Cost-Effectiveness of Early Intervention in Psychosis: A Modeling Study
Abstract
Objective:
Programs for early intervention in psychosis have shown clinical efficacy. The authors aimed to evaluate the cost-effectiveness of early intervention programs compared with standard care for the treatment of first-episode psychosis in the United States.
Methods:
A decision-analytic model integrating published data on clinical efficacy, costs, and health utilities was developed to evaluate early intervention versus standard care over the lifetime of patients after their first psychotic episode. Model input data were derived from meta-analyses, clinical trials, and U.S. national data. The main outcomes included hospitalizations, employment rate, quality-adjusted life years (QALYs), lifetime health care costs, and incremental cost-effectiveness ratios (ICERs).
Results:
Compared with patients receiving standard care, patients in the early intervention strategy had 3.2 fewer hospitalizations and 2.7 more years of employment over the course of their remaining life expectancy. From a health care perspective, early intervention had an ICER of approximately $51,600 per QALY. From a societal perspective, early intervention saved costs (i.e., yielded greater health benefits and had lower costs compared with standard care). Results were sensitive to the effect of early intervention on suicide, cost of standard care, cost of early intervention, and the effect (relative risk) of early intervention on employment. A scenario analysis that excluded the effect (i.e., hazard ratio) of early intervention on suicide yielded an ICER of approximately $197,000 per QALY.
Conclusions:
These results suggest that it is economically beneficial to fund early intervention in psychosis programs in the United States. The findings indicate that early intervention in psychosis saves costs (from the societal perspective) and is cost-effective (health care sector perspective).
HIGHLIGHTS
This study is the first computer simulation–based economic evaluation of early intervention in psychosis to use U.S. national data.
Model input data were derived from published meta-analyses, clinical trials, and U.S. data, and the main outcomes included hospitalizations, employment rate, quality-adjusted life years, lifetime health care costs, and incremental cost-effectiveness ratios.
The findings suggest that early intervention in psychosis saves costs from the societal perspective and is cost-effective from the health care sector perspective.
First-episode psychosis (FEP) is defined as the first time that an individual meets criteria for a primary psychotic disorder, typically in adolescence or early adulthood (1), and is a vulnerable time in the life of a young adult. The hallmark of psychosis is impaired evaluation of reality testing, when one has difficulty distinguishing between what is real and what is not, which can include delusions and hallucinations (2). FEP can be a harbinger for chronic primary psychotic disorders, including schizophrenia (2).
Schizophrenia is a severe and persistent mental illness that affects approximately 1% of the U.S. population, or roughly 3.7 million people (3). This diagnosis predicts poor health outcomes, including a mortality rate that is 3.5 times higher than the rate in the general population (4). It also predicts poor quality-of-life outcomes, including educational attainment and gainful employment (2). Consequently, schizophrenia imposes a significant societal economic burden. In 2013, the estimated economic cost of managing schizophrenia in the United States totaled $155.7 billion (5). Therefore, there is burgeoning interest in interventions to optimize the management of FEP and schizophrenia.
Early intervention in psychosis with medication management and psychosocial support has yielded marked improvements in clinical outcomes, including medication compliance, hospitalization rates, and symptom severity. It has also improved educational attainment and employment (6). Furthermore, imaging studies suggest that the longer the duration of untreated psychosis, the greater the damage to the brain (7). Taken together, primary evidence is in favor of early intervention in psychosis; yet, the question remains, Is early intervention cost-effective?
A recent systematic review of cost-effectiveness analyses of early intervention suggests that it is (8). However, of the highest-quality cost-effectiveness analyses included in the review, only one used U.S. data and costs. There is a need for more U.S.-based cost-effectiveness analyses to inform mental health policy. Additionally, most of these studies took a narrow economic perspective, with a focus on health care costs and limited inclusion of societal costs. In this study, we performed a cost-effectiveness analysis of early intervention compared with standard care in the United States by using both a health care sector perspective and a broader societal perspective.
