Antipsychotic Medications for Low-Income Preschoolers: Long Duration and Psychotropic Medication Polypharmacy
Abstract
Objective:
This study aimed to evaluate prescribing patterns of antipsychotic medication and factors that predict duration of use among low-income, preschool-age children.
Methods:
State Medicaid claims from 2012 to 2017 were used to identify antipsychotic medication use for children <6 years old. ICD-9 and ICD-10 codes were used to describe child diagnoses. Descriptive and multivariable analyses were used to determine patterns of antipsychotic medication use and factors that predicted duration of use.
Results:
In 2012, 316 children <6 years of age started an antipsychotic medication in a southeastern state. Most were non-Hispanic White (N=202, 64%) and boys (N=231, 73%). Diagnoses included attention-deficit hyperactivity disorder (N=288, 91%), neurodevelopmental disorders (N=208, 66%), anxiety and trauma-related diagnoses (N=202, 64%), and autism spectrum disorders (ASDs) (N=137, 43%). The mean±SD duration of exposure to antipsychotic medication for children in the cohort was 2.6±1.7 years, but 86 children (27%) had >4 years of exposure. Almost one-third (N=97, 31%) received polypharmacy of four or more medication classes, and 42% (N=131) received metabolic screening. Being male, being in foster care, and having a diagnosis of ASD or disruptive mood dysregulation disorder were significantly associated with duration of use of antipsychotic medications; race-ethnicity was not significantly associated with duration of use. Emergency department visits (N=277, 88%) and inpatient hospitalizations (N=107, 34%) were observed during the study period.
Conclusions:
Many preschoolers received antipsychotic medications for substantial periods. Further research is needed to identify evidence-based practices to reduce medication use and improve outcomes.
HIGHLIGHTS
Preschoolers who are started on antipsychotic medications often remain on them for long periods.
Some preschoolers treated with antipsychotic medications do not see a psychiatrist, and many do not receive metabolic monitoring.
Preschoolers treated with antipsychotic medications experience concerning levels of polypharmacy.
The use of antipsychotic medication for children <6 years of age is mostly off-label and is concerning because of the lack of empirical evidence related to efficacy and long-term impacts of such use on children’s health (1). As such, there is a need to better understand the demographic and other risk factors associated with the use of antipsychotic medications for preschool-age children to inform treatment guidelines.
Concerns have been raised about the use of antipsychotic medications in this population. Olfson et al. (2) found that the annualized rate of antipsychotic use per 1,000 children ages 2–5 years with private insurance increased from 0.78 in 1999–2001 to 1.59 in 2007. Fewer than half of the young children treated with these medications received a mental health assessment (41%) or attended a psychotherapy visit (41%) or a visit with a psychiatrist (43%) during 1 year of antipsychotic use. Preschoolers started on antipsychotics often remain on them for long periods and have complex or severe problems that are managed with other classes of psychotropic medications (3).
In a southeastern state, the use of second-generation antipsychotics for children <6 years of age covered by Medicaid increased over the 2001–2010 period, with a peak rate of about 1% in 2004. Older and male children were more likely to receive second-generation antipsychotics, and risperidone accounted for two-thirds of the prescriptions filled. Only 32% of the prescriptions were written by psychiatrists. Geographic analysis showed significantly higher use in some Medicaid service regions in the western part of the state (4).
More recent data suggest that overall prescriptions of psychotropic medications to preschoolers ages 2–4 years have decreased in recent years (1, 5). Overall prevalence rates of antipsychotic use have also decreased significantly for youths ages 0–17, but the largest decrease was observed for the youngest children, ages 0–5 years. Rates fell from 9.06 per 1,000 children to 3.79 per 1,000 children from 2008 to 2013 (6). Despite these decreases, rates are still higher for children and youths from low-income families and for those in foster care (7, 8).
Despite these recent trends, concerns remain. The full extent of adverse effects of antipsychotic medications in preschoolers is unknown and concerns exist about increased risks of weight gain and metabolic problems (9, 10). The purpose of this longitudinal cohort study was to describe ongoing use over a 6-year period among children <6 years old with an antipsychotic medication prescription in 2012 and to determine the covariates for duration of use.
Methods
Sample
We reviewed Medicaid pharmacy and medical claims for one U.S. state for 2012–2017. A total of 465 children <6 years old received an antipsychotic medication in 2012, and 316 were continuously enrolled in Medicaid through 2017 (see Figure S1 in the online supplement). The duration of exposure to antipsychotic medication was measured by summing the total days of pharmacy claims for each cohort member for the years of the study.
