: A randomized trial of assertive community treatment for homeless persons with severe mental illness. Archives of General Psychiatry 54:1038–1043, 1997Crossref, Medline, Google Scholar
58. Burns BJ, Santos AB: Assertive community treatment: an update of randomized trials. Psychiatric Services 46:669–675, 1995Link, Google Scholar
59. Fenton WS, Blyler CR, Heinssen RK: Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophrenia Bulletin 23:637–651, 1997Crossref, Medline, Google Scholar
60. Haynes RB, McDonald H, Garg AX, et al: Interventions for helping patients to follow prescriptions for medications, in The Cochrane Library, Issue 2. New York, Oxford University Press, 2003Google Scholar
61. Garety PA, Fowler D, Kuipers E: Cognitive-behavioral therapy for medication-resistant symptoms. Schizophrenia Bulletin 26:73–86, 2000Crossref, Medline, Google Scholar
62. Sensky T, Turkington D, Kingdon D, et al: A randomized controlled trial of cognitive-behavioral therapy for persistant symptoms of schizophrenia resistant to medication. Archives of General Psychiatry 57:165–172, 2000Crossref, Medline, Google Scholar
63. Regier DA, Boyd JH, Burke JD, et al: One-month prevalence of mental-disorders in the United States based on 5 epidemiologic catchment-area sites. Archives of General Psychiatry 45:977–986, 1988Crossref, Medline, Google Scholar
64. Drake RE, Mueser KT: Psychosocial approaches to dual diagnosis. Schizophrenia Bulletin 26:105–118, 2000Crossref, Medline, Google Scholar
65. Bridging Science and Service. Bethesda, Md, National Institute of Mental Health, 1999Google Scholar
66. Drake RE, Goldman HH, Leff HS: Implementing evidence-based practices in routine mental health service settings. Psychiatric Services 52:179–182, 2001Link, Google Scholar
67. Schoenwald SK, Hoagwood K: Effectiveness, transportability, and dissemination of interventions: what matters when? Psychiatric Services 52:1190–1197, 2001Google Scholar
68. Newman FL, Tejeda MJ: The need for research that is designed to support decisions in the delivery of mental health services. American Psychologist 51:1040–1049, 1996Crossref, Medline, Google Scholar