Methods
Model Overview
We developed a computer simulation–based, state-transition model that projected hospitalizations, employment, quality-adjusted life years (QALYs), lifetime health care costs, and productivity benefits for a cohort of 20-year-old men with a diagnosis of FEP that developed into schizophrenia (Figure 1). Health states (i.e., the Markov states used in the simulation model, which could relate to health or employment status) included employed, unemployed, suicide death, and nonsuicide death. Employment reflected average time of being employed. Hospitalizations occurred as events with an associated decrement in quality of life, assuming a length of stay of 10.9 days (9). The model compared the lifetime cost-effectiveness and net benefits of early intervention in psychosis with those of standard care.
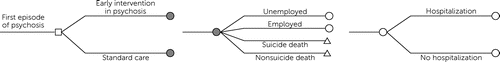
FIGURE 1. Overview of the disease model used in this studya
aIn the model, patients (20-year-old men) with a first psychotic episode enter and receive early intervention or standard care. Patients start unemployed and may transition to employment, suicide, or nonsuicide death. Hospitalization is a clinical event with an annualized disutility and cost that can occur in the employed and unemployed health states.
The costs and QALYs projected for each strategy were used to calculate incremental cost-effectiveness ratios (ICERs), defined as the additional cost of a particular strategy divided by the additional health benefits (i.e., QALYs gained) compared with the next less-costly strategy. The resulting ICERs were compared with a cost-effectiveness threshold of $100,000 per QALY, a commonly used willingness-to-pay threshold in the United States (10). A “cost-saving” ICER result is defined as a strategy that produces more health benefits (i.e., QALYs gained) at a lower cost. We conducted the analysis by using a lifetime horizon with all costs in 2018 U.S. dollars from a health care sector perspective (including only direct medical costs) and a societal perspective (direct medical costs and productivity benefits). Future health care costs and QALYs were discounted at 3% annually (11). We programmed the model with TreeAge Pro (Health Care) 2019, version 2.0, software.
Clinical Strategies Evaluated
In this analysis, patients entering the model were assumed to be male, age 20 years, and diagnosed as having FEP, which developed into schizophrenia. The early intervention strategy involves a specialized clinic with an emphasis on medication management and psychosocial support. In addition to seeing a psychiatrist, the patient is assigned a case manager who provides psychosocial support such as securing employment or enrolling in school. The standard strategy involves a general psychiatric clinic with a focus on medication management alone. This strategy involves no case managers; therefore, there is minimal to no psychosocial support.
The annual rate of nonsuicide death (110 per 100,000 people) was derived from the Centers for Disease Control and Prevention 2015 male life table (12) and was increased by a risk ratio of 3.3, per findings that used Medicaid data from Olfson et al. (4). This risk ratio was not assumed to differ between the two strategies. All estimates for annual transition probabilities between health states were derived from peer-reviewed published literature, with priority given to randomized controlled trials, cohort trials, and meta-analyses.
For suicide rates, the best available estimates were found in Chan et al.’s (13) study, which relied on data from Hong Kong. The authors reported suicide probabilities over a 12-year period. Assuming constant suicide rates over this period, annualized probabilities for suicide were calculated to be 0.37% for early intervention and 0.65% for standard care. The annual probability of hospitalization was 32.3% for early intervention and 42.4% for standard care (6). Each hospitalization was assumed to last 10.9 days according to Medicaid data (9). The 1-year transition probability of employment from the unemployed health state was 52.5% for early intervention and 45.3% for standard care (6). In the early intervention strategy, the probability of employment in the year of a patient’s hospitalization was assumed to be that of standard care (45.3%) to reflect a reduction in capacity for employment posthospitalization. All model inputs are summarized in Table 1 and are based on the studies listed in this table (4–6, 9, 10, 13–19).