Definitions and Covariates
Diagnoses were identified by using ICD-9 and ICD-10 codes within the medical claims. Antipsychotic medications were indicated by the National Drug Code description found in Medicaid pharmacy data (see Tables S1 and S2 in the online supplement). Polypharmacy was defined as patients concurrently receiving at least two psychotropic medications from at least two classes of medication for at least 90 consecutive days, allowing for a single 15-day lag (11). Polypharmacy with three psychotropic medication classes and with at least four classes were also identified. Psychosocial therapy was identified as the presence of behavioral health therapy at least once within a calendar year, indicated in the medical claims by one of the following Current Procedural Terminology codes: 90832–34, 90836–40, 90840, 90845–49, 90853, 90875–76, or 90880. These codes include both inpatient and outpatient visits for individual or group therapy.
Race and ethnicity were obtained from the Medicaid enrollment data for 2012–2017. Geography of child residence was coded by using 2013 Rural Urban Continuum Codes (RUCCs), as defined by the U.S. Department of Agriculture, such that county of residence RUCCs of 1–3 were classified as “metro” and 4–9 codes as “nonmetro.” Metabolic dysfunction was defined as the presence of a medical claim within the 6-year follow-up period with a diagnosis of at least one of the following conditions: insulin resistance, type 2 diabetes, prediabetes, or hyperlipidemia.
The provider categories were created by combining the National Provider Identifier from claims and the classification from the National Uniform Claim Committee. The final provider classifications were then categorized into binary variables indicating whether the child had been seen at least once or not seen by a primary care provider (PCP) or psychiatrist, including general or child and adolescent psychiatrists.
Statistical Analysis
A multivariable Poisson regression model was used to determine the demographic and diagnostic characteristics present in 2012 that were associated with the duration of antipsychotic medication treatment. A multivariable Poisson regression model was used because the primary outcome was count data (total days with antipsychotic medication treatment during the 6-year period). A Pearson scaling factor, which computes a scaling factor (i.e., the Pearson Q statistic divided by its degrees of freedom), was used to adjust for overdispersion. The University of Louisville Institutional Review Board approved the study protocol. The state’s Cabinet for Health and Human Services provided the Medicaid data.
Results
Descriptive Characteristics
A total of 316 children <6 years old, with a mean±SD age of 4.6 ±0.64 years, were started on an antipsychotic medication in 2012 in the state. The demographic characteristics of our sample were as follows: 202 children (64%) were non-Hispanic White; 32 (10%) were non-Hispanic Black; 15 (5%) were Hispanic; five (2%) were American Indian, Native Hawaiian, or other race; and 62 (20%) had no race-ethnicity provided. The sample was 73% (N=231) boys. These children experienced substantial polypharmacy, with 25% (N=79) of the cohort receiving concurrent medications of two classes, 34% (N=107) receiving three classes, and 31% (N=97) receiving four or more classes over the study period (Table 1). The mean duration of exposure to antipsychotic medication for children in the cohort was 2.6±1.7 years, but 86 children (27%) had >4 years of exposure, and about 7% (N=22) of the children had near-continuous antipsychotic exposure (Figure 1). The median annual duration of antipsychotic medication use was 256 days, and the annual median duration of use of at least two medication classes was 266 days. The distribution of days on antipsychotic medication in our cohort was not significant among race-ethnicity categories (see Figure S2 in the online supplement).
| Total exposure to antipsychotic medication (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (N=316) | 0–2 (N=126) | 2–4 (N=104) | 4–6 (N=86) | ||||||
| Variable | N | % | N | % | N | % | N | % | pb |
| Age in 2017 (M±SD years) | 9.6±.6 | 9.6±.7 | 9.7±.6 | 9.5±.7 | .027 | ||||
| Exposure to antipsychotic (M±SD years) | 2.6±1.7 | .8±.6 | 3.1±.6 | 4.6±.4 | <.001 | ||||
| Foster care | 63 | 20 | 23 | 18 | 17 | 16 | 23 | 27 | .169 |
| Nonmetro area | 202 | 64 | 87 | 69 | 67 | 64 | 48 | 56 | .142 |
| Median comorbid psychiatric diagnosesc | 2.5 | 2.0 | 3.0 | 3.0 | <.001 | ||||
| Diagnosis | |||||||||
| Schizophrenia and other psychotic processes | 30 | 10 | 5 | 4 | 13 | 13 | 12 | 14 | .023 |
| Autism spectrum disorders | 137 | 43 | 31 | 25 | 51 | 49 | 55 | 64 | <.001 |
| Other neurodevelopmental disordersd | 208 | 66 | 68 | 54 | 70 | 67 | 70 | 81 | .000 |
| Disruptive behavior disorders (total) | 293 | 93 | 116 | 92 | 101 | 97 | 76 | 88 | .065 |
| Attention-deficit hyperactivity disorder | 288 | 91 | 112 | 89 | 100 | 96 | 76 | 88 | .089 |
| Oppositional defiant disorder | 165 | 52 | 63 | 50 | 54 | 52 | 48 | 56 | .706 |
| Conduct disorder | 173 | 55 | 67 | 53 | 56 | 54 | 50 | 58 | .756 |
| Mood disorders (total) | 203 | 64 | 69 | 55 | 74 | 71 | 60 | 70 | .016 |
| Depressive disorders | 174 | 55 | 54 | 43 | 69 | 66 | 51 | 59 | .001 |
| Bipolar disorder | 88 | 28 | 26 | 21 | 35 | 34 | 27 | 31 | .063 |
| Mood disorder not otherwise specified | 162 | 51 | 48 | 38 | 63 | 61 | 51 | 59 | .001 |
| Disruptive mood dysregulation disorder | 90 | 29 | 21 | 17 | 42 | 40 | 27 | 31 | <.001 |
| Anxiety and trauma-related disorders (total) | 202 | 64 | 78 | 62 | 66 | 63 | 58 | 67 | .707 |
| PTSD | 70 | 22 | 23 | 18 | 26 | 25 | 21 | 24 | .395 |
| Tic disorders | 24 | 8 | 10 | 8 | 6 | 6 | 8 | 9 | .647 |
| Epilepsy and seizure disorders | 70 | 22 | 23 | 18 | 26 | 25 | 21 | 24 | .395 |
| Polypharmacye | |||||||||
| 2 medication classes | 79 | 25 | 50 | 40 | 22 | 21 | 7 | 8 | .004 |
| 3 medication classes | 107 | 34 | 34 | 27 | 42 | 40 | 31 | 36 | <.001 |
| ≥4 medication classes | 97 | 31 | 11 | 9 | 39 | 38 | 47 | 55 | <.001 |
| Prescribed by a psychiatrist | 266 | 84 | 99 | 79 | 91 | 88 | 76 | 88 | .083 |
| Prescribed by a primary care provider | 300 | 95 | 118 | 94 | 97 | 93 | 85 | 99 | .153 |
| Visit with a primary care provider | 297 | 94 | 118 | 94 | 97 | 93 | 82 | 95 | .818 |
| Emergency department visit | 277 | 88 | 109 | 87 | 88 | 85 | 80 | 93 | .189 |
| Inpatient hospitalizationf | 107 | 34 | 27 | 21 | 44 | 42 | 36 | 42 | .001 |
| Metabolic screeningg | 131 | 42 | 32 | 25 | 52 | 50 | 47 | 55 | <.001 |
| Lipid screening | 150 | 48 | 42 | 33 | 57 | 55 | 51 | 59 | <.001 |
| Blood glucose screening | 236 | 75 | 81 | 64 | 83 | 80 | 72 | 84 | .002 |
| Metabolic dysfunctionh | 28 | 9 | 5 | 4 | 11 | 11 | 12 | 14 | .032 |
TABLE 1. Characteristics of preschool-age children (N=316) started on an antipsychotic medication in 2012, by years of exposure from 2012 to 2017a
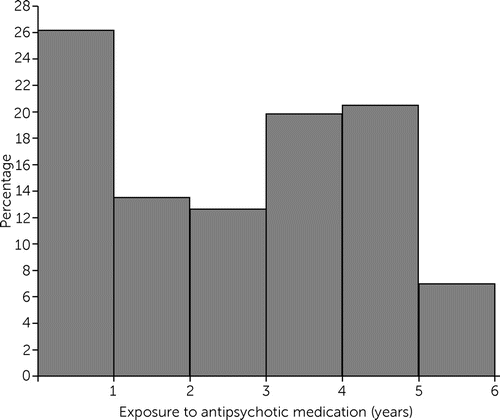
FIGURE 1. Proportion of preschool-age children (N=316) prescribed antipsychotic medication, by years of exposure, 2012–2017
Diagnoses
The most common diagnoses over the study period for the cohort who received an antipsychotic were attention-deficit hyperactivity disorder (ADHD) (N=288, 91%), neurodevelopmental disorders (N=208, 66%), and anxiety and trauma-related diagnoses (N=202, 64%). Autism spectrum disorders (ASDs) were observed among 43% (N=137) of the cohort, and schizophrenia and other psychotic processes were present among 10% (N=30) (Table 1). Children exposed to at least 2 years of antipsychotic medication had a higher median number of diagnoses and more diagnoses of ASD, other neurodevelopmental disorders, depressive disorders, mood disorders not otherwise specified, and disruptive mood dysregulation disorder (DMDD).