| Variable | Base case value | Sensitivity analysis range | Probability distribution for probabilistic sensitivity analyses | Source/reference |
|---|---|---|---|---|
| Age in years | 20 | NA | NA | Assumption |
| Mortality ratio for men with schizophrenia | 3.3 | 3.3–3.3 | NA | 4 |
| Hospital length of stay | 10.9 | 10.6–11.2 | Lognormal | 9 |
| Risk ratio of employment | 1.13 | 1.03–1.24 | Lognormal | 6 |
| Risk ratio of hospitalization | .74/.87b | .61 to .90/.71 to 1.07b | Lognormal | 6 |
| Hazard ratio of suicide | .57 | .36–.91 | Lognormal | 13 |
| Early intervention program | ||||
| Annual probability of hospitalization | .323 | .296–.350 | Beta | 6 |
| Annual probability of employment | .525 | .496–.554 | Beta | 6 |
| Annual probability of employment (posthospitalization) | .453 | .422–.484 | Beta | 6 |
| Annual probability of suicide | .004 | NA | NA | 13 |
| Standard care | ||||
| Annual probability of hospitalization | .424 | .394–.455 | Beta | 6 |
| Annual probability of employment | .453 | .422–.484 | Beta | 6 |
| Annual probability of suicide | .007 | NA | NA | 13 |
| Costsc | ||||
| Cost of early intervention program clinic | 15,036 | 12,646–17,426 | Gamma | 14 |
| Cost of standard care clinic | 12,768 | 10,096–15,440 | Gamma | 14 |
| Cost of psychiatric hospitalization | 10,380 | 10,070–10,690 | Gamma | 10 |
| Other direct costs of schizophreniad | 6,974 | 3,487–10,461 | NA | 5 |
| Quality-of-life valuese | NA | |||
| Employed | .89 | .83–.92 | NA | 16 |
| Unemployed | .76 | .60–.92 | NA | 17 |
| Hospitalization (from employed)f | −.29 | −.43 to −.14 | NA | 17 |
| Hospitalization (from unemployed)f | −.16 | −.24 to −.08 | NA | 17 |
| Productivity | ||||
| Average earnings | See online supplement | NA | NA | Authors’ calculationg |
| Average rates of fringe benefits in % | 46.2 | NA | NA | 18 |
| Individual effective income tax rates in % | 9.2 | NA | NA | 19 |
| Absenteeism | .11 | NA | NA | 17 |
| Presenteeism | .15 | NA | NA | 17 |
| Adjustment factor for presenteeism and absenteeism associated with mental illness | .76 | NA | NA | Author’s calculationh |
TABLE 1. Model variables with base case values and ranges used in one-way sensitivity analysis to evaluate early intervention versus standard care over the lifetime of patients after their first psychotic episodea
Costs and Utilities
Information regarding costs and utilities is available in an online supplement to this article.
Sensitivity Analysis
Parameters were varied individually in one-way sensitivity analyses to evaluate the influence of plausible variations in model inputs (Table 1). Overall model uncertainty was evaluated with probabilistic sensitivity analysis, conducted by using 10,000 random draws from probability distributions (Table 1) for each variable simultaneously and by recalculating the cost-effectiveness of each strategy as the ICER of the mean costs and mean benefits over the 10,000 draws. To be consistent with the logical constraints of each parameter, we established parameters by using beta distributions for probabilities, lognormal distributions for risk ratios, and gamma distributions for cost values (20). Although expert guidance for cost-effectiveness analysis recommends using a lifetime horizon to capture all relevant cost and health outcomes (15, 21), we used 10-year and 5-year analytic time horizons in sensitivity analyses to assess the degree to which extrapolating these outcomes influenced our main conclusions.
Beyond the health care sector and societal perspective, we analyzed three additional scenarios. Because our estimates for absenteeism and presenteeism were based on “depression-sadness-mental illness” from Goetzel et al. (16), we performed a scenario analysis where these estimates were doubled (absenteeism, 0.214, and presenteeism, 0.306) to create an adjustment factor for the expected reduced productivity for a population affected by psychosis. We investigated the effect of differential suicide rates on the outcomes of the two selected economic perspectives. In these two scenarios, we used equal suicide rates (i.e., the annual transition probability of suicide in standard care, or 0.65%) for both the early intervention and standard care strategies. All sensitivity analyses were repeated for these scenarios to assess whether the impact of uncertainty on cost-effectiveness results was different for patients with the adjusted productivity and equal suicide rates.
For both base case scenarios (health care sector and societal perspective), an adjusted estimate for the risk ratio of hospitalization that accounts for publication bias (6) was incorporated and reanalyzed. An accompanying probabilistic sensitivity analysis was performed for the societal perspective.