Safety and Monitoring
Most, but not all, children in the cohort had an antipsychotic prescribed by a psychiatrist (N=266, 84%) or PCP (N=300, 95%) (Table 1). Only 42% (N=131) of the children received metabolic screening (defined as either a glucose or hemoglobin A1c and lipid panel) over the entire study period. Glucose testing (N=236, 75%) was more common than lipid testing (N=150, 48%). Metabolic screening was more common among children exposed to longer durations of antipsychotic medication, and the annual rate of metabolic screening in the cohort increased from 9% (N=28) in 2012 to 17% (N=55) in 2017. Almost all children (N=277, 88%) had an emergency department (ED) visit, and 34% (N=107) had an inpatient visit over the study period, for any reason. Children with a longer duration of exposure to antipsychotic medication were significantly more likely to have an inpatient hospitalization. Metabolic dysfunction (defined by codes for insulin resistance, type 2 diabetes mellitus, prediabetes, or hyperlipidemia) was found in 28 (9%) of our cohort overall but in 14% (N=12) of the children exposed to ≥4 years of antipsychotics.
Children received antipsychotics from a variety of providers. Psychiatrists accounted for 5,507 (45%) antipsychotic prescriptions dispensed to the study cohort, and various PCPs prescribed 4,112 (34%). Nurse practitioners wrote prescriptions for antipsychotics to 127 children and accounted for 1,635 dispenses (13%).
Types of Medications
Risperidone was the most utilized medication in our cohort, prescribed to 93% (N=294) of children, followed by clonidine (N=230, 73%), amphetamine-based stimulants (N=229, 72%), and guanfacine (N=212, 67%). The most common antidepressant, trazodone, was prescribed to 95 children (30%), and hydroxyzine was prescribed to 83 children (26%). Alpha-agonists were the most common additional drug class, prescribed to 286 children (91%), followed by psychostimulants (N=275, 87%) and antidepressants (N=200, 63%) (Table 2). Among children with ≥4 years of exposure to antipsychotics, the most common combinations of medications were antipsychotics, alpha-agonists, and stimulants. Anticonvulsants and mood stabilizers were prescribed to 157 children (50%), and 70 children (22%) of our cohort had epilepsy and seizure disorders.
| Medication | N | % |
|---|---|---|
| Antipsychotics | 316 | 100 |
| First generation | 17 | 5 |
| Second generation | 315 | >99 |
| Risperidone | 294 | 93 |
| Aripiprazole | 100 | 32 |
| Quetiapine | 85 | 27 |
| Stimulants | 275 | 87 |
| Amphetamine based | 229 | 72 |
| Methylphenidate | 194 | 61 |
| Dexmethylphenidate | 95 | 30 |
| Alpha-agonists | 286 | 91 |
| Clonidine | 230 | 73 |
| Guanfacine | 212 | 67 |
| Antidepressants | 200 | 63 |
| Trazodone | 95 | 30 |
| Sertraline | 67 | 21 |
| Mirtazapine | 53 | 17 |
| Fluoxetine | 50 | 16 |
| Anticonvulsants and mood stabilizers | 157 | 50 |
| Valproic acid | 76 | 24 |
| Oxcarbazepine | 59 | 19 |
| Lamotrigine | 55 | 17 |
| Lithium | 13 | 4 |
| Benzodiazepines, anxiolytics, and hypnotics | 115 | 36 |
| Hydroxyzine | 83 | 26 |
TABLE 2. Types of medications prescribed to preschool-age children (N=316) started on antipsychotic medication in 2012, between 2012 and 2017
Predictors of Duration of Antipsychotic Medication
Descriptive factors were examined as predictors of the duration (total days) of antipsychotic medication use over 2012–2017 (Table 3). Being male, being in foster care, and having a diagnosis of ASD or DMDD were significantly associated with the duration of use. Receiving an antipsychotic from a psychiatrist in 2012 was not associated with treatment duration. A Poisson regression sensitivity analysis examining race-ethnicity (non-Hispanic White vs. all other racial-ethnic categories) as a predictor of the duration of exposure to antipsychotic medications was not statistically significant.
| Variable | IRRa | 95% CI | p |
|---|---|---|---|
| Male (reference: female) | 1.25 | 1.04–1.49 | .017* |
| Nonmetro residence (reference: metro residence) | .95 | .82–1.11 | .536 |
| Prescribed by a psychiatrist | .91 | .78–1.06 | .230 |
| Child in foster care in 2012 | 1.25 | 1.00–1.56 | .046* |
| Schizophrenia and other psychotic processes | 1.04 | .68–1.59 | .855 |
| Autism spectrum disorders | 1.26 | 1.03–1.54 | .022* |
| Other neurodevelopmental disordersb | 1.14 | .90–1.45 | .272 |
| Disruptive behavior disorders | |||
| Attention-deficit hyperactivity disorder | .98 | .80–1.20 | .440 |
| Oppositional defiant disorder | 1.08 | .89–1.32 | .096 |
| Conduct disorder | .83 | .67–1.03 | .827 |
| Mood disorders | |||
| Depressive disorders | 2.12 | .90–4.97 | .084 |
| Bipolar disorder | 1.24 | .94–1.64 | .126 |
| Mood disorder not otherwise specified | .50 | .21–1.16 | .108 |
| Disruptive mood dysregulation disorder | .38 | .15–.98 | .044* |
| Anxiety and trauma-related disorders | |||
| PTSD | 1.18 | .86–1.63 | .302 |
| Tic disorders | .84 | .40–1.77 | .644 |
| Epilepsy and seizure disorders | 1.01 | .77–1.31 | .956 |
| At least one inpatient code in 2012 | 1.16 | .89–1.50 | .265 |
| At least one emergency department visit in 2012 | 1.01 | .87–1.17 | .904 |
| N of mental disorders in 2012 | 1.00 | .90–1.10 | .967 |
TABLE 3. Factors associated with duration of treatment for preschool-age children (N=316) started on antipsychotic medication in 2012, from 2012 to 2017
Discussion
The current longitudinal study detailed antipsychotic medication use and duration in a cohort of preschoolers in a southeastern state from 2012 to 2017 who were receiving Medicaid and were prescribed an antipsychotic in 2012. These children were treated with antipsychotics for long durations, with significant rates of concurrent medication use. The mean duration of exposure to antipsychotic medications in our cohort was 2.6±1.7 years, but 86 children (27%) had at least 4 years exposure. This group was notable for having increased comorbid conditions and being treated with polypharmacy.
Little evidence related to the efficacy or safety of using antipsychotics for preschool children is available. Few antipsychotics have been approved by the U.S. Food and Drug Administration (FDA) for use in this population. The only FDA-approved second-generation antipsychotic for children <6 years old is risperidone for the treatment of irritability associated with ASD (12), and aripiprazole has similar indications starting at age 6.
Psychostimulants and alpha-agonists were the most common additional categories of medications used, suggesting that providers are diagnosing and managing ADHD and disruptive behavior among young children. Also, the diagnosis of DMDD was significantly associated with duration of antipsychotic use. This diagnosis, added to DSM-5 in 2013, includes criteria of temper outbursts out of proportion to the situation and inconsistent with developmental level (13). This diagnosis should not be used before the age of 6 years, so the number of preschool children in our sample treated with antipsychotic medications for DMDD and the lack of evidence-based treatment guidelines for this diagnosis raise concerns about care quality (14). Recent studies, such as the Treatment of Severe Childhood Aggression study (15), suggest that adding risperidone, after initial implementation of psychostimulants and parent-management training, to treat complex ADHD can be beneficial. But the efficacy and safety for children <6 years are unknown. Insomnia is also a frequent target of treatment, given the number of children receiving clonidine and trazodone.