Results
In the base case analysis, patients with FEP who underwent early intervention were estimated to have 12.2 hospitalizations over the course of their remaining life expectancy, whereas patients who underwent standard care were estimated to have 15.4 hospitalizations (Table 2). Employment estimates were 19.1 years for patients in early intervention and 16.4 years for patients in standard care. From a health care sector perspective, early intervention was estimated to have higher total lifetime costs and QALYs, with an ICER of $51,600 per QALY, compared with standard care. From a societal perspective, early intervention had greater total lifetime discounted QALYs and lower costs than standard care and was therefore predicted to be a cost-saving strategy.
| Strategy | N of hospitalizations | Years of employment | QALYsb | Health care costs ($)b | Productivity benefits ($)b | Consumption costs ($)b | Total (net) costs ($)b | ICER ($/QALY) |
|---|---|---|---|---|---|---|---|---|
| Societal perspective | ||||||||
| Standard care | 15.4 | 16.4 | 16.9 | 499,480 | 780,080 | 268,100 | −12,490 | Reference |
| Early intervention in psychosis | 12.2 | 19.1 | 17.8 | 548,040 | 895,500 | 307,960 | −39,500 | Cost-saving |
| Societal with adjusted productivity | ||||||||
| Standard care | 15.4 | 16.4 | 16.9 | 499,480 | 662,430 | 268,100 | 105,150 | Reference |
| Early intervention in psychosis | 12.2 | 19.1 | 17.8 | 548,040 | 760,500 | 307,960 | 95,500 | Cost-saving |
| Societal perspective with no suicide effect for early intervention | ||||||||
| Standard care | 15.4 | 16.4 | 16.9 | 499,480 | 780,080 | 268,110 | −12,490 | Reference |
| Early intervention in psychosis | 11.4 | 17.9 | 17.0 | 522,660 | 849,510 | 291,970 | −34,880 | Cost-saving |
| Health care perspective | ||||||||
| Standard care | 15.4 | 16.4 | 16.9 | 499,480 | 0 | 0 | 499,480 | Reference |
| Early intervention in psychosis | 12.2 | 19.1 | 17.8 | 548,040 | 0 | 0 | 548,044 | 51,600 |
| Health care perspective with no suicide effect for early intervention | ||||||||
| Standard care | 15.4 | 16.4 | 16.9 | 499,480 | 0 | 0 | 499,480 | Reference |
| Early intervention in psychosis | 11.4 | 17.9 | 17.0 | 522,660 | 0 | 0 | 522,664 | 197,020 |
TABLE 2. The cost-effectiveness results of the base case analyses (societal and health care perspectives) and several scenarios to evaluate early intervention versus standard care over patients’ lifetimes after first-episode psychosisa
When the risk ratio for hospitalization was updated to an estimate that accounted for publication bias (i.e., less favorable for the early intervention strategy), the results for each base case scenario were reevaluated. From the societal perspective, early intervention had greater total lifetime discounted QALYs and lower costs than standard care; therefore, it was predicted to be a cost-saving strategy but less so than the original base case scenario. From a health care sector perspective, early intervention had higher total lifetime costs and QALYs, with an ICER of $60,980 per QALY, compared with standard care (see the online supplement).
Table 2 also displays the cost-effectiveness results for a variety of scenario analyses. In a scenario analysis from the societal perspective, where absenteeism and presenteeism were doubled to evaluate an expected worse productivity for patients with psychosis (because our base case estimates were derived from individuals with depression), early intervention was predicted to save costs compared with standard care. When suicide rates were adjusted equally for both interventions (i.e., the suicide rate of standard care was applied to both strategies), early intervention remained a cost-saving treatment but less so than the adjusted productivity scenario. In an equal–suicide rate scenario for our health care perspective, early intervention had an ICER of approximately $197,000 per QALY.
Use of a 10-year analytic time horizon did not change our main cost-effectiveness results for the societal perspective (early intervention remained a health-improving and cost-saving strategy) or the health care perspective (the ICER for early intervention remained between $50,000 and $100,000 per QALY). With a 5-year time horizon, the ICER for early intervention changed to $37,270 per QALY for the societal perspective and $136,920 per QALY for the health care perspective (see online supplement). In one-way sensitivity analyses, the cost-effectiveness of early intervention compared with standard care was sensitive to the effect of early intervention on suicide, the costs of standard care and early intervention clinics, the relative risk of early intervention on employment, and the utility (health-related quality of life) of unemployment (see online supplement).