Polypharmacy was a significant concern for our cohort. About one-third of our population received polypharmacy, with four or more classes of medications at some point in our study, including 55% (N=47) of the children with the most exposure to antipsychotic medications. Safety risks of polypharmacy include increased adverse events, drug interactions, and weight gain (16). Furthermore, the addition of antipsychotics to medication combinations may lower the seizure threshold, which could be a particular concern for children with known seizures, autism, and neurodevelopmental concerns (17, 18). There is very little evidence to guide the efficacy and safety of polypharmacy among preschool-age children, so research and problem solving are clearly needed (19).
The diagnoses identified in our cohort suggest that the children in our sample had complex mental health problems, including developmental, emotional, and behavioral difficulties, and 20% (N=63) were in foster care. Despite receiving multiple forms of treatment, the children in our cohort struggled with high rates of inpatient and ED utilization: 277 children (88%) had an ED visit, and 107 (34%) had an inpatient hospitalization over the study period. Our study did not separately consider the reasons for these visits, but other researchers have documented increases in inpatient hospitalizations and ED visits for mental health reasons among this population (20).
Our data suggest that professional guidelines for monitoring young children receiving antipsychotics are not being consistently followed. Some children (N=50, 16%) did not have antipsychotics prescribed by a psychiatrist, and 6% (N=19) had no medical claims from a PCP over the study period. In our cohort, 25% (N=80) of the children did not undergo a glucose test, and over half (N=166, 53%) did not have a lipid test. Preschool children can experience known adverse effects of antipsychotics, including rapid weight gain and metabolic dysfunction (21–25). The National Committee for Quality Assurance has recommended metabolic monitoring and use of first-line psychosocial care as measures for safe and judicious antipsychotic medication use among youths, but implementation varies by health care plan (26), and a minority of young children on Medicaid may receive psychosocial treatments before receiving antipsychotic treatment (27). Providers may be less likely to monitor children on antipsychotics for metabolic adverse effects (28). Younger children are less likely than teenagers to have baseline glucose screening (29) even though younger children may be more likely than older individuals to have a significant increase in glucose within the first 6 months of treatment (30).
Concerns have been raised about the duration of antipsychotic use among youths receiving Medicaid (31) as a contributing factor to the development of long-term adverse effects, such as type 2 diabetes (9, 32). In a cohort study, <3% of children ages 5–15 years were diagnosed as having insulin resistance (33), but children <10 years comprise a small but increasing number of children diagnosed as having type 2 diabetes mellitus (34), which has been reported among children as young as 4 years (35). Because only 42% (N=131) of our cohort received recommended metabolic monitoring over the years of the study, rates of metabolic abnormalities could be higher. We found that 9% (N=28) of our cohort had metabolic dysfunction, but it is difficult to compare this finding with those of other reports describing rates of metabolic abnormalities in preschool populations.
Many safety concerns exist for the use of antipsychotics for preschool-age children. Variations in pharmacokinetics may place children at higher risk for some adverse effects (36). The potential neurobehavioral toxicity to youths treated with these medications, especially preschool-age children, is unknown. As the developing brain matures, multiple synapses and circuits form to create the foundation for the adult brain. Immature brains have a great degree of plasticity, meaning the brain is very sensitive to environmental stimulation. In the case of psychotropic medication, an adult brain may temporarily accommodate the effect of a drug, but the juvenile brain assimilates the drug into its system, producing permanent alterations in the system (37). The administration of psychotropic medications, including antipsychotics, may have lifelong effects on brain function and structure (38).
Because of the complex nature of investigating these questions in humans, much of our understanding on the long-term effects of antipsychotics on the brain and behavior come from animal studies that raise questions about potential alterations in brain structure, executive functioning, emotional regulation, and maternal-offspring interaction (39–43). These findings suggest that chronic antipsychotic use in pediatric populations (e.g., treatment for the symptoms of autism) could modify brain development and alter neural set points for specific behaviors during adulthood. More research is needed to explore these questions.
Additional research is also needed to identify the best strategies for treating young children with complex mental health problems. Our results show that children received antipsychotic prescriptions from multiple prescribers over the course of the study. This pattern highlights a need for communication among all treating clinicians and parents or guardians to understand the rationale and indications behind ongoing antipsychotic use among children and to review alternative treatment options. Systems-of-care treatment approaches that focus on the environment of the child and offer case management services could help (44). In addition to targeting social determinants of health risk factors, such a system could ensure that children and families receive collaborative care based on evidence-based therapies and individualized support to increase rates of metabolic monitoring and guidance for nutrition and exercise management.
Increased psychotropic medication monitoring is an element of improved health care oversight and coordination for children (45). Increased attention to antipsychotic use within the preschool population may identify cases where additional services can be implemented to reduce the use of these medications and improve long-term outcomes. Given these concerns, many states have built a prior authorization process that detects and limits the use of antipsychotics for children <6 years (6, 46). In our state, all Medicaid services were organized into managed care organizations in 2013, and various prior authorization procedures for antipsychotic medications were introduced in later years.
This study utilized administrative claims data, with known limitations (47). We studied only children with continuous enrollment over the study period to examine long-term covariates, so our population may have had additional risk factors compared with children who were not continuously enrolled. Children entered the cohort if they had received an antipsychotic medication at any time in 2012, and we studied descriptive variables for the subsequent years when children received an antipsychotic, potentially reducing the accuracy of our longitudinal findings. Diagnostic data were reported by clinicians and limited to four diagnoses per claim, raising the possibility of errors in billing procedures. In addition, the introduction of DSM-5 in 2013 may have altered the associations between diagnoses and antipsychotic use in our cohort. The accuracy of provider identification was limited, and our provider information did not distinguish child psychiatrists as a separate provider type. We did not separate out inpatient and ED claims data by nature of the visit nor did we examine other eligibility categories. Pharmacy claims were generated only if a prescription was filled, so actual prescribing practices by providers could not be determined. Administrative data did not allow us to determine the appropriateness of the diagnosis or treatments. Our study represents Medicaid claims from one state, so it is unknown whether our findings are generalizable to other states.
Conclusions
Some preschool-age children receive antipsychotic medications and concurrent treatment with other drug classes for substantial periods. Further study is needed to identify evidence-based practices to improve care quality and outcomes for this vulnerable population.
1 : Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry 2015; 72:867–874Crossref, Medline, Google Scholar
2 : Trends in antipsychotic drug use by very young, privately insured children. J Am Acad Child Adolesc Psychiatry 2010; 49:13–23Crossref, Medline, Google Scholar
3 : Exposure to antipsychotic medications over a 4-year period among children who initiated antipsychotic treatment before their sixth birthday. Pharmacoepidemiol Drug Saf 2012; 21:152–160Crossref, Medline, Google Scholar
4 : Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol 2015; 25:440–443Crossref, Medline, Google Scholar
5 : Psychotropic medication in children and adolescents in the United States in the year 2004 vs 2014. Daru 2018; 26:5–10Crossref, Medline, Google Scholar
6 : Trends in antipsychotic prescribing in Medicaid-eligible youth. J Am Acad Child Adolesc Psychiatry 2017; 56:59–66Crossref, Medline, Google Scholar
7 : Rapid growth of antipsychotic prescriptions for children who are publicly insured has ceased, but concerns remain. Health Aff 2016; 35:974–982Crossref, Google Scholar
8 : The relationship between mental health diagnosis and treatment with second-generation antipsychotics over time: a national study of US Medicaid-enrolled children. Health Serv Res 2012; 47:1836–1860Crossref, Medline, Google Scholar
9 : Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry 2013; 70:1067–1075Crossref, Medline, Google Scholar
10 : A comparison of risperidone-induced weight gain across the age span. J Clin Psychopharmacol 2004; 24:429–436Crossref, Medline, Google Scholar
11 : Psychotropic polypharmacy among children and youth receiving Medicaid, 2012–2015. J Manag Care Spec Pharm 2018; 24:736–744Crossref, Medline, Google Scholar
12 : Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002; 347:314–321Crossref, Medline, Google Scholar
13 Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA, American Psychiatric Association, 2013Google Scholar
14 : Focus on disruptive mood dysregulation disorder: a review of the literature. Psychiatry Res 2019; 279:323–330Crossref, Medline, Google Scholar
15 : What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry 2014; 53:47–60.e1Crossref, Medline, Google Scholar
16 : Combination pharmacotherapy for psychiatric disorders in children and adolescents: prevalence, efficacy, risks and research needs. Paediatr Drugs 2013; 15:377–391Crossref, Medline, Google Scholar