Figure 2 shows the cost-effectiveness acceptability curve results for the probabilistic sensitivity analysis. The early intervention strategy was likely to be optimal in 90.6% of probabilistic sensitivity analysis iterations when a cost-effectiveness threshold of $100,000 per QALY was used. A figure showing the cost-effectiveness acceptability curve results for the probabilistic sensitivity analysis for the base case scenario, which includes an adjusted risk ratio for hospitalization to account for publication bias, is available as an online supplement to this article. The early intervention strategy was likely to be optimal in 87.8% of probabilistic sensitivity analysis iterations when a cost-effectiveness threshold of $100,000 per QALY was used.
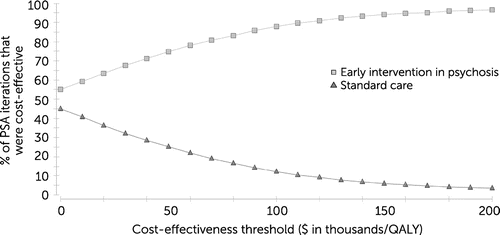
FIGURE 2. Cost-effectiveness acceptability curves obtained with probabilistic sensitivity analysis (PSA) indicating societal perspective results for early intervention in psychosis and standard carea
aThe early intervention strategy was most likely to be optimal in the PSA when a cost-effectiveness threshold of $100,000 per quality-adjusted life year (QALY) was used. Early intervention was optimal in 90.6% of PSA iterations; standard care was optimal in 9.4% of PSA iterations.
Discussion
We developed a decision-analytic model to compare two clinical treatment strategies for the management of FEP among young adults. From a societal perspective, early intervention saved costs (i.e., yielded more QALYs at a lower cost). Additionally, in scenarios where productivity was adjusted with increased absenteeism and presenteeism, and where we removed the effect of early intervention on suicide, the early intervention strategy remained a cost-saving strategy relative to standard care. From a health care perspective and a willingness-to-pay threshold of $100,000 per QALY, early intervention was cost-effective with an ICER of approximately $51,600 per QALY. When the beneficial effect of early intervention on suicide was removed, early intervention was no longer cost-effective, with an ICER of $197,000 per QALY.
Our results were also sensitive to using a relatively short analytic time horizon (5 years rather than 10 years or lifetime) in combination with a health care perspective (ICER of $136,920 per QALY for early intervention vs. standard care); however, early intervention had an ICER of <$50,000 per QALY when an analytic time horizon of 5 years in combination with a societal perspective was used.
To our knowledge, this model-based economic evaluation of early intervention in psychosis is the first to use U.S. data including costs, where available. It is also the first to consider a lifetime horizon from a societal perspective, include data from the first retrospective cohort study evaluating the effect of early intervention in psychosis on suicide outcomes (13), and conduct a scenario analysis including estimated adjusted productivity for people living with a primary psychotic illness. The only other model-based, comparative cost-effectiveness analyses for early intervention in psychosis were performed in the United Kingdom (22, 23). These studies found early intervention to be cost-effective but had data limitations and methodological challenges. In Perez et al.’s (22) study, the cost of “treatment as usual” (or “standard care”) was assumed to be zero, which is unlikely; we focused on comparing the true incremental costs of early intervention with standard care in our cost-effectiveness analyses. Park et al. (23) built three decision-analytic models to evaluate early intervention versus standard care for employment and education, as well as homicide and suicide. The authors also used European trial-based and observational data, and their time horizon was limited to the effectiveness data available (i.e., 2 years for employment and education and 10 years for homicide and suicide).
A recent systematic review of cost-effectiveness analyses of early intervention in psychosis identified 16 studies worldwide and revealed that most economic evaluations used a narrow economic perspective (i.e., the health care sector), which fails to account for the broader economic effects of psychosis (8). In this study, we attempted to address this limitation and included productivity benefits as recommended by the Second Panel on Cost-Effectiveness in Health and Medicine (15). The inclusion of productivity benefits can have a significant impact on cost-effectiveness results. In one systematic review that evaluated the effects of including productivity costs in economic evaluations of the treatment of depression, approximately 69% of studies ignored productivity costs (24). For the studies that included them, productivity costs accounted for 60% of total costs per treatment arm. Moreover, the review found that the inclusion of productivity costs (for the studies that did not do so) in most cases substantially affected the incremental costs such that the optimal decision changed. Therefore, the choice of perspective on cost has significant implications on cost-effectiveness results, which, in turn, can affect subsequent decision making. Our findings highlight the importance of including productivity costs or benefits in the economic evaluation of psychiatric disorders, especially because these diseases have broad and ubiquitous societal impact.
To account for the reduction in productivity associated with mental illness, we used estimates for absenteeism and presenteeism from a U.S. study that relied on administrative data of medical treatment, employee absence and disability, and self-report surveys (16); we found that early intervention was well within conventional standards for cost-effectiveness in the United States. However, the estimates for absenteeism and presenteeism were for “depression-sadness-mental illness,” a vague definition that likely includes minimal to no experiences of psychosis. Because the hallmark symptoms of psychotic illness include difficulty with reality testing and disorganized thought processes (2), and because the adverse effects of antipsychotic medications include sedation (25), we would expect lower productivity for persons living with psychosis than suggested by the estimates in the study by Goetzel and colleagues (16). In our scenario analysis, we therefore doubled the values for absenteeism and presenteeism and found that early intervention remained a cost-saving treatment, although less so than our base case analysis.
One of the most important clinical outcomes for early intervention in psychosis is prevention of suicide. In theory, early intervention should reduce suicide attempts and completions through rapid psychiatric assessment, close follow-up, and better treatment adherence (6). To our knowledge, there are no trial-based evaluations of suicide outcomes after early intervention versus standard care in the United States. We found the best estimates of these outcomes in a retrospective cohort trial in Hong Kong (13). The inclusion of these data in this study represented both a strength and a limitation of our model. Without the inclusion of suicide outcomes, our model would have missed important clinical events and misrepresented the lethal risk of psychosis. Conversely, including the Hong Kong data may have not accurately represented suicide outcomes of FEP in the United States and may have introduced bias into our results. To address this limitation, we included a scenario analysis without differential suicide rates between treatment strategies. This scenario was the only one in which, when assuming a limited health care perspective, early intervention was not cost-effective.
Our study had several other limitations. For example, because of the drawbacks associated with the available key data sources that informed the model inputs on the effectiveness of early intervention, our findings may have reduced generalizability. We may have underestimated hospitalization costs because we relied on Medicaid data (because these data were available) instead of Medicare or private insurance data. Our transition probability for employment came from an estimate of employment or school enrollment in early intervention versus treatment as usual (6); therefore, this calculation may have overestimated employment in this population. As with all model-based, cost-effectiveness analyses, our study required combining data from various sources. Our literature review revealed a paucity of important data for early intervention in psychosis. However, a recent meta-analysis by Correll et al. (6) provided well-powered estimates for employment and hospitalization rates for patients in early intervention and standard care, which were included in this analysis. The authors included studies from North America, Europe, and Asia. Although the Correll et al. study provides the best available evidence for early intervention leading to a variety of improved outcomes, it may not accurately represent these outcomes in the United States, which was the focus for our study. In practice, when input assumptions are needed for a decision-modeling analysis but are unavailable in a specific country context, the necessary values may be borrowed from neighboring countries or settings with similar characteristics (15). Typically, these parameters are then varied in a sensitivity analysis to identify threshold values based on relevant cost-effectiveness criteria that would result in no differences among strategies; decision makers can then interpret how plausible these threshold values are for their setting.
Important data gaps remain regarding incarceration, homelessness, caregiver time and productivity costs, social assistance rates, and annual wage earnings for people living with psychosis. Access to these outcomes for early intervention and standard care clinical strategies would provide an opportunity for more comprehensive cost-effectiveness analyses. Until then, decision-analytic models such as ours can synthesize the existing information to inform policy decisions regarding the value of early intervention for psychosis.
Another limitation was our assumption regarding long-term suicide, hospitalization, and employment rates across the life span for patients in this model, namely, that these rates remain constant year to year. We made this assumption for two reasons. First, annualized rates for these outcomes over the life course do not exist. Second, we assumed that early intervention services are not time limited for young adults who later exhibit schizophrenia and other primary psychotic disorders. We assumed that individuals receive psychosocial support in the form of case management across their life span and modeled lifelong treatment costs accordingly.