17 : Effects of psychotropic drugs on seizure threshold. Drug Saf 2002; 25:91–110Crossref, Medline, Google Scholar
18 : Autism. Lancet 2013; 383:896–910. doi: 10.1016/S0140-6736(13)61539-1Crossref, Medline, Google Scholar
19 : Family adverse experiences and psychotropic polypharmacy among us youth: 2009-2015. Pediatrics 2020; 145:e20192705Crossref, Medline, Google Scholar
20 : Annual report on health care for children and youth in the United States: national estimates of cost, utilization and expenditures for children with mental health conditions. Acad Pediatr 2015; 15:19–35Crossref, Medline, Google Scholar
21 : Metabolic and cardiovascular adverse events associated with antipsychotic treatment in children and adolescents. Arch Pediatr Adolesc Med 2008; 162:929–935Crossref, Medline, Google Scholar
22 : Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs 2013; 15:139–150Crossref, Medline, Google Scholar
23 : Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 2009; 302:1765–1773Crossref, Medline, Google Scholar
24 : Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry 2011; 26:144–158Crossref, Medline, Google Scholar
25 : Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: comprehensive review of prospective head-to-head and placebo-controlled comparisons. Eur Neuropsychopharmacol 2011; 21:621–645Crossref, Medline, Google Scholar
26 : Quality measures for managing prescription of antipsychotic medication among youths: factors associated with health plan performance. Psychiatr Serv 2019; 70:1020–1026Link, Google Scholar
27 : Access to psychosocial services prior to starting antipsychotic treatment among Medicaid-insured youth. J Am Acad Child Adolesc Psychiatry 2016; 55:69–76.e3Crossref, Medline, Google Scholar
28 : Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med 2010; 164:344–351Crossref, Medline, Google Scholar
29 : Adherence to guidelines for glucose assessment in starting second-generation antipsychotics. Pediatrics 2014; 134:e1308–e1314Crossref, Medline, Google Scholar
30 Second-generation antipsychotic use in children and adolescents: a six-month prospective cohort study in drug-naive patients. J Am Acad Child Adolesc Psychiatry 2014; 53:1179–1190Crossref, Medline, Google Scholar
31 : Atypical antipsychotic use among Medicaid-insured children and adolescents: duration, safety, and monitoring implications. J Child Adolesc Psychopharmacol 2014; 24:112–119Crossref, Medline, Google Scholar
32 : Risk for incident diabetes mellitus following initiation of second-generation antipsychotics among Medicaid-enrolled youths. JAMA Pediatr 2015; 169:e150285Crossref, Medline, Google Scholar
33 : Insulin resistance in a cohort of 5–15-year-old children in urban Sri Lanka. BMC Res Notes 2017; 10:347Crossref, Medline, Google Scholar
34 : Incidence of diabetes in youth in the United States. JAMA 2007; 297:2716–2724Crossref, Medline, Google Scholar
35 : Type 2 diabetes in a four-year-old child. CMAJ 2017; 189:E888–E890Crossref, Medline, Google Scholar
36 : Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet 2006; 45:1077–1097Crossref, Medline, Google Scholar
37 : Annual research review: new frontiers in developmental neuropharmacology: can long-term therapeutic effects of drugs be optimized through carefully timed early intervention? J Child Psychol Psychiatry 2011; 52:476–503Crossref, Medline, Google Scholar
38 : Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry 2020; 77:674–683Crossref, Medline, Google Scholar
39 : Olanzapine treatment of adolescent rats causes enduring specific memory impairments and alters cortical development and function. PLoS One 2013; 8:e57308Crossref, Medline, Google Scholar
40 : Olanzapine treatment of adolescent rats alters adult reward behaviour and nucleus accumbens function. Int J Neuropsychopharmacol 2013; 16:1599–1609Crossref, Medline, Google Scholar
41 : Effects of risperidone on dopamine receptor subtypes in developing rat brain. Eur Neuropsychopharmacol 2007; 17:448–455Crossref, Medline, Google Scholar
42 : Adult rats treated with risperidone during development are hyperactive. Exp Clin Psychopharmacol 2013; 21:259–267Crossref, Medline, Google Scholar
43 : Early-life risperidone administration alters maternal-offspring interactions and juvenile play fighting. Pharmacol Biochem Behav 2015; 130:90–96Crossref, Medline, Google Scholar
44 ,
45 : Fostering psychotropic medication oversight for children in foster care: a national examination of states’ monitoring mechanisms. Adm Policy Ment Health 2017; 44:243–257Crossref, Medline, Google Scholar
46 : Second-generation antipsychotic prescribing patterns for pediatric patients enrolled in West Virginia Medicaid. Psychiatr Serv 2017; 68:1061–1067Link, Google Scholar
47 Strengths and Limitations of CMS Administrative Data in Research. Minneapolis, University of Minnesota, Research Data Assistance Center, 2018Google Scholar