We chose to build our model with these assumptions because studies have shown that the long-term benefits of early intervention in psychosis all but disappear at 10-year follow-up, likely because of the lack of long-term, follow-up care (26). These clinical findings combined with this model-based, cost-effectiveness evaluation speak to the importance and economic necessity of long-term support for individuals living with psychotic disorders.
Conclusions
In summary, our findings show that when using a societal perspective, early intervention for FEP is a cost-saving treatment, even when reduced productivity and no differential suicide effect are assumed in the model. Early intervention was found to be cost-effective from the narrower health care perspective, but it was no longer cost-effective when the treatment effect on suicide likelihood was removed. Taken together, our findings show that the analytic perspective matters when evaluating early intervention in psychosis and that it is economically favorable to fund this treatment program in the United States.
1 : Treatment implications of the schizophrenia prodrome. Curr Top Behav Neurosci 2010; 4:97–121Crossref, Medline, Google Scholar
2 Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA, American Psychiatric Publishing, 2013Google Scholar
3 : The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry 1993; 50:85–94Crossref, Medline, Google Scholar
4 : Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015; 72:1172–1181Crossref, Medline, Google Scholar
5 : The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry 2016; 77:764–771Crossref, Medline, Google Scholar
6 : Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry 2018; 75:555–565Crossref, Medline, Google Scholar
7 : Brain imaging of first-episode psychosis. Encephale 2013; 39(suppl 2):S93–S98Crossref, Medline, Google Scholar
8 : Cost-effectiveness of early intervention in psychosis: systematic review. Br J Psychiatry 2019; 215:388–394Crossref, Medline, Google Scholar
9 : An examination of costs, charges, and payments for inpatient psychiatric treatment in community hospitals. Psychiatr Serv 2012; 63:666–671Link, Google Scholar
10 : The value of medical spending in the United States, 1960–2000. N Engl J Med 2006; 355:920–927Crossref, Medline, Google Scholar
11 : Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996; 276:1253–1258Crossref, Medline, Google Scholar
12 United States Life Tables, 2015. Atlanta,
13 : Association of an early intervention service for psychosis with suicide rate among patients with first-episode schizophrenia-spectrum disorders. JAMA Psychiatry 2018; 75:458–464Crossref, Medline, Google Scholar
14 : Cost-effectiveness of comprehensive, integrated care for first episode psychosis in the NIMH RAISE early treatment program. Schizophr Bull 2016; 42:896–906Crossref, Medline, Google Scholar
15 : Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016; 316:1093–1103Crossref, Medline, Google Scholar
16 : Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting US employers. J Occup Environ Med 2004; 46:398–412Crossref, Medline, Google Scholar
17 : Cost-effectiveness of 3-month paliperidone therapy for chronic schizophrenia in the Netherlands. J Med Econ 2017; 20:1187–1199Crossref, Medline, Google Scholar
18 Employer Costs for Employee Compensation Historical Listing: March 2004–March 2017. Washington, DC,
19 T13-0174: Average Effective Federal Tax Rates by Filing Status; by Expanded Cash Income Percentile, 2014. Washington, DC,
20 : Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ 2005; 14:339–347Crossref, Medline, Google Scholar
21 : Conceptualizing a model: a report of the ISPOR–SMDM Modeling Good Research Practices Task Force–2. Med Decis Making 2012; 32:678–689Crossref, Medline, Google Scholar
22 : Clinical effectiveness and cost-effectiveness of tailored intensive liaison between primary and secondary care to identify individuals at risk of a first psychotic illness (the LEGs study): a cluster-randomised controlled trial. Lancet Psychiatry 2015; 2:984–993Crossref, Medline, Google Scholar
23 : Early intervention for first-episode psychosis: broadening the scope of economic estimates. Early Interv Psychiatry 2016; 10:144–151Crossref, Medline, Google Scholar
24 : Do productivity costs matter? The impact of including productivity costs on the incremental costs of interventions targeted at depressive disorders. Pharmacoeconomics 2011; 29:601–619Crossref, Medline, Google Scholar
25 : Schizophrenia in Adults: Maintenance Therapy and Side Effect Management. Waltham, MA,
26 : Ten-year follow-up of the OPUS specialized early intervention trial for patients with a first episode of psychosis. Schizophr Bull 2015; 41:617–626Crossref, Medline, Google Scholar



